Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SAMSON OKIKIOLA OPARANTI | -- | 1641 | 2023-01-04 00:31:46 | | | |
| 2 | Catherine Yang | Meta information modification | 1641 | 2023-01-04 01:54:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Oparanti, S.O.; Rao, U.M.; Fofana, I. Natural Esters Additives to Improve the Properties. Encyclopedia. Available online: https://encyclopedia.pub/entry/39703 (accessed on 07 February 2026).
Oparanti SO, Rao UM, Fofana I. Natural Esters Additives to Improve the Properties. Encyclopedia. Available at: https://encyclopedia.pub/entry/39703. Accessed February 07, 2026.
Oparanti, Samson Okikiola, Ungarala Mohan Rao, Issouf Fofana. "Natural Esters Additives to Improve the Properties" Encyclopedia, https://encyclopedia.pub/entry/39703 (accessed February 07, 2026).
Oparanti, S.O., Rao, U.M., & Fofana, I. (2023, January 04). Natural Esters Additives to Improve the Properties. In Encyclopedia. https://encyclopedia.pub/entry/39703
Oparanti, Samson Okikiola, et al. "Natural Esters Additives to Improve the Properties." Encyclopedia. Web. 04 January, 2023.
Copy Citation
Regardless of their environmental and technical merits, natural esters have some limitations that are slowing down their total acceptance by transformer owners and utilities. Critical limitations and concerns include esters’ pour point, viscosity, oxidative stability, and ionization resistance.
green transformers
natural esters
additives and chemical modifications
1. Pour Point Depressants
The addition of a certain type of pour point depressants to natural esters has also proven to be an alternative means of reducing natural esters’ pour point to a fitting value that can withstand low-temperature performance. The addition of pour point depressants into the base liquid does not by any means affect the temperature of crystallization of wax in the solution, and it also has nothing to do with the percentage of wax that precipitates. The pour point depressant co-crystallizes with the wax of the base oil and reforms the structure of the wax crystal’s growth pattern [1]. It also keeps the wax crystals apart and prevents them from forming a three-dimensional structure that could resist the flow of the liquid. The most common depressant used by oil manufacturers is poly methyl methacrylate (PMMA). This has been reported to reduce the pour point of natural insulating oil by 10 °C when less than 1 % weight of it is applied [2]. The depressant helps in suppressing the development of large crystals during the solidification of the natural ester at low temperatures. Polymethacrylate, among others, stands out as a good pour point depressant. It has little or no effect on the chemical and dielectric properties of the base liquid (natural ester). It has a negligible effect on the acid value and dielectric loss of natural esters. The comparison of other pour point depressants with polymethacrylate can be seen in [1]. The Evonik Viscoplex 10 series is designed for use as an environmentally friendly depressant [3]. This depressant makes vegetable oil stable for 15 days without losing its fluidity, but the fresh oil (untreated) solidifies after 24 h. However, the temperature details are not discussed by authors.
A thermomechanical method known as crystalizing fractionation is also a systematic method of improving the pour point of natural esters. In this method, freezing crystallization is used to separate triglycerides with high melting points from those with low melting points [1][4]. In this method, a diatomite can be added to increase nucleation. This process is performed in two steps, viz., crystallization and filtration, respectively. The fractionation process is reversible, and the steps can be seen in Figure 1.
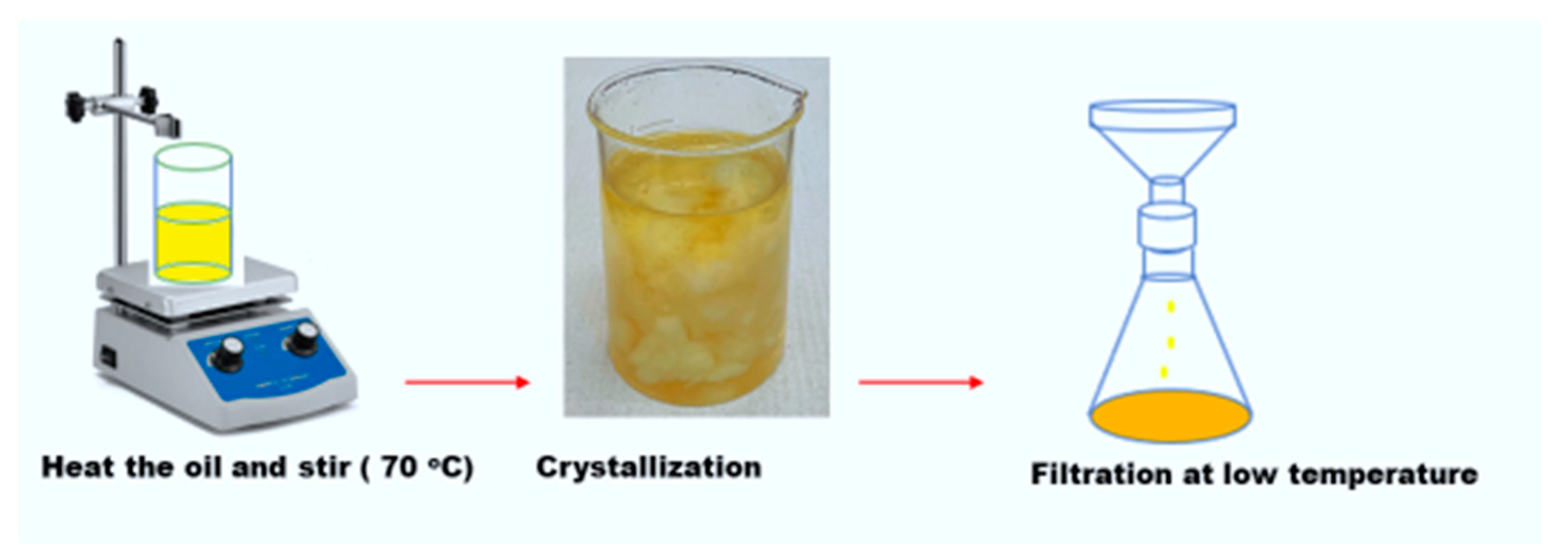
Figure 1. Fractional crystallization of natural esters.
Recently, crystallizing fractionation used on soybean oil drastically reduced the pour point of the oil from −13.5 °C to −20.3 °C [1]. However, the problem with this method is the reduction in the oxidation resistance due to the removal of triglycerides with high melting points [1]. The pour point property of an insulating oil needs proper study after aging to understand the potential of the pour point depressant even after aging. The pour point in [1] had a positive effect on oil crystallization properties after aging. This prevents the crystallization of the wax and the agglomeration of oxidation polar molecules. However, the effect of this pour point depressant on other physicochemical or dielectric properties of natural ester liquid has not yet been explored. Further, despite the enhancement of the pour point property, there is still a need for further study on the compatibility of pour point depressants with impregnated cellulose paper.
2. Oxidation Inhibitors
The oxidation in vegetable oil occurs in three different stages, viz., initiation, propagation, and termination. At the initiation stage, the alkyl free radical is formed due to some external factors such as heat, metals, or light. In the propagation stage, the free alkyl radicals react with oxygen molecules to form peroxyl free radicals. Thereafter, the peroxyl free radicals react with oil molecules to form hydroperoxide and a new alkyl free radical [5]. This process can be a continuous one in a cyclic manner. The peroxide generated in this stage is often termed a primary oxidation byproduct, which can be decomposed into a secondary oxidation byproduct (acids, ketones, and aldehydes) when subjected to thermal and electrical stress. The last stage is the termination stage. At this stage, the free radicals are joined together to form a stable compound [6]. Figure 2 shows a schematic diagram of the oxidation process. A radical hydroperoxide scavenger additive known as an antioxidant is used to protect materials from free radicals and oxidation. The antioxidant can be a donor or an acceptor. The chemistry of the reaction between the antioxidant (acceptor and donor) and the free radicals in the oil can be seen in Figure 3. The letters with asterisk mark in Figure 2 and Figure 3 are the radicals.
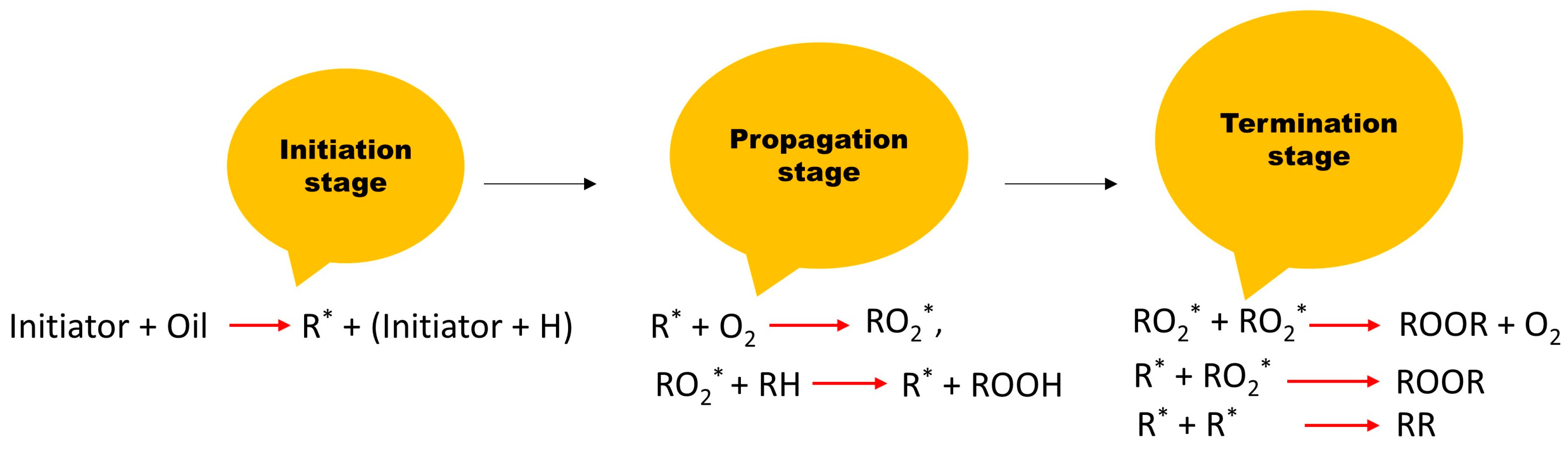
Figure 2. Stages of oil oxidation in vegetable oil.
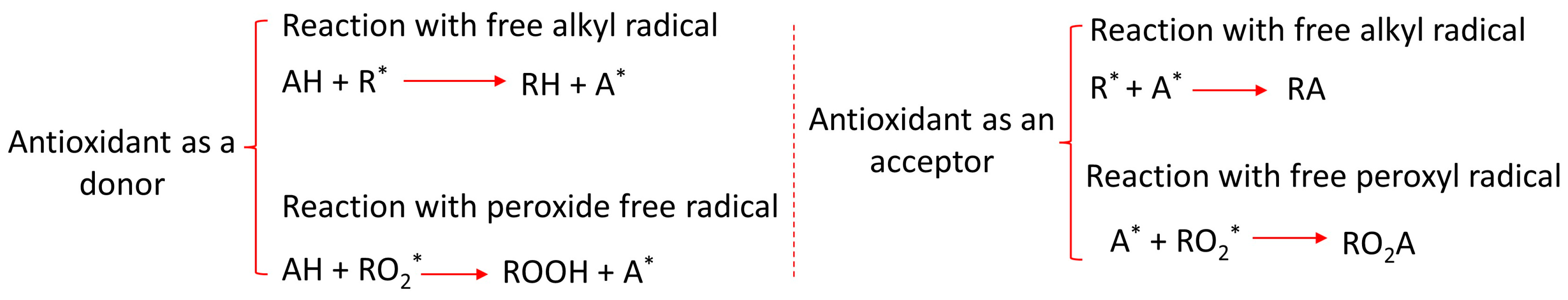
Figure 3. Reaction between antioxidant and the free radicals in the oil.
Over the decades, butylated hydroxytoluene (BTH) has been the main choice of manufacturers, because it increases the melting point and oxidative stability of insulating oil [7][8][9]. It is reported that despite the addition of this antioxidant to natural esters, the oxidative stability is still below that of mineral oil [10]. Researchers also utilized the tertiary butyl hydroxyquinone (TBHQ) as an antioxidant additive in alkyl esters. As reported, the choice of TBHQ was based on the fact that ester treated with TBHQ has less susceptibility to oxidation relative to butylated hydroxytoluene (BHT), butylated hydroxy anisole (BHA), and propyl gallate (PrG) α—tocopherols [11][12][13]. It is important to optimize the antioxidant loading, because excessive antioxidants can affect the quality of the base liquid. The optimization of TBHQ on palm kernel-based liquid was achieved in [14] by varying the percentage loading from 1 wt.% to 5 wt.%. It is reported that the optimum performance at 4 wt.% was observed when the alkyl ester was doped with TBHQ before epoxidation. In the same vein, a 3 wt.% optimum concentration was recorded for the epoxidized and side-branched alkyl esters. This is an indication that the antioxidant was able to protect the oxidative degradation of the long and branched carbon chains against much higher temperatures. In [15], the effect of different loading of antioxidants (BHT) on mineral oil, sunflower, and rapeseed oil was observed. Viscosity, AC breakdown analysis, dielectric dissipation factor, and partial discharge tests were used as factors for analyzing the rate of oxidation between inhibited and uninhibited samples. The sample preparation was achieved through the addition of a 0.25%, 0.3%, and 0.35% weight concentration into the base liquid, and copper metal was added to the glass vessel as a catalyst. An increase in the acidity of the uninhibited sample was observed, which was attributed to the oxidation products. This, consequently, increases the dielectric loss and decreases the breakdown voltage. The properties of the natural ester with inhibitors are moderately higher than base liquids, with an outstanding performance in sunflower oil apart from the viscosity value [15].
Several researchers have tried a mixture of different antioxidants, and the report showed a promising property [16][17]. The oxidation stability of rapeseed oil was enhanced in [17] through additive optimization using a two-level factorial design. Propyl gallate and citric acid were used as an antioxidant; the choice of selecting propyl gallate and citric acid was related to radical scavenging and hydroperoxide scavenging, respectively. The loading of equal proportion of antioxidants (0.25 wt%) gave an optimum performance based on the average oxidation induction time. The work reported in [16] also confirmed the possibility of mixing antioxidants for the oxidation stability enhancement of natural esters. A 0.3 wt.% of T501 (2, 6-ditert-butyl-4-methylphenol) and a 0.3 wt.% of L06 (high purity alkylation-α- naphthylamine) were mixed with natural ester liquid, and an increase in the oxidation onset temperature was reported. The effect of the addition of antioxidants on other properties, especially the dielectric properties of transformer insulating oil, also needs a proper inspection.
The effect of antioxidants on the dielectric properties of natural ester insulating liquid has been explored, and it was reported that antioxidants enhance the AC dielectric strength of the liquid as well as some physical properties such as viscosity, fire point, and flash point [2]. The improvement in the dielectric strength is attributed to the nature of the antioxidants being mono-aromatic compounds. It is proved beyond a reasonable doubt that mono-aromatic compounds enhance the dielectric strength of natural ester insulating liquid and give a low partial discharge, since they could enhance gas absorption under electrical stress [18][19][20][21]. In this regard, it may be concluded from the previous research that the enhancement of the oxidative stability of natural esters can be achieved by antioxidants without any pronounced negative effect on the electrical properties of the base liquid.
3. Electronic Scavengers
The challenges of natural esters’ poor ionizing potential have been addressed using some additives such as dimethylaniline and azobenzene, which showed an improvement in streamer propagation and impulse breakdown [2]. Table 1 shows a summary of previous additives used on natural esters. Additives with low ionization potential and low first excitation energy are desired for proper enhancement of the base liquids. It was hypothetically stated that the streamer propagation and the impulse breakdown strength were increased due to the difference in ionization potential and the first excitation energy values that occur between the additive and the dielectric liquid [22]. Despite this outstanding enhancement, it is important to notice that the addition of low-ionization potential additives affects other properties of the insulating oil. Further, it is to be noted that some types of additives are toxic and highly harmful to humans and the environment [23]. It is highly imperative to seek another means of curbing the menace of poor ionizing potential using an environmentally friendly material. An attempt to mitigate this effect is considered in the next section of this paper.
Table 1. Some additives used in the literature and their effect on natural esters.
| Reference | Additives | Factors | Percentage Increment |
|---|---|---|---|
| Unge et. al., 2013 [24] | dimethylaniline (DMA) (1 wt%) | Breakdown voltage and acceleration voltage | 32% and 90%, respectively |
| Unge, et. al., 2013 [25] | dimethylaniline, azobenzene (5 wt.%) | Acceleration voltage | 40% |
| Liang, et. al., 2019 [26] | (i) 4ethoxycarbonylphenyl-n-methyl-n-phenylformamidine, (ii) 2-(4′-diethylamino-2′-hydroxybenzoyl) benzoic acid hexyl ester (5wt.%) |
Light impulse voltage and AC breakdown strength | (i) 15% and 60%, 17% respectively (ii) 4.7% and 18.4%. |
References
- Yang, T.; Wang, F.; Yao, D.; Li, J.; Zheng, H.; Yao, W.; Lv, Z.; Huang, Z. Low-Temperature Property Improvement on Green and Low-Carbon Natural Ester Insulating Oil. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1459–1464.
- Ab Ghani, S.; Muhamad, N.A.; Noorden, Z.A.; Zainuddin, H.; Abu Bakar, N.; Talib, M.A. Methods for improving the workability of natural ester insulating oils in power transformer applications: A review. Electr. Power Syst. Res. 2018, 163, 655–667.
- Evonik Publication. Pour Point Depressants, A Treatise on Performance and Selection; Evonik Publication: Allentown, PA, USA, 2015.
- Zaliha, O.; Chong, C.L.; Cheow, C.S.; Norizzah, A.R.; Kellens, M.J. Crystallization properties of palm oil by dry fractionation. Food Chem. 2004, 86, 245–250.
- Bandara, K.; Ekanayake, C.; Saha, T.K.; Annamalai, P.K. Understanding the ageing aspects of natural ester based insulation liquid in power transformer. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 246–257.
- Xu, Y.; Qian, S.; Liu, Q.; Wang, Z. Oxidation stability assessment of a vegetable transformer oil under thermal aging. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 683–692.
- Kuliev, R.S.; Mamedov NSh Musaev, G.T. Effect of the antioxidant additive ionol on the properties of transformer oils. Chem. Technol. Fuels Oils 1970, 6, 300–301.
- Krishnamoorthy, P.R.; Vijayakumari, S.; Sankaralingam, S. Effect of antioxidants and metal deactivator on the oxidation of transformer oil. IEEE Trans. Electr. Insul. 1992, 27, 271–277.
- Rabelo Neto, R.C.; Lima, D.O.; Pinheiro, T.D.S.; Almeida, R.F.; Castro Dantas, T.N.; Dantas, M.S.G.; Araújo, M.A.S.; Cavalcante, C.L.; Azevedo, D.C.S. Thermo-Oxidative Stability of Mineral Naphthenic Insulating Oils: Combined Effect of Antioxidants and Metal Passivator. Ind. Eng. Chem. Res. 2004, 43, 7428–7434.
- Wu, J.; Zhang, J. Research and Development of Natural Vegetable Insulating Oil Based on Jatropha curcas Seed Oil. Energies 2020, 13, 4319.
- Abdelmalik, A.A. Chemically modified palm kernel oil ester: A possible sustainable alternative insulating fluid. Sustain. Mater. Technol. 2014, 1–2, 42–51.
- Sarin, A.; Arora, R.; Singh, N.P.; Sarin, R.; Malhotra, R.K. Oxidation Stability of Palm Methyl Ester: Effect of Metal Contaminants and Antioxidants. Energy Fuels 2010, 24, 2652–2656.
- Ryu, K. Effect of antioxidants on the oxidative stability and combustion characteristics of biodiesel fuels in an indirect-injection (IDI) diesel engine. J. Mech. Sci. Technol. 2009, 23, 3105–3113.
- Abdelmalik, A.A. The Feasibility of Using a Vegetable Oil-Based Fluid as Electrical Insulating Oil. Doctoral Dissertation, University of Leicester, Leicester, UK, 2012.
- Madavan, R.; Balaraman, S. Comparison of antioxidant influence on mineral oil and natural ester properties under accelerated aging conditions. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2800–2808.
- Hao, J.; Liao, R.; Liang, S.; Yang, L.; Zhu, M. Choice of antioxidants and their synergistic effect study towards new insulation oil. In Proceedings of the IEEE 9th International Conference on the Properties and Applications of Dielectric Materials, Harbin, China, 19–23 July 2009; pp. 53–56.
- Ab Ghani, S.; Muhamad, N.A.; Noorden, Z.A.; Zainuddin, H.; Talib, M.A. Oxidation stability enhancement of natural ester insulation oil: Optimizing the antioxidants mixtures by two-level factorial design. ARPN J. Eng. Appl. Sci. 2017, 12, 1694–1700.
- Azis, N.; Jasni, J.; Kadir, M.Z.A.A.; Mohtar, M.N. Suitability of Palm Based Oil as Dielectric Insulating Fluid in Transformers. J. Electr. Eng. Technol. 2014, 9, 662–669.
- Walker, J.; Valot, A.; Wang, Z.D.; Yi, X.; Liu, Q. M/DBT, new alternative dielectric liquids for transformers. In CIGRE D1-107 Colloquium; CIGRE: Paris, France, 2012; pp. 1–10.
- Evangelou, C.; Zaky, A.A.; Megahed, I.Y. The effect of organic additives on the breakdown strength of transformer oil. J. Phys. D Appl. Phys. 1973, 6, L60–L62.
- Zaky, A.A.; Megahed, I.Y.; Evangelou, C. The effect of organic additives on the breakdown and gassing properties of mineral oils. J. Phys. D Appl. Phys. 1976, 9, 841–849.
- Davari, N.; Åstrand, P.-O.; Ingebrigtsen, S.; Unge, M. Excitation energies and ionization potentials at high electric fields for molecules relevant for electrically insulating liquids. J. Appl. Phys. 2013, 113, 143707.
- Singha, S.; Viertel, J.; Unge, M.; Karlsson, J.; Johansson, K.; Faleke, H. Development of a natural ester liquid with significantly enhanced dielectric characteristics. In Proceedings of the IEEE 18th International Conference on Dielectric Liquids (ICDL), Bled, Slovenia, 29 June–3 July 2014.
- Unge, M.; Singha, S.; Van Dung, N.; Linhjell, D.; Ingebrigtsen, S.; Lundgaard, L.E. Enhancements in the lightning impulse breakdown characteristics of natural ester dielectric liquids. Appl. Phys. Lett. 2013, 102, 172905.
- Unge, M.; Singha, S.; Ingebrigtsen, S.; Linhjell, D.; Lundgaard, L.E. Influence of molecular additives on positive streamer propagation in ester liquids. In Proceedings of the IEEE 18th International Conference on Dielectric Liquids (ICDL), Bled, Slovenia, 29 June–3 July 2014.
- Liang, S.; Wang, F.; Huang, Z.; Chen, W.; Wang, Y.; Li, J. Significantly Improved Electrical Breakdown Strength of Natural Ester Liquid Dielectrics by Doping Ultraviolet Absorbing Molecules. IEEE Access 2019, 7, 73448–73454.
More
Information
Subjects:
Engineering, Manufacturing
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
04 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




