Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yevgeniya Kushchayeva | -- | 1031 | 2023-01-03 07:42:55 | | | |
| 2 | Conner Chen | Meta information modification | 1031 | 2023-01-03 08:39:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kushchayeva, Y.; Pestun, I.; Kushchayev, S.; Radzikhovska, N.; Lewiecki, E.M. Fracture Healing in Healthy Bone. Encyclopedia. Available online: https://encyclopedia.pub/entry/39674 (accessed on 07 February 2026).
Kushchayeva Y, Pestun I, Kushchayev S, Radzikhovska N, Lewiecki EM. Fracture Healing in Healthy Bone. Encyclopedia. Available at: https://encyclopedia.pub/entry/39674. Accessed February 07, 2026.
Kushchayeva, Yevgeniya, Iryna Pestun, Sergiy Kushchayev, Nataliia Radzikhovska, E. Michael Lewiecki. "Fracture Healing in Healthy Bone" Encyclopedia, https://encyclopedia.pub/entry/39674 (accessed February 07, 2026).
Kushchayeva, Y., Pestun, I., Kushchayev, S., Radzikhovska, N., & Lewiecki, E.M. (2023, January 03). Fracture Healing in Healthy Bone. In Encyclopedia. https://encyclopedia.pub/entry/39674
Kushchayeva, Yevgeniya, et al. "Fracture Healing in Healthy Bone." Encyclopedia. Web. 03 January, 2023.
Copy Citation
Fracture healing is a complex, multistage, coordinated process commencing autonomously in the bone fracture area. There are two principal histological types of bone healing: primary and secondary healing.
osteoporosis
bone-related surgeries
1. Introduction
Osteoporosis (OP) is a major public health concern that affects approximately 200 million people globally. The clinical consequences of this systemic disorder are an increased risk of fractures with an increased fracture severity [1]. Poor bone quality due to OP seriously complicates the surgical treatment of these fractures and stability of the bone fixation construct. Patients with OP having spine surgery are at an increased risk of pedicle screw loosening, instrumentation failure, pseudoarthrosis, vertebral fractures (VFs), proximal junctional kyphosis, and revision surgery [2]. Since OP is a disorder of aging and both aging and OP may affect the normal bone healing process, it is difficult to separate their negative effects on bone tissue [1].
2. Fracture Healing in Healthy Bone
Fracture healing is a complex, multistage, coordinated process commencing autonomously in the bone fracture area [3]. There are two principal histological types of bone healing: primary and secondary healing. Primary healing is rare and is based on the attempt of the cortical bone cells to re-establish the disrupted continuity directly, therefore requiring absolute stability and contact of the fragments, as may occur with a stress fracture and fractures treated with open reduction and internal fixation (ORIF) with plates and screws [4]. In contrast, secondary bone healing takes place in the majority of bony injuries and involves both intramembranous and endochondral ossification with the activation of committed osteoprogenitor cells of the periosteum and undifferentiated multipotent mesenchymal stem cells (MSCs). This type of bone healing involves callus formation [4]. Four stages typically describe the bone healing process: hematoma formation with inflammation, fibrocartilaginous callus formation, bony callus formation, and bone remodeling [5] (Figure 1).
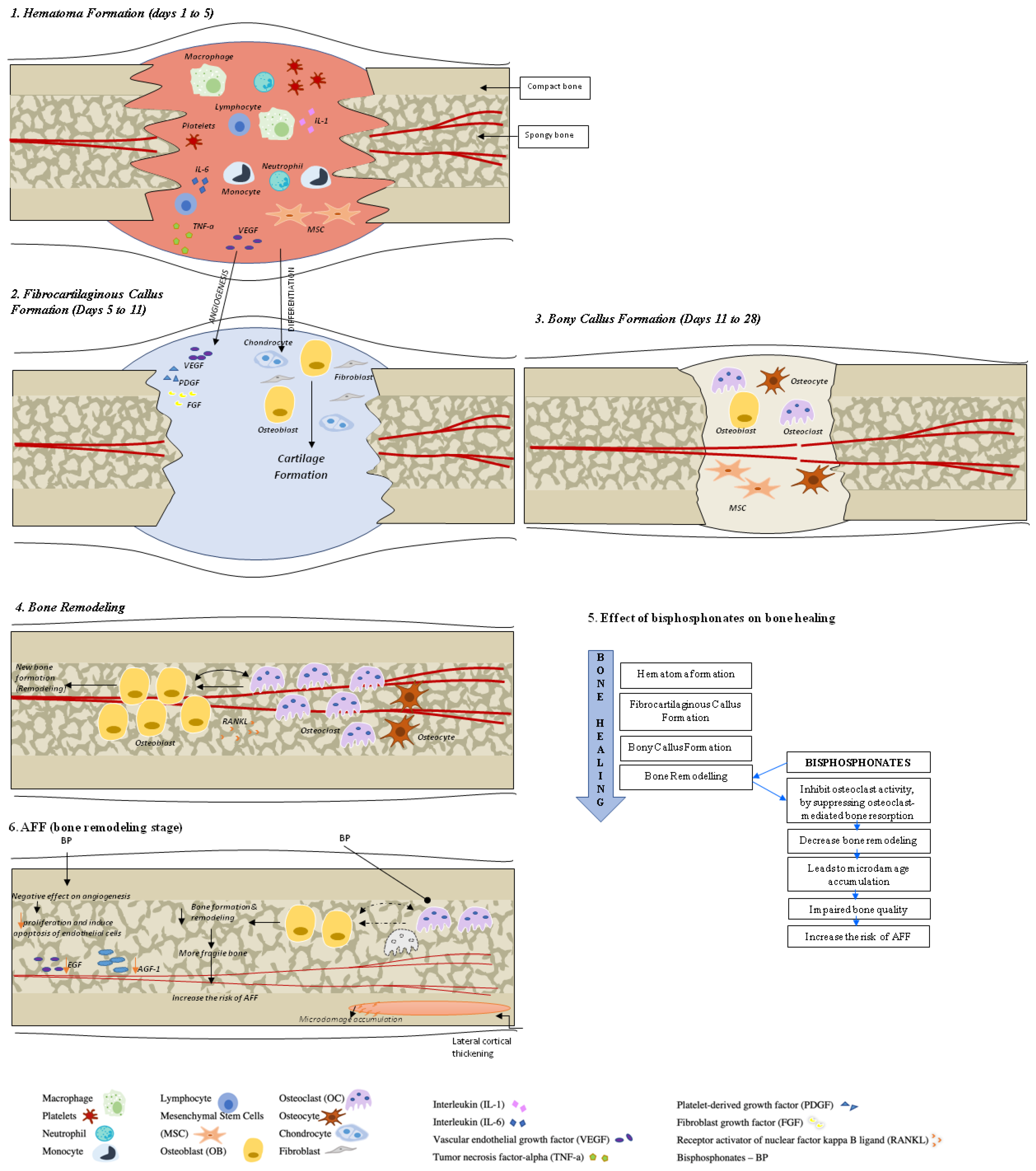
Figure 1. Bone healing in healthy bone and after atypical femoral fracture (AFF). There are four stages that describe the bone healing process: (1) hematoma formation with inflammation, (2) fibrocartilaginous callus formation, (3) bony callus formation, and (4) bone remodeling. During the first stage, a formed hematoma is composed of bone marrow and peripheral and intramedullary blood cells. The inflammatory cells (macrophages, neutrophils, lymphocytes, monocytes) and degranulating platelets infiltrate the hematoma between the fracture ends, causing acute inflammation and releasing cytokines and growth factors to stimulate the fracture healing process. During the fibrocartilaginous callus formation (stage 2), the soft callus is developed. The soft callus is a semi-rigid tissue able to provide mechanical support to the fracture and act as a template for the bony callus. The cartilaginous matrix is produced until the whole fibrinous/granulation tissue is replaced by cartilage. Angiogenic factors amplify the process of fracture healing vascularization. Further progress of bone regeneration occurs with the replacement of the primary soft cartilaginous callus with a hard bony callus during stage 3 (bony callus formation stage). The last stage represents bone remodeling that is characterized by high levels of bone resorption and formation markers and the migration of osteoblasts and osteoclasts with the hard callus that undergoes repeated remodeling. During this process, the central part of the callus is finally replaced by compact bone, whereas the callus edges are replaced by lamellar bone. See the test for more details.
Hematoma Formation (Days 1 to 7). A hematoma is formed immediately after the fracture; it is composed of peripheral and intramedullary blood cells, and bone marrow cells. The inflammatory response, necessary for healing to progress, peaks within 24 h and is completed in 7 days. Hematoma coagulation within the medulla and between/around the fracture ends sets up a template for callus formation [6]. Inflammatory cells (macrophages, neutrophils, lymphocytes, monocytes) and degranulating platelets infiltrate the hematoma between the fracture ends, causing acute inflammation and releasing cytokines and growth factors that stimulate fracture healing [3][5][7].
Fibrocartilaginous Callus Formation (Days 5 to 11). Chondrocytes and fibroblasts dominate on a cellular level at this stage; however, specific proportions of different cell types can vary among fractures. The soft callus produced by these cells is a semi-rigid tissue that is able to provide mechanical support to the fracture. At the same time, the soft callus acts as a template for the bony callus that will supersede it. The cartilaginous matrix is synthesized by proliferating chondrocytes derived from mesenchymal progenitors. This process lasts until the whole fibrinous/granulation tissue is replaced by cartilage [7].
Angiogenic factors, such as fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) amplify the process of the fracture healing vascularization [3][8][9][10].
Bony Callus Formation (Days 11 to 28). Further progress of bone regeneration occurs with the replacement of primary soft cartilaginous callus with a hard bony callus [6]. When endochondral ossification of the cartilaginous callus begins, the receptor activator of nuclear factor kappa B ligand (RANKL) is expressed. This stimulates the further differentiation of chondroblasts, osteoblasts, and osteoclasts, resulting in the resorption and calcification of the cartilaginous callus. At the same time, woven bone is laid down subperiosteally. The proliferation of newly formed blood vessels continues, allowing for the further migration of mesenchymal stem cells. At the end of this stage, a hard, calcified callus of immature bone is formed [5].
Fracture callus chondrocytes become hypertrophic in the course of their proliferation, while the extracellular matrix becomes calcified. This sequence of events is primarily controlled by macrophage colony-stimulating factor (M-CSF), RANKL, and osteoprotegerin (OPG), while the resorption of this mineralized cartilage is initiated by TNF-α [6][8]. It is also likely that, during this process, M-CSF, RANKL, and OPG help to recruit bone cells and osteoclasts to form woven bone [6].
Bone Remodeling (lasting from months to years). The remodeling of bone tissue is the final stage of bone repair, characterized by high levels of bone resorption and bone formation markers [11]. Osteoblasts and osteoclasts continue migrating, and the hard callus undergoes repeated “coupled remodeling”, a process involving a balance of osteoclastic bone resorption and osteoblastic bone formation until the bone returns to its original state [5].
During the remodeling process, immature woven bone and underlying cartilage matrix are resorbed by osteoclasts and replaced with the lamellar bone. The fate of osteoblasts after completing bone formation is to undergo apoptosis, become bone lining cells, or embed themselves into the bone matrix as osteocytes. Cellular functions of both osteoclasts and osteoblasts are regulated by cytokines, which include RANKL and OPG [3].
References
- Hak, D.J. The biology of fracture healing in osteoporosis and in the presence of anti-osteoporotic drugs. Injury 2018, 49, 1461–1465.
- Sardar, Z.M.; Coury, J.R.; Cerpa, M.; DeWald, C.J.; Ames, C.P.; Shuhart, C.; Watkins, C.; Polly, D.W.; Dirschl, D.R.; Klineberg, E.O.; et al. Best Practice Guidelines for Assessment and Management of Osteoporosis in Adult Patients Undergoing Elective Spinal Reconstruction. Spine 2022, 47, 128–135.
- Lafuente-Gracia, L.; Borgiani, E.; Nasello, G.; Geris, L. Towards in silico Models of the Inflammatory Response in Bone Fracture Healing. Front. Bioeng Biotechnol. 2021, 9, 703725.
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, 3–6.
- Sheen, J.R.; Garla, V.V. Fracture Healing Overview. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021.
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555.
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466.
- Barnes, G.L.; Kostenuik, P.J.; Gerstenfeld, L.C.; Einhorn, T.A. Growth factor regulation of fracture repair. J. Bone Miner. Res. 1999, 14, 1805–1815.
- Carano, R.A.; Filvaroff, E.H. Angiogenesis and bone repair. Drug Discov. Today 2003, 8, 980–989.
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P. Molecular aspects of fracture healing: Which are the important molecules? Injury 2007, 38, 11–25.
- Cox, G.; Einhorn, T.A.; Tzioupis, C.; Giannoudis, P.V. Bone-turnover markers in fracture healing. J. Bone Jt. Surg. Br. 2010, 92, 329–334.
More
Information
Subjects:
Orthopedics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.7K
Revisions:
2 times
(View History)
Update Date:
03 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




