
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammet Çağrı OĞUZ | -- | 1810 | 2022-12-31 19:29:30 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1810 | 2023-01-03 02:20:09 | | | | |
| 3 | Beatrix Zheng | -3 word(s) | 1807 | 2023-01-03 08:52:17 | | |
Video Upload Options
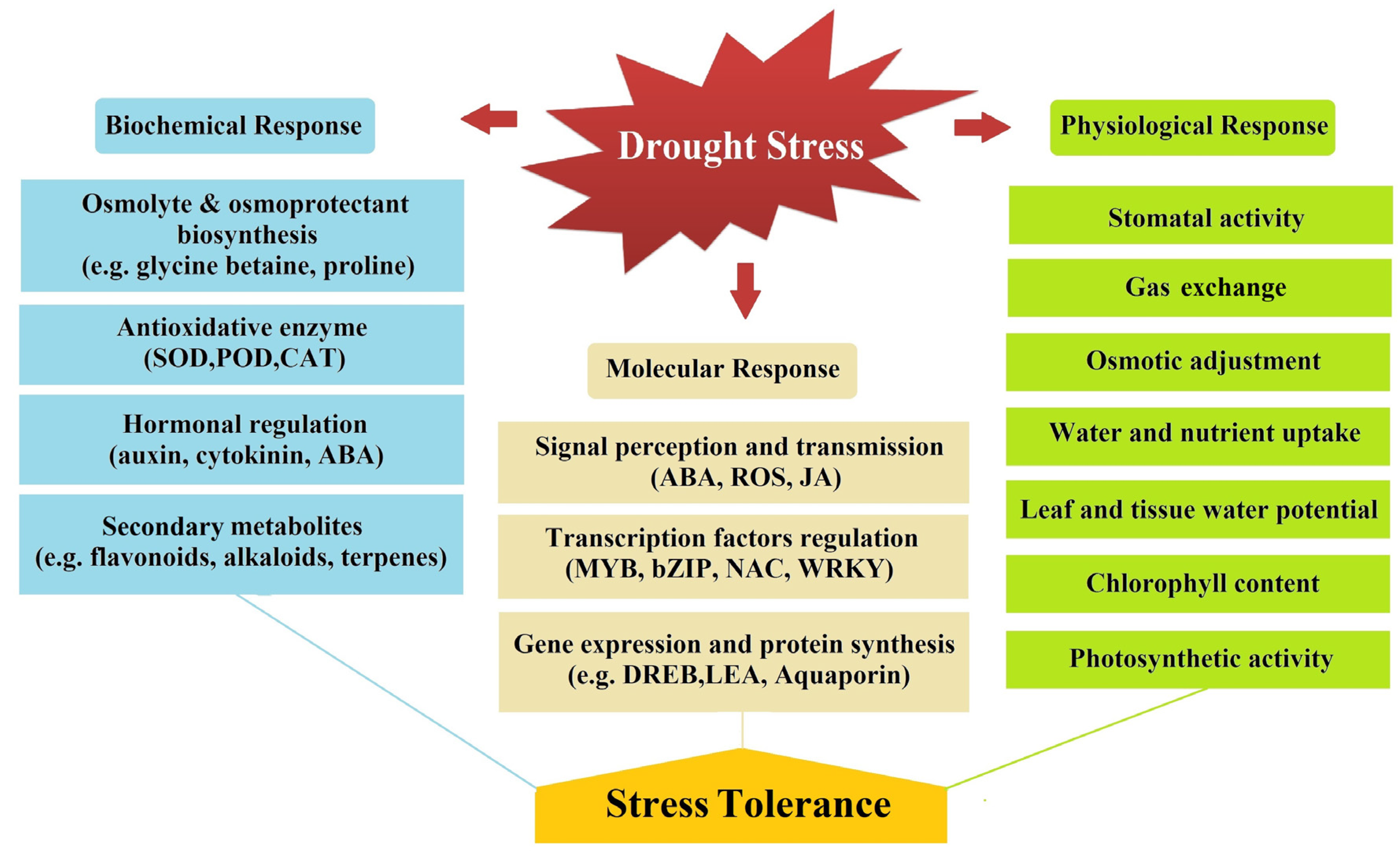
Drought is an important abiotic stress factor limiting crop productivity worldwide and its impact is increasing with climate change. Regardless of the plant growth period, drought has a deadly and yield-reducing effect on the plant at every stage of development. As with many environmental stressors, drought-exposed plants trigger a series of molecular, biochemical, and physiological responses to overcome the effect of drought stress. Currently, researchers are trying to determine the complex functioning of drought stress response in plants with different approaches. Plants are more sensitive to drought stress during certain critical stages like germination, seedling formation, flowering, fertilization, and grain formation periods. Plants have high success in reducing the effects of drought stress in vegetative development periods with the activity of tolerance mechanisms. On the other hand, drought stress during the generative period can cause irreversible losses in yield.
1. Introduction
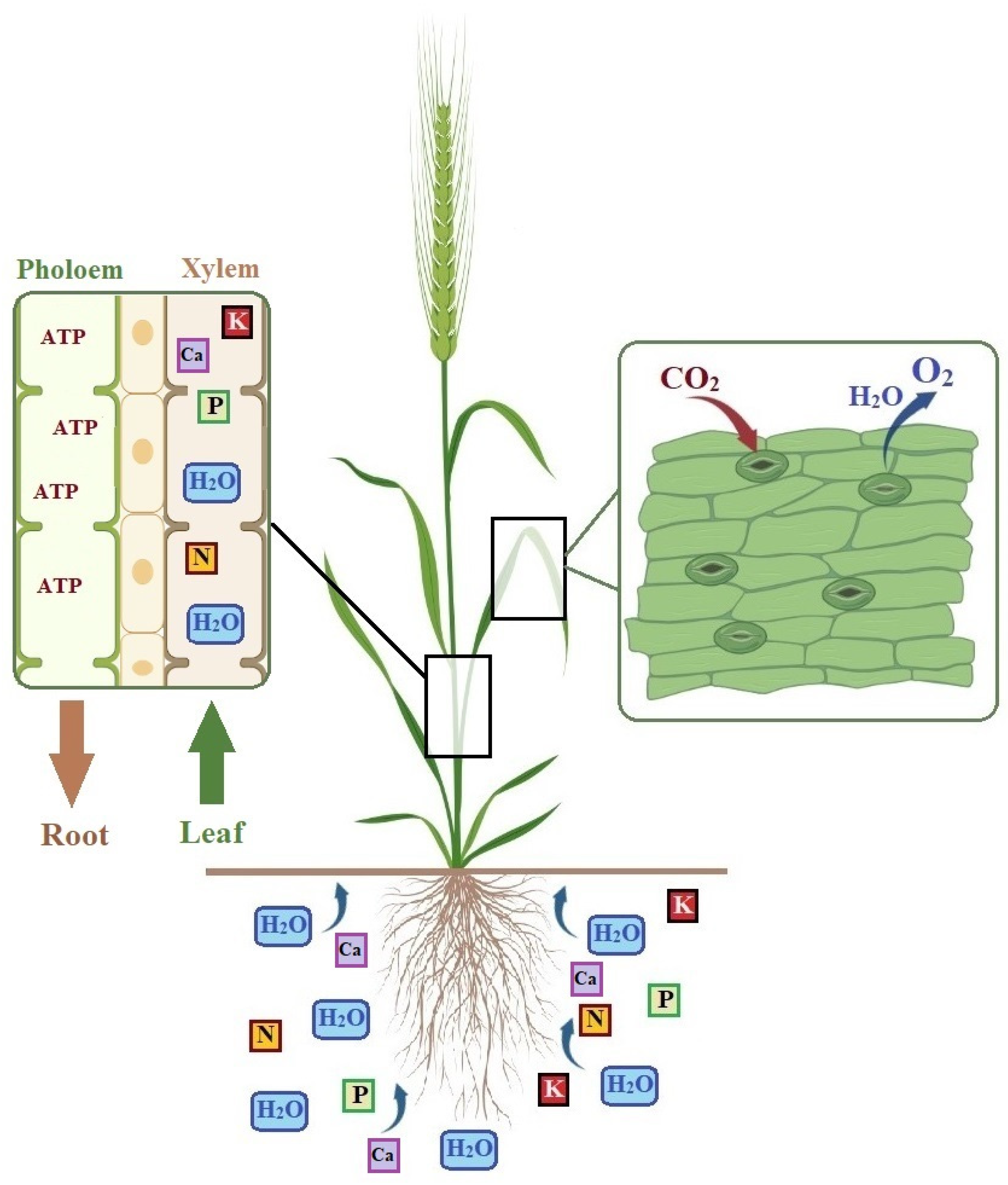
2. Physiological Responses and Mechanisms of Plants against Drought Stress
3. Management of Drought Stress in Plants
References
- Yohannes, G.; Kidane, L.; Abraha, B.; Beyene, T. Effect of Salt Stresses on Seed Germination and Early Seedling Growth of Camelina sativa L. Momona Ethiop. J. Sci. 2020, 12, 1–19.
- Billah, M.; Aktar, S.; Brestic, M.; Zivcak, M.; Khaldun, A.B.M.; Uddin, M.S.; Bagum, S.A.; Yang, X.; Skalicky, M.; Mehari, T.G.; et al. Progressive Genomic Approaches to Explore Drought- and Salt-Induced Oxidative Stress Responses in Plants under Changing Climate. Plants 2021, 10, 1910.
- el Haddad, N.; Choukri, H.; Ghanem, M.E.; Smouni, A.; Mentag, R.; Rajendran, K.; Hejjaoui, K.; Maalouf, F.; Kumar, S. High-Temperature and Drought Stress Effects on Growth, Yield and Nutritional Quality with Transpiration Response to Vapor Pressure Deficit in Lentil. Plants 2022, 11, 95.
- Oo, A.T.; van Huylenbroeck, G.; Speelman, S. Measuring the Economic Impact of Climate Change on Crop Production in the Dry Zone of Myanmar: A Ricardian Approach. Climate 2020, 8, 9.
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and Physiological Plant Responses to Drought Stress in Thymus citriodorus. Int. J. Agron. 2016, 2016, 4165750.
- Kumar, S.; Sachdeva, S.; Bhat, K.V.; Vats, S. Plant Responses to Drought Stress: Physiological, Biochemical and Molecular Basis. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–25. ISBN 978-981-10-9029-5.
- Pamungkas, S.S.T.; Suwarto; Suprayogi; Farid, N. Drought Stress: Responses and Mechanism in Plants. Rev. Agric. Sci. 2022, 10, 168–185.
- Hossain, A.; Farooq, M.; el Sabagh, A.; Hasanuzzaman, M.; Erman, M.; Islam, T. Morphological, Physiobiochemical and Molecular Adaptability of Legumes of Fabaceae to Drought Stress, with Special Reference to Medicago sativa L. In The Plant Family Fabaceae; Springer: Berlin/Heidelberg, Germany, 2021; pp. 289–317.
- Sinclair, T.R. Challenges in Breeding for Yield Increase for Drought. Trends Plant Sci. 2011, 16, 289–293.
- Gahlaut, V.; Jaiswal, V.; Singh, S.; Balyan, H.S.; Gupta, P.K. Multi-Locus Genome Wide Association Mapping for Yield and Its Contributing Traits in Hexaploid Wheat under Different Water Regimes. Sci. Rep. 2019, 9, 19486.
- Ballesta, P.; Mora, F.; del Pozo, A. Association Mapping of Drought Tolerance Indices in Wheat: QTL-Rich Regions on Chromosome 4A. Sci. Agric. 2020, 77, e20180153.
- Swamy, B.P.M.; Kaladhar, K.; Anuradha, K.; Batchu, A.K.; Longvah, T.; Sarla, N. QTL Analysis for Grain Iron and Zinc Concentrations in Two O. Nivara Derived Backcross Populations. Rice Sci. 2018, 25, 197–207.
- Cormier, F.; le Gouis, J.; Dubreuil, P.; Lafarge, S.; Praud, S. A Genome-Wide Identification of Chromosomal Regions Determining Nitrogen Use Efficiency Components in Wheat (Triticum aestivum L.). Theor. Appl. Genet. 2014, 127, 2679–2693.
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide Application Improves the Drought Tolerance in Maize Through Modulation of Enzymatic Antioxidants and Leaf Gas Exchange. J. Agron. Crop Sci. 2011, 197, 177–185.
- Haworth, M.; Elliott-Kingston, C.; McElwain, J.C. Co-Ordination of Physiological and Morphological Responses of Stomata to Elevated in Vascular Plants. Oecologia 2013, 171, 71–82.
- Conesa, M.R.; de la Rosa, J.M.; Domingo, R.; Bañon, S.; Pérez-Pastor, A. Changes Induced by Water Stress on Water Relations, Stomatal Behaviour and Morphology of Table Grapes (Cv. Crimson Seedless) Grown in Pots. Sci. Hortic. 2016, 202, 9–16.
- Ammar, M.H.; Anwar, F.; El-Harty, E.H.; Migdadi, H.M.; Abdel-Khalik, S.M.; Al-Faifi, S.A.; Farooq, M.; Alghamdi, S.S. Physiological and Yield Responses of Faba Bean (Vicia faba L.) to Drought Stress in Managed and Open Field Environments. J. Agron. Crop Sci. 2015, 201, 280–287.
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought Tolerance in Wheat. Sci. World J. 2013, 2013, 610721.
- Mahla, R.; Madan, S.; Kaur, V.; Munjal, R.; Behl, R.K.; Midathala, R. Activities of Sucrose to Starch Metabolizing Enzymes during Grain Filling in Late Sown Wheat under Water Stress. J. Appl. Nat. Sci. 2017, 9, 338–343.
- Akram, R.; Fahad, S.; Masood, N.; Rasool, A.; Ijaz, M.; Ihsan, M.Z.; Maqbool, M.M.; Ahmad, S.; Hussain, S.; Ahmed, M.; et al. Plant Growth and Morphological Changes in Rice under Abiotic Stress. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biwas, J.K., Eds.; Woodhead Publishing: Shaxton, UK, 2018; pp. 69–85.
- Queiroz, M.S.; Oliveira, C.E.S.; Steiner, F.; Zuffo, A.M.; Zoz, T.; Vendruscolo, E.P.; Silva, M.V.; Mello, B.F.F.R.; Cabral, R.C.; Menis, F.T. Drought Stresses on Seed Germination and Early Growth of Maize and Sorghum. J. Agri. Sci. 2019, 11, 310–318.
- Sarshad, A.; Talei, D.; Torabi, M.; Rafiei, F.; Nejatkhah, P. Morphological and Biochemical Responses of Sorghum bicolor (L.) Moench under Drought Stress. SN Appl. Sci. 2021, 3, 81.
- Sanjari, S.; Shobbar, Z.S.; Ghanati, F.; Afshari-Behbahanizadeh, S.; Farajpour, M.; Jokar, M.; Khazaei, A.; Shahbazi, M. Molecular, Chemical, and Physiological Analyses of Sorghum Leaf Wax under Post-Flowering Drought Stress. Plant Physiol. Biochem. 2021, 159, 383–391.
- Hammad, H.M.; Farhad, W.; Abbas, F.; Fahad, S.; Saeed, S.; Nasim, W.; Bakhat, H.F. Maize Plant Nitrogen Uptake Dynamics at Limited Irrigation Water and Nitrogen. Environ. Scie Pollut. Res. 2017, 24, 2549–2557.
- Goodarzian Ghahfarokhi, M.; Mansurifar, S.; Taghizadeh-Mehrjardi, R.; Saeidi, M.; Jamshidi, A.M.; Ghasemi, E. Effects of Drought Stress and Rewatering on Antioxidant Systems and Relative Water Content in Different Growth Stages of Maize (Zea mays L.) Hybrids. Arch. Agron. Soil Sci. 2015, 61, 493–506.
- Kulczycki, G.; Sacała, E.; Chohura, P.; Załuska, J. Maize and Wheat Response to Drought Stress under Varied Sulphur Fertilisation. Agronomy 2022, 12, 1076.
- Jin, N.; Ren, W.; Tao, B.; He, L.; Ren, Q.; Li, S.; Yu, Q. Effects of Water Stress on Water Use Efficiency of Irrigated and Rainfed Wheat in the Loess Plateau, China. Sci. Total Environ. 2018, 642, 1–11.
- Yang, X.; Wang, B.; Chen, L.; Li, P.; Cao, C. The Different Influences of Drought Stress at the Flowering Stage on Rice Physiological Traits, Grain Yield, and Quality. Sci. Rep. 2019, 9, 3742.
- Prakash, M.; Sunilkumar, B.; Sathiyanarayanan, G.; Gokulakrishnan, J. Screening for Drought Tolerance in Mungbean. Legume Rese 2017, 40, 423–428.
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R.; Bhat, K.V. Morphophysiological and Biochemical Response of Mungbean Varieties at Different Developmental Stages under Drought Stress. Turk. J. Biol. 2019, 43, 58–69.
- Jincy, M.; Prasad, V.B.R.; Jeyakumar, P.; Senthil, A.; Manivannan, N. Evaluation of Green Gram Genotypes for Drought Tolerance by PEG (Polyethylene Glycol) Induced Drought Stress at Seedling Stage. Legume Res. 2021, 44, 684–691.
- Felisberto, G.; Schwerz, F.; Umburanas, R.C.; Dourado-Neto, D.; Reichardt, K. Physiological and Yield Responses of Soybean under Water Deficit. J. Crop Sci. Biotechnol. 2022.
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A Study on Soybean Responses to Drought Stress and Rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017.
- Çakir, R. Effect of Water Stress at Different Development Stages on Vegetative and Reproductive Growth of Corn. Field Crop. Res. 2004, 89, 1–16.
- Tarafdar, M.; Bahadur, V.; Rana, S.; Singh, R.K. A Review: Abiotic Stress on Transpiration, Stomatal Diffusive Resistance and Photosynthetic Rate. Pharma Innov. J. 2022, 11, 1632–1635, ISSN (E) 2277-7695.
- Tardieu, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759.
- Demidchik, V. ROS-Activated Ion Channels in Plants: Biophysical Characteristics, Physiological Functions and Molecular Nature. Int. J. Mol. Sci. 2018, 19, 1263.
- Batool, S.; Uslu, V.V.; Rajab, H.; Ahmad, N.; Waadt, R.; Geiger, D.; Malagoli, M.; bin Xiang, C.; Hedrich, R.; Rennenberg, H.; et al. Sulfate Is Incorporated into Cysteine to Trigger ABA Production and Stomatal Closure. Plant Cell 2018, 30, 2973–2987.
- Roblero, M.; Pineda, J.; León, C.; Castellanos, J.S. Oxygen in the Root Zone and Its Effect on Plants. Rev. Mex. Cienc. Agric. 2020, 11, 931–943.
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Zahid Nabi, S.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant Drought Stress Tolerance: Understanding Its Physiological, Biochemical and Molecular Mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925.
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Dordrech, The Netherlands, 2009; pp. 153–188.
- Razi, K.; Muneer, S. Drought Stress-Induced Physiological Mechanisms, Signaling Pathways and Molecular Response of Chloroplasts in Common Vegetable Crops. Crit. Rev. Biotechnol. 2021, 41, 669–691.
- Schachtman, D.P.; Goodger, J.Q.D. Chemical Root to Shoot Signaling under Drought. Trends Plant Sci. 2008, 13, 281–287.
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70.
- Rameau, C.; Goormachtig, S.; Cardinale, F.; Bennett, T.; Cubas, P. Strigolactones as Plant Hormones. In Strigolactones—Biology and Applications; Springer: Cham, Switzerland, 2019; pp. 47–87.
- Yamada, Y.; Umehara, M. Possible Roles of Strigolactones during Leaf Senescence. Plants 2015, 4, 664–677.
- Visentin, I.; Vitali, M.; Ferrero, M.; Zhang, Y.; Ruyter-Spira, C.; Novák, O.; Strnad, M.; Lovisolo, C.; Schubert, A.; Cardinale, F. Low Levels of Strigolactones in Roots as a Component of the Systemic Signal of Drought Stress in Tomato. New Phytol. 2016, 212, 954–963.
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of Drought Stress in Grapevine by Foliar-Applied Strigolactones. Plant Physiol. Biochem. 2019, 135, 99–110.
- Wang, L.; Wang, B.; Yu, H.; Guo, H.; Lin, T.; Kou, L.; Wang, A.; Shao, N.; Ma, H.; Xiong, G.; et al. Transcriptional Regulation of Strigolactone Signalling in Arabidopsis. Nature 2020, 583, 277–281.
- Foyer, C.H.; Noctor, G. Oxidant and Antioxidant Signalling in Plants: A Re-Evaluation of the Concept of Oxidative Stress in a Physiological Context. Plant Cell Environ. 2005, 28, 1056–1071.
- Jaspers, P.; Kangasjärvi, J. Reactive Oxygen Species in Abiotic Stress Signaling. Physiol. Plant 2010, 138, 405–413.
- Oğuz, M.Ç.; Mujtaba, M.; Yüksel Özmen, C.; Kibar, U.; Kumlay, A.M.; Ergül, A. Expression Analysis of Transcription-Factor Genes Related to Endoplasmic Reticulum Stress Signaling Pathway in Alfalfa (Medicago sativa L.). Acta Physiol. Plant 2022, 44, 37.
- Hartmann, H.; Ziegler, W.; Kolle, O.; Trumbore, S. Thirst Beats Hunger—Declining Hydration during Drought Prevents Carbon Starvation in Norway Spruce Saplings. New Phytol. 2013, 200, 340–349.
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560.
- Murata, Y.; Mori, I.C. Stomatal Regulation of Plant Water Status. In Plant Abiotic Stress, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 47–67.
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; von Korff, M. Leaf Proteome Alterations in the Context of Physiological and Morphological Responses to Drought and Heat Stress in Barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212.
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147.
- Bray, E.A. Classification of Genes Differentially Expressed during Water-Deficit Stress in Arabidopsis thaliana: An Analysis Using Microarray and Differential Expression Data. Ann. Bot. 2002, 89, 803–811.
- Mumm, P.; Wolf, T.; Fromm, J.; Roelfsema, M.R.G.; Marten, I. Cell Type-Specific Regulation of Ion Channels within the Maize Stomatal Complex. Plant Cell Physiol. 2011, 52, 1365–1375.
- Potopová, V.; Boroneanţ, C.; Boincean, B.; Soukup, J. Impact of Agricultural Drought on Main Crop Yields in the Republic of Moldova. Int. J. Climatol. 2016, 36, 2063–2082.
- Zhang, Q. Strategies for Developing Green Super Rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409.
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942.
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes under Abiotic Stress. Biomolecules 2019, 9, 285.
- Sevanto, S. Phloem Transport and Drought. J. Exp. Bot. 2014, 65, 1751–1759.
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C3 Plants. Plant Biol. 2004, 6, 269–279.
- Amin Kheradmand, M.; Shahmoradzadeh Fahraji, S.; Fatahi, E.; Mahdi Raoofi, M. Effect of Water Stress on Oil Yield and Some Characteristics of Brassica napus. Int. Res. J. Appl. Basic Sci. 2014, 8, 1447–1453.
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant Growth under Drought Stress: Significance of Mineral Nutrients. In Water Stress and Crop Plants: A Sustainable Approach; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 649–668. ISBN 978-1-1190-5436-8.
- Bhargava, S.; Sawant, K. Drought Stress Adaptation: Metabolic Adjustment and Regulation of Gene Expression. Plant Breed. 2013, 132, 21–32.
- Ying, Y.; Yue, Y.; Huang, X.; Wang, H.; Mei, L.; Yu, W.; Zheng, B.; Wu, J. Salicylic Acid Induces Physiological and Biochemical Changes in Three Red Bayberry (Myric rubra) Genotypes under Water Stress. Plant Growth Regul. 2013, 71, 181–189.
- Rivas, R.; Falcão, H.M.; Ribeiro, R.V.; Machado, E.C.; Pimentel, C.; Santos, M.G. Drought Tolerance in Cowpea Species Is Driven by Less Sensitivity of Leaf Gas Exchange to Water Deficit and Rapid Recovery of Photosynthesis after Rehydration. S. Afr J. Bot. 2016, 103, 101–107.
- Nayyar, H.; Gupta, D. Differential Sensitivity of C3 and C4 Plants to Water Deficit Stress: Association with Oxidative Stress and Antioxidants. Environ. Exp. Bot. 2006, 58, 106–113.
- Georgii, E.; Jin, M.; Zhao, J.; Kanawati, B.; Schmitt-Kopplin, P.; Albert, A.; Winkler, J.B.; Schäffner, A.R. Relationships between Drought, Heat and Air Humidity Responses Revealed by Transcriptome-Metabolome Co-Analysis. BMC Plant Biol. 2017, 17, 120.
- Alghabari, F.; Ihsan, M.Z.; Hussain, S.; Aishia, G.; Daur, I. Effect of Rht Alleles on Wheat Grain Yield and Quality under High Temperature and Drought Stress during Booting and Anthesis. Environ. Sci. Pollut. Res. 2015, 22, 15506–15515.
- Sun, X.L.; Sun, M.; Luo, X.; Ding, X.D.; Ji, W.; Cai, H.; Bai, X.; Liu, X.F.; Zhu, Y.M. A Glycine Soja ABA-Responsive Receptor-like Cytoplasmic Kinase, GsRLCK, Positively Controls Plant Tolerance to Salt and Drought Stresses. Planta 2013, 237, 1527–1545.
- Nikinmaa, E.; Hölttä, T.; Hari, P.; Kolari, P.; Mäkelä, A.; Sevanto, S.; Vesala, T. Assimilate Transport in Phloem Sets Conditions for Leaf Gas Exchange. Plant Cell Environ. 2013, 36, 655–669.
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190.
- Sun, Q.; Zybailov, B.; Majeran, W.; Friso, G.; Olinares, P.D.B.; van Wijk, K.J. PPDB, the Plant Proteomics Database at Cornell. Nucleic Acids Res. 2009, 37, D969–D974.
- Faisal, S.; Mujtaba, S.M.; Asma; Mahboob, W. Polyethylene Glycol Mediated Osmotic Stress Impacts on Growth and Biochemical Aspects of Wheat (Triticum aestivum L.). J. Crop Sci. Biotechnol. 2019, 22, 213–223.
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34.
- Karavidas, I.; Ntatsi, G.; Vougeleka, V.; Karkanis, A.; Ntanasi, T.; Saitanis, C.; Agathokleous, E.; Ropokis, A.; Sabatino, L.; Tran, F.; et al. Agronomic Practices to Increase the Yield and Quality of Common Bean (Phaseolus vulgaris L.): A Systematic Review. Agronomy 2022, 12, 271.
- Abebe, A.; Tsige, A.; Work, M.; Enyew, A. Optimizing Irrigation Frequency and Amount on Yield and Water Productivity of Snap Bean (Phaseolus vulgaris L.) in NW Amhara, Ethiopia: A Case Study in Koga and Ribb Irrigation Scheme. Cogent Food Agric. 2020, 6, 1773690.
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-Based Fertilizers: A Practical Approach towards Circular Economy. Bioresour. Technol. 2020, 295, 122223.
- Deligios, P.A.; Chergia, A.P.; Sanna, G.; Solinas, S.; Todde, G.; Narvarte, L.; Ledda, L. Climate Change Adaptation and Water Saving by Innovative Irrigation Management Applied on Open Field Globe Artichoke. Sci. Total Environ. 2019, 649, 461–472.
- Marcinkowski, P.; Piniewski, M. Effect of Climate Change on Sowing and Harvest Dates of Spring Barley and Maize in Poland. Int. Agrophys. 2018, 32, 265–271.
- Cooper, M.; van Eeuwijk, F.; Chapman, S.C.; Podlich, D.W.; Löffler, C. Genotype-by environment interactions under water-limited conditions. In Drought Adaptaton in Cereals; CRC Press: New York, NY, USA, 2006; pp. 51–96. ISBN 978-1-5602-2278-1.




