Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marino Paroli | -- | 1889 | 2022-12-31 00:43:14 | | | |
| 2 | Rita Xu | Meta information modification | 1889 | 2023-01-03 03:25:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Paroli, M.; Caccavale, R.; Fiorillo, M.T.; Spadea, L.; Gumina, S.; Candela, V.; Paroli, M.P. IL-17-Member Family. Encyclopedia. Available online: https://encyclopedia.pub/entry/39642 (accessed on 07 March 2026).
Paroli M, Caccavale R, Fiorillo MT, Spadea L, Gumina S, Candela V, et al. IL-17-Member Family. Encyclopedia. Available at: https://encyclopedia.pub/entry/39642. Accessed March 07, 2026.
Paroli, Marino, Rosalba Caccavale, Maria Teresa Fiorillo, Luca Spadea, Stefano Gumina, Vittorio Candela, Maria Pia Paroli. "IL-17-Member Family" Encyclopedia, https://encyclopedia.pub/entry/39642 (accessed March 07, 2026).
Paroli, M., Caccavale, R., Fiorillo, M.T., Spadea, L., Gumina, S., Candela, V., & Paroli, M.P. (2022, December 31). IL-17-Member Family. In Encyclopedia. https://encyclopedia.pub/entry/39642
Paroli, Marino, et al. "IL-17-Member Family." Encyclopedia. Web. 31 December, 2022.
Copy Citation
T-helper 17 (Th17) cells represent a subpopulation of CD4+ T lymphocytes that play an essential role in defense against pathogens. Th17 cells are distinguished from Th1 and Th2 cells by their ability to produce members of the interleukin-17 (IL-17) family, namely IL-17A and IL-17F. IL-17 in turn induces several target cells to synthesize and release cytokines, chemokines, and metalloproteinases, thereby amplifying the inflammatory cascade. Th17 cells reside predominantly in the lamina propria of the mucosa.
Th17
pathogens
immunopathology
1. Introduction
T helper 17 cells represent a subset of CD4+ T lymphocytes discovered about 20 years ago [1][2]. It was evident from the beginning that this population played a key role in defense against bacteria, viruses, and fungi. It was shown that the defensive action of Th17 cells occurred primarily through the production of members of the IL-17 family, in particular IL-17A and IL-17F [3][4]. It was also found that the specific cytokine signature of Th17 cells was closely dependent on IL-23 produced by cells of innate immunity in response to stimulation by microbial agents. The dependence of IL-17 production on IL-23 led to the formulation of the term “IL-23/IL-17 axis” [5].
Although Th17 cells were shown to be essential in preserving the integrity of the mucosal barrier from attack by infectious agents, subsequent studies revealed that these cells were also involved in immunopathological processes. Indeed, in an appropriate inflammatory microenvironment, they were found to be responsible for immunopathology, promoting the induction and maintenance of inflammatory and autoimmune diseases [6][7][8][9][10].
2. Th17 Cell Discovery: A Novel CD4+ T-Helper Cell Paradigm
For several years since the second half of the 1980s, CD4+ T cells have been subdivided into two subpopulations, T-helper 1 (Th1) cells and Th2 cells [11]. Th1 cells were characterized by their ability to produce interferon (IFN)-γ, cooperate with B cells to produce immunoglobulin (Ig)G, and give rise to delayed-type T-cell responses [12]. Conversely, Th2 cells were characterized by the ability to produce interleukin (IL)-4, which is required to promote isotope switching by B cells for IgE production [13][14]. While Th1 cells predominantly provided defense against intracellular pathogens, Th2 cells provided defense against parasites. In 1989, the so-called Th1/Th2 paradigm was thus formulated [15].
Subsequently, however, several pieces of evidence showed that IFN-γ production by Th1 cells did not fully explain the presence of pro-inflammatory CD4+ T-cell mediated responses in mouse models [16][17][18]. The Th1/Th2 paradigm was therefore destined to be overcome.
3. IL-17-Member Family
A critical event that preceded the discovery of Th17 cells was the cloning of a new interleukin with pro-inflammatory properties in 1993 [19][20]. This interleukin was initially defined as CTLA8 and later as interleukin-17A. That interleukin showed sequence homology with an open reading frame of the Herpesvirus saimiri, a herpes virus capable of infecting T cells [21][22]. Interleukin 17A was characterized by not having sequence homology with other known cytokines and deserved the definition of cytokine because it could induce the production of immune-active soluble factors by target cells [23]. Other cytokines structurally similar to interleukin 17A were then identified in sequence homology studies [24][25][26]. All these molecules were then grouped into a family defined as IL-17 that presently includes six members from 17A to IL-17F [27][28]. Five specific receptors expressed on different cell types were then cloned [29][30][31][32]. These receptors have molecular peculiarities that differentiate them from other interleukin receptors. In particular, they contain an intracytoplasmic motif termed SEFIR (SEF/IL-17 receptor) that has some similarities with a domain present in the Toll/IL-1 receptor (TIR) [26]. IL-17R signaling initiates with the recruitment of Act1 adaptor molecule, with subsequent IL-17R/Act-1 association [33][34][35] thus amplifying the signal transduction. This interaction is critical in the response to pathogens as demonstrated in murine models of knockout mice for the gene encoding IL-17RA as well as in humans with ACT1 mutations or with congenital deficiency of IL-17A or IL-17C, where a high susceptibility to fungal infections is observed [36]. Act1 possesses the peculiar ability to bind to E3 Ubiquitin [37]. Through this interaction, TNF-receptor associated factor(TRAF)6 is then recruited [34][38], resulting in activation of the nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) and subsequent gene transcription of several antimicrobial proteins [20][38][39][40][41].
In the year 2000, a discovery would prove essential for the subsequent identification of new pro-inflammatory CD4+ T cells in addition to the classic Th1 and Th2 cells [42]. Indeed, a new cytokine called p19 was discovered. This cytokine was able to form a heterodimer with the p40 chain of IL-12. The association of these two proteins originated a new interleukin called IL-23. Interleukin-23 was able to bind a receptor to IL-23R constituted by IL23R/IL-12β1 heterodimer [43]. Later studies showed that IL-23 was produced mainly by dendritic cells after activation by prostaglandin E and adenosine triphosphate [44][45]. It was shown that IL-23 was able to induce IL-17 production by a subpopulation of CD4+ T cells distinct from both Th1 and Th2 cells [46][47]. Therefore, this subpopulation was termed Th17 [1][2]. The peculiar differentiation and function characteristics of this subpopulation were further defined [7]. Th17 cells predominantly produce a member of the IL-17 family, namely IL-17A. However, subsequent study showed that other soluble factors involved in the inflammatory response, such as IL-17F, IL-21, IL-22, IL-26, and the chemokines CXCL8 and CCL20, were also produced [5]. It was found that a peculiar feature of Th17 cells was that their differentiation depended on specific transcription factors retinoic-acid orphan gamma receptor t (RORγt) in mice and its human isoform retinoic-acid orphan receptor C (RORC) [48][49]. These factors in turn induced il17a gene transcription [6][50]. Th17 cells were shown to characteristically express CCR6 chemokine receptor on their surface [4]. Further studies identified the existence of an intermediate cell subtype termed Th1Th17 able to produce both IL-17 and IFN-γ [3][51][52].
4. Differentiation of Th17 Cells
Differentiation of Th17 cells is a rather intricate molecular process that has been clarified at least in part only recently. This process requires TCR engagement of naïve CD4+ T cells together with the action of different cytokines present in the microenvironment. In more detail, IL-6 and tissue growth factor-β (TGF-β) provide to chromatin remodeling of the il17 gene locus [17][53][54]. IL-6 enhances retinoic-acid orphan receptors (RORγt) transcription through signal transducer and activator of transcription 3 (STAT3) phosphorylation [17], whereas TGF-β regulates Th17 differentiation via Staufen1 (STAU1)-mediated mRNA decay (SMD)-dependent or -independent pathways. It is noteworthy that TGF-β is also capable to promote regulatory T-cell (Treg) differentiation, which in turn suppresses Th17 through the function of forkhead box P3 (FOXP3). However, this activity is counteracted by IL-6-phosphorylated STAT3, which downregulates FOXP3, with consequent induction of RORγt and transformation of inducible Tregs into Th17 cells [50]. An important role in the early stages of Th17 cell differentiation is also played by IL-1β. This interleukin upregulates the expression of interferon regulatory factor (IRF) 4 [55] and RORγt [56][57][58]. Once differentiated, Th17 cells express IL23R on their surface. Interaction with IL-23 present in the inflammatory microenvironment is required to maintain the phenotype of Th17 cells, increasing RORγt and IL-17 expression by the intervention of STAT3 [59], but does not participate in the Th17 differentiation process [8][60]. Figure 1 shows the differentiation process of Th17 cells.
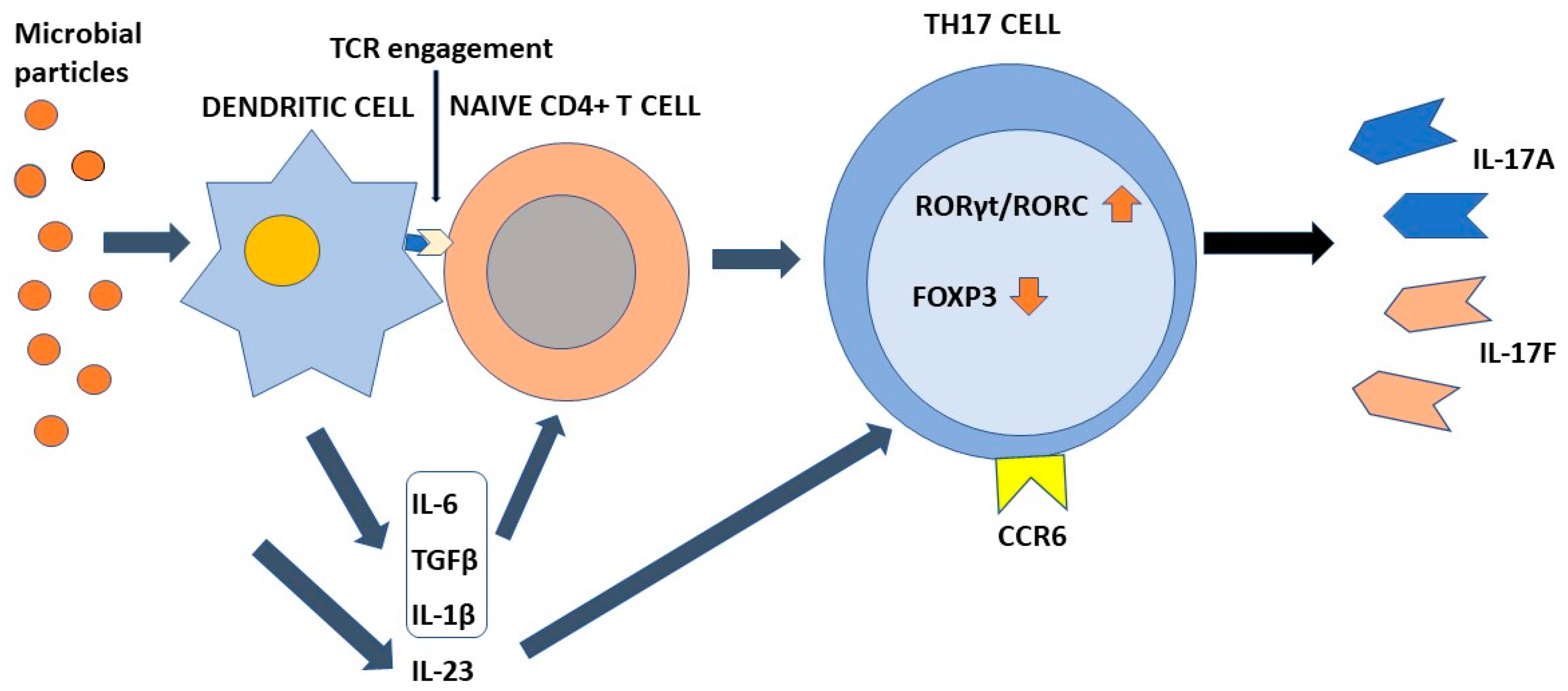
Figure 1. The process of Th17 cell differentiation. Dendritic cells after activation by microbial components activate CD4+ naive T cells in an antigen-dependent manner. In the presence of the appropriate cytokine environment, such cells acquire a Th17 phenotype by upregulating RORγt/RORC and downregulating Foxp3. IL-23 is required to stabilize their phenotype. Th17 cells are characterized by the ability to synthesize and secrete IL-17A and IL-17F in addition to other soluble factors. IL-6, TGFβ, and IL-1β contribute to the differentiation of naïve T cells into Th17 cells. IL-23 is required to stabilize their phenotype. Chemokine receptor CCR6 directs Th17 cells to barrier tissues.
5. Plasticity of Th17
An important feature of Th17 cells is their plasticity, defined by their ability to acquire functional and phenotypic characteristics of other CD4+ T lymphocyte subpopulations. Indeed, Th-17 cells can acquire the characteristics of Th1 cells in the presence of interleukin 12, which downregulates the expression of RORγt/RORC and induces the expression of T-bet, a major transcription factor of IFN-γ [61][62][63]. In a microenvironment rich in IL-4, they can acquire a Th2 phenotype [64]. Th17 cells present in the Peyer’s patches can acquire the follicular T cells (Tfh) phenotype and induce immunoglobulin (Ig) A production by geminal center B lymphocytes [65]. Finally, differentiation of naïve CD4+ T cells into Treg or Th17 is bidirectional. Interconnection between these two subpopulations depends on FOXP3/ RORγt/RORC balance, as demonstrated in numerous studies. This is regulated by the relative activity of several cytokines, including TGF-β, IL-6, IL-21, IL1β, and IL-23 [66][67][68]. Th17 cells exert their function on several cellular targets that are not only part of the immune system, but also express receptors for cytokines they produce.
6. The Physiological Function of Th17
The final effect of Th17-produced cytokines, mainly IL-17A, induces the consequent production of chemokines, interleukins, and chemokines by IL17R+ cells. These factors participate to the recruitment of neutrophils to the inflammatory site and induce secretion of anti-bacterial substances by epithelial cells [7]. Importantly, Th17 is localized mainly at the mucosal level where it exerts protective activity against bacteria and fungi [69]. The primary function of Th17 cells is therefore to maintain immune control of infection at the mucosal level and in the skin [70][71]. As discussed above, the maintenance of their differentiative state over time is strictly dependent on the cytokine environment. To this end, an important role is played by low levels of IL-1β produced by macrophage cells stimulated by intestinal commensal bacteria [72]. In the skin, commensal bacteria including S. epidermidis contribute to Th17 cell stability [71]. The presence of several metabolites including tryptophan can also favor the differentiation state of Th17 cells [73]. At the intestinal level, Th17 cells produce IL-22 and IL-21 in addition to IL-17. IL-17 and IL-22 locally exert an antimicrobial action through the production of bactericidal proteins [74]. Either in experimental animal models or rare primary immunodeficiencies in humans, alteration in the production of interleukin 17 or its receptors as well as in the case of RORγt mutations, loss of immunological defense against C. albicans and S. aureus has been observed at the skin, nail, and genital mucosa levels [75][76]. The observation that commensal segmented filamentous bacteria (SFBs) can induce a vigorous Th17 response in the intestine appears to be of considerable importance [77]. Such bacteria have a special ability to penetrate through the mucus that protects mucous membranes and thus can resist their removal by epithelial cell turnover and digestive processes [78]. It has been proposed that this SFB property can indirectly facilitate the transformation of Th17 cells from defensive to pathogenic. Importantly, Th17 cells play their role in the mucosal defense against pathogens not only in the gut or at the skin level, but also in the lung [79][80]. Figure 2 shows the main pro-inflammatory activity of Th17 cells.
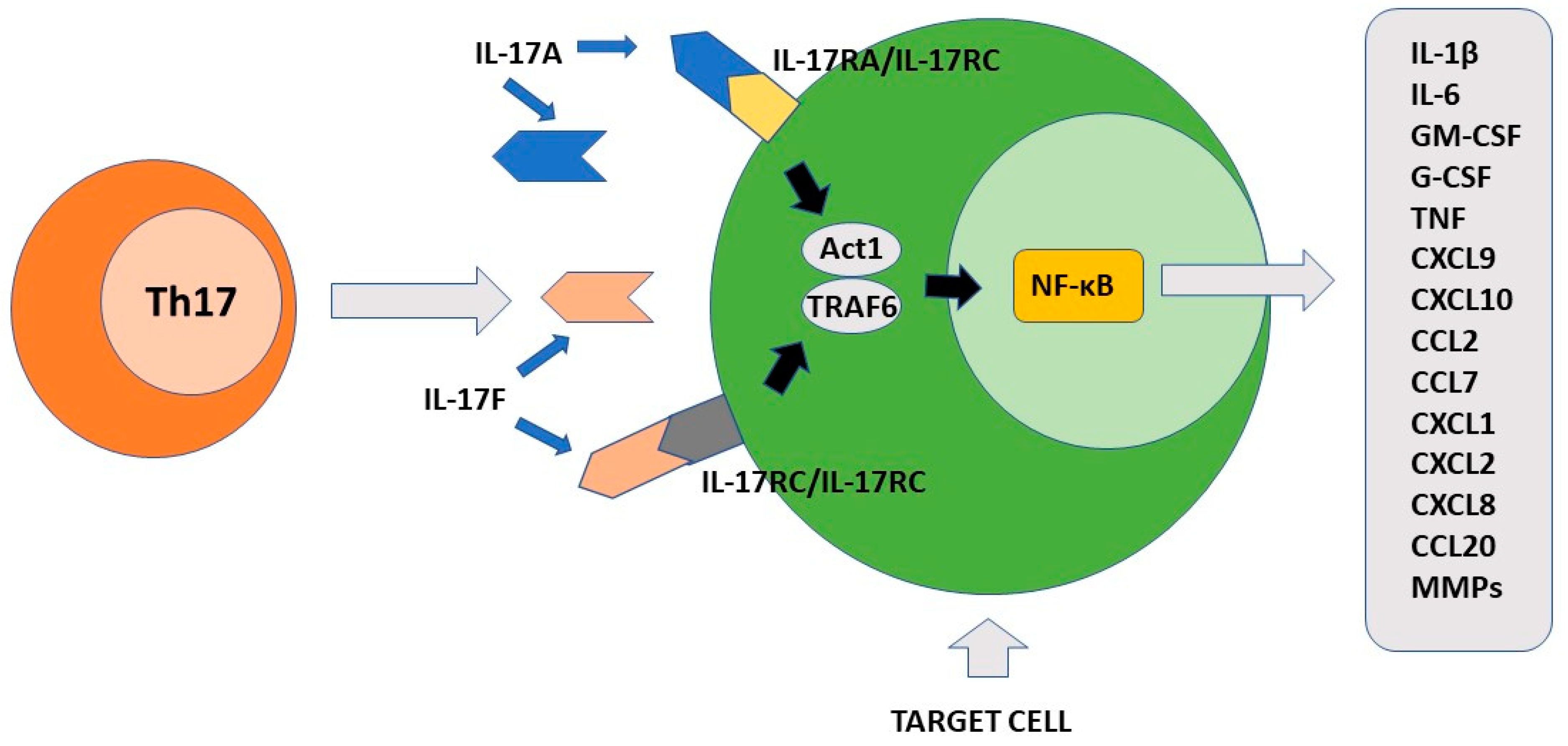
Figure 2. The pro-inflammatory function of Th17 cells. IL-17A and IL-17F produced by Th17 cells recognize different cellular targets that express their specific receptors. After activation, the signal transduction is triggered with formation of the ACT1/TRAF6 complex. The final event of this process includes activation of transcription factor NF-κB. Different pro-inflammatory factors are synthesized by the stimulated cells including cytokines, chemokines, growth factors and matrix metalloproteinases. IL-17A/IL-17F target cells include keratinocytes, fibroblasts, osteoblasts, epithelial cells, endothelial cells and macrophages.
References
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132.
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141.
- Acosta-Rodriguez, E.V.; Rivino, L.; Geginat, J.; Jarrossay, D.; Gattorno, M.; Lanzavecchia, A.; Sallusto, F.; Napolitani, G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007, 8, 639–646.
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Fili, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861.
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600.
- Capone, A.; Volpe, E. Transcriptional Regulators of T Helper 17 Cell Differentiation in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 348.
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517.
- Toussirot, E. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm. Allergy Drug Targets 2012, 11, 159–168.
- Paroli, M.; Spadea, L.; Caccavale, R.; Spadea, L.; Paroli, M.P.; Nante, N. The Role of Interleukin-17 in Juvenile Idiopathic Arthritis: From Pathogenesis to Treatment. Medicina 2022, 58, 1552.
- Chimenti, M.S.; Fonti, G.L.; Conigliaro, P.; Sunzini, F.; Scrivo, R.; Navarini, L.; Triggianese, P.; Peluso, G.; Scolieri, P.; Caccavale, R.; et al. One-year effectiveness, retention rate, and safety of secukinumab in ankylosing spondylitis and psoriatic arthritis: A real-life multicenter study. Expert Opin. Biol. Ther. 2020, 20, 813–821.
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone, I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357.
- Cher, D.J.; Mosmann, T.R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J. Immunol. 1987, 138, 3688–3694.
- Coffman, R.L.; Carty, J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J. Immunol. 1986, 136, 949–954.
- Hu-Li, J.; Shevach, E.M.; Mizuguchi, J.; Ohara, J.; Mosmann, T.; Paul, W.E. B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J. Exp. Med. 1987, 165, 157–172.
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173.
- Krakowski, M.; Owens, T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 1996, 26, 1641–1646.
- Bettelli, E.; Sullivan, B.; Szabo, S.J.; Sobel, R.A.; Glimcher, L.H.; Kuchroo, V.K. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 2004, 200, 79–87.
- Duong, T.T.; Finkelman, F.D.; Singh, B.; Strejan, G.H. Effect of anti-interferon-gamma monoclonal antibody treatment on the development of experimental allergic encephalomyelitis in resistant mouse strains. J. Neuroimmunol. 1994, 53, 101–107.
- Rouvier, E.; Luciani, M.F.; Mattei, M.G.; Denizot, F.; Golstein, P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993, 150, 5445–5456.
- Yao, Z.; Painter, S.L.; Fanslow, W.C.; Ulrich, D.; Macduff, B.M.; Spriggs, M.K.; Armitage, R.J. Human IL-17: A novel cytokine derived from T cells. J. Immunol. 1995, 155, 5483–5486.
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567.
- Yao, Z.; Fanslow, W.C.; Seldin, M.F.; Rousseau, A.M.; Painter, S.L.; Comeau, M.R.; Cohen, J.I.; Spriggs, M.K. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 1995, 3, 811–821.
- Hymowitz, S.G.; Filvaroff, E.H.; Yin, J.P.; Lee, J.; Cai, L.; Risser, P.; Maruoka, M.; Mao, W.; Foster, J.; Kelley, R.F.; et al. IL-17s adopt a cystine knot fold: Structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001, 20, 5332–5341.
- Buckley, K.M.; Ho, E.C.H.; Hibino, T.; Schrankel, C.S.; Schuh, N.W.; Wang, G.; Rast, J.P. IL17 factors are early regulators in the gut epithelium during inflammatory response to Vibrio in the sea urchin larva. Elife 2017, 6, e23481.
- Han, Q.; Das, S.; Hirano, M.; Holland, S.J.; McCurley, N.; Guo, P.; Rosenberg, C.S.; Boehm, T.; Cooper, M.D. Characterization of Lamprey IL-17 Family Members and Their Receptors. J. Immunol. 2015, 195, 5440–5451.
- Novatchkova, M.; Leibbrandt, A.; Werzowa, J.; Neubuser, A.; Eisenhaber, F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem. Sci. 2003, 28, 226–229.
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162.
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906.
- Goepfert, A.; Lehmann, S.; Blank, J.; Kolbinger, F.; Rondeau, J.M. Structural Analysis Reveals that the Cytokine IL-17F Forms a Homodimeric Complex with Receptor IL-17RC to Drive IL-17RA-Independent Signaling. Immunity 2020, 52, 499–512.e495.
- Su, Y.; Huang, J.; Zhao, X.; Lu, H.; Wang, W.; Yang, X.O.; Shi, Y.; Wang, X.; Lai, Y.; Dong, C. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci. Immunol. 2019, 4, eaau9657.
- Reynolds, J.M.; Lee, Y.H.; Shi, Y.; Wang, X.; Angkasekwinai, P.; Nallaparaju, K.C.; Flaherty, S.; Chang, S.H.; Watarai, H.; Dong, C. Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity 2015, 42, 692–703.
- Huang, J.; Lee, H.Y.; Zhao, X.; Han, J.; Su, Y.; Sun, Q.; Shao, J.; Ge, J.; Zhao, Y.; Bai, X.; et al. Interleukin-17D regulates group 3 innate lymphoid cell function through its receptor CD93. Immunity 2021, 54, 673–686.e674.
- Liu, C.; Swaidani, S.; Qian, W.; Kang, Z.; Sun, P.; Han, Y.; Wang, C.; Gulen, M.F.; Yin, W.; Zhang, C.; et al. A CC’ loop decoy peptide blocks the interaction between Act1 and IL-17RA to attenuate IL-17- and IL-25-induced inflammation. Sci. Signal. 2011, 4, ra72.
- Qian, Y.; Liu, C.; Hartupee, J.; Altuntas, C.Z.; Gulen, M.F.; Jane-Wit, D.; Xiao, J.; Lu, Y.; Giltiay, N.; Liu, J.; et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 2007, 8, 247–256.
- Sonder, S.U.; Saret, S.; Tang, W.; Sturdevant, D.E.; Porcella, S.F.; Siebenlist, U. IL-17-induced NF-kappaB activation via CIKS/Act1: Physiologic significance and signaling mechanisms. J. Biol. Chem. 2011, 286, 12881–12890.
- Conti, H.R.; Gaffen, S.L. IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J. Immunol. 2015, 195, 780–788.
- Liu, C.; Qian, W.; Qian, Y.; Giltiay, N.V.; Lu, Y.; Swaidani, S.; Misra, S.; Deng, L.; Chen, Z.J.; Li, X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci. Signal. 2009, 2, ra63.
- Schwandner, R.; Yamaguchi, K.; Cao, Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 2000, 191, 1233–1240.
- Karlsen, J.R.; Borregaard, N.; Cowland, J.B. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-beta nor C/EBP-delta. J. Biol. Chem. 2010, 285, 14088–14100.
- Ruddy, M.J.; Wong, G.C.; Liu, X.K.; Yamamoto, H.; Kasayama, S.; Kirkwood, K.L.; Gaffen, S.L. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 2004, 279, 2559–2567.
- Tohyama, M.; Shirakata, Y.; Hanakawa, Y.; Dai, X.; Shiraishi, K.; Murakami, M.; Miyawaki, S.; Mori, H.; Utsunomiya, R.; Masuda, K.; et al. Bcl-3 induced by IL-22 via STAT3 activation acts as a potentiator of psoriasis-related gene expression in epidermal keratinocytes. Eur. J. Immunol. 2018, 48, 168–179.
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000, 13, 715–725.
- Chyuan, I.T.; Lai, J.H. New insights into the IL-12 and IL-23: From a molecular basis to clinical application in immune-mediated inflammation and cancers. Biochem. Pharmacol. 2020, 175, 113928.
- Schnurr, M.; Toy, T.; Shin, A.; Wagner, M.; Cebon, J.; Maraskovsky, E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: A novel role for the cAMP pathway. Blood 2005, 105, 1582–1589.
- Sheibanie, A.F.; Tadmori, I.; Jing, H.; Vassiliou, E.; Ganea, D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 2004, 18, 1318–1320.
- Aggarwal, S.; Ghilardi, N.; Xie, M.H.; de Sauvage, F.J.; Gurney, A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003, 278, 1910–1914.
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748.
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133.
- Unutmaz, D. RORC2: The master of human Th17 cell programming. Eur. J. Immunol. 2009, 39, 1452–1455.
- Yang, X.O.; Pappu, B.P.; Nurieva, R.; Akimzhanov, A.; Kang, H.S.; Chung, Y.; Ma, L.; Shah, B.; Panopoulos, A.D.; Schluns, K.S.; et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 2008, 28, 29–39.
- Zielinski, C.E.; Mele, F.; Aschenbrenner, D.; Jarrossay, D.; Ronchi, F.; Gattorno, M.; Monticelli, S.; Lanzavecchia, A.; Sallusto, F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 2012, 484, 514–518.
- Duhen, T.; Campbell, D.J. IL-1beta promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J. Immunol. 2014, 193, 120–129.
- Veldhoen, M.; Hirota, K.; Westendorf, A.M.; Buer, J.; Dumoutier, L.; Renauld, J.C.; Stockinger, B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008, 453, 106–109.
- Akimzhanov, A.M.; Yang, X.O.; Dong, C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 2007, 282, 5969–5972.
- Huber, M.; Brustle, A.; Reinhard, K.; Guralnik, A.; Walter, G.; Mahiny, A.; von Low, E.; Lohoff, M. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc. Natl. Acad. Sci. USA 2008, 105, 20846–20851.
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009, 30, 576–587.
- Ikeda, S.; Saijo, S.; Murayama, M.A.; Shimizu, K.; Akitsu, A.; Iwakura, Y. Excess IL-1 signaling enhances the development of Th17 cells by downregulating TGF-beta-induced Foxp3 expression. J. Immunol. 2014, 192, 1449–1458.
- Mailer, R.K.; Joly, A.L.; Liu, S.; Elias, S.; Tegner, J.; Andersson, J. IL-1beta promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci. Rep. 2015, 5, 14674.
- Gooderham, M.J.; Papp, K.A.; Lynde, C.W. Shifting the focus—The primary role of IL-23 in psoriasis and other inflammatory disorders. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1111–1119.
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489.
- Rivino, L.; Messi, M.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F.; Geginat, J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 2004, 200, 725–735.
- Abromson-Leeman, S.; Bronson, R.T.; Dorf, M.E. Encephalitogenic T cells that stably express both T-bet and ROR gamma t consistently produce IFNgamma but have a spectrum of IL-17 profiles. J. Neuroimmunol. 2009, 215, 10–24.
- Boniface, K.; Blumenschein, W.M.; Brovont-Porth, K.; McGeachy, M.J.; Basham, B.; Desai, B.; Pierce, R.; McClanahan, T.K.; Sadekova, S.; de Waal Malefyt, R. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J. Immunol. 2010, 185, 679–687.
- Cosmi, L.; Maggi, L.; Santarlasci, V.; Capone, M.; Cardilicchia, E.; Frosali, F.; Querci, V.; Angeli, R.; Matucci, A.; Fambrini, M.; et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J. Allergy. Clin. Immunol. 2010, 125, 222–230.e4.
- Hirota, K.; Turner, J.E.; Villa, M.; Duarte, J.H.; Demengeot, J.; Steinmetz, O.M.; Stockinger, B. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 2013, 14, 372–379.
- Koenen, H.J.; Smeets, R.L.; Vink, P.M.; van Rijssen, E.; Boots, A.M.; Joosten, I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 2008, 112, 2340–2352.
- Valmori, D.; Raffin, C.; Raimbaud, I.; Ayyoub, M. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc. Natl. Acad. Sci. USA 2010, 107, 19402–19407.
- Hoechst, B.; Gamrekelashvili, J.; Manns, M.P.; Greten, T.F.; Korangy, F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood 2011, 117, 6532–6541.
- Wacleche, V.S.; Landay, A.; Routy, J.P.; Ancuta, P. The Th17 Lineage: From Barrier Surfaces Homeostasis to Autoimmunity, Cancer, and HIV-1 Pathogenesis. Viruses 2017, 9, 303.
- Wong, M.T.; Ong, D.E.; Lim, F.S.; Teng, K.W.; McGovern, N.; Narayanan, S.; Ho, W.Q.; Cerny, D.; Tan, H.K.; Anicete, R.; et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 2016, 45, 442–456.
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119.
- Shaw, M.H.; Kamada, N.; Kim, Y.G.; Nunez, G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 2012, 209, 251–258.
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017, 17, 535–544.
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; de Sauvage, F.J.; et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008, 14, 282–289.
- Puel, A.; Cypowyj, S.; Bustamante, J.; Wright, J.F.; Liu, L.; Lim, H.K.; Migaud, M.; Israel, L.; Chrabieh, M.; Audry, M.; et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 2011, 332, 65–68.
- Hernandez-Santos, N.; Huppler, A.R.; Peterson, A.C.; Khader, S.A.; McKenna, K.C.; Gaffen, S.L. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013, 6, 900–910.
- Wu, H.J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010, 32, 815–827.
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Setoyama, H.; Nagamori, T.; et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 2015, 163, 367–380.
- Bystrom, J.; Al-Adhoubi, N.; Al-Bogami, M.; Jawad, A.S.; Mageed, R.A. Th17 lymphocytes in respiratory syncytial virus infection. Viruses 2013, 5, 777–791.
- Chen, K.; Kolls, J.K. T cell-mediated host immune defenses in the lung. Annu. Rev. Immunol. 2013, 31, 605–633.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
736
Revisions:
2 times
(View History)
Update Date:
03 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





