Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Agnieszka Gęgotek | -- | 1516 | 2022-12-30 21:27:48 | | | |
| 2 | Jessie Wu | Meta information modification | 1516 | 2023-01-03 07:58:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gęgotek, A.; Skrzydlewska, E. The Role of ABC Transporters in the Skin. Encyclopedia. Available online: https://encyclopedia.pub/entry/39640 (accessed on 07 February 2026).
Gęgotek A, Skrzydlewska E. The Role of ABC Transporters in the Skin. Encyclopedia. Available at: https://encyclopedia.pub/entry/39640. Accessed February 07, 2026.
Gęgotek, Agnieszka, Elżbieta Skrzydlewska. "The Role of ABC Transporters in the Skin" Encyclopedia, https://encyclopedia.pub/entry/39640 (accessed February 07, 2026).
Gęgotek, A., & Skrzydlewska, E. (2022, December 30). The Role of ABC Transporters in the Skin. In Encyclopedia. https://encyclopedia.pub/entry/39640
Gęgotek, Agnieszka and Elżbieta Skrzydlewska. "The Role of ABC Transporters in the Skin." Encyclopedia. Web. 30 December, 2022.
Copy Citation
ABC transporters are expressed in skin cells to protect them against harmful xenobiotics. These transmembrane proteins have a number of additional functions that ensure skin homeostasis.
ABC transporters
skin
1. ABC Transporters
ABC transporters are expressed in many epithelial and endothelial barrier tissues/cells, limiting the penetration of the xenobiotics between the body’s compartments. They are located, among other places, in the cells of the liver, kidneys, the epithelium of the small intestine, the blood–brain barrier, and the blood–retina barrier; they are present, however, not only in the plasma membrane, but also in intracellular membranes surrounding cell organelles, e.g., peroxisomes, lysosomes, mitochondria, and the endoplasmic reticulum [1][2][3]. They are involved in the elimination of metabolic byproducts from cells and protection against xenobiotics, including toxins, carcinogens, cytotoxic components of the diet, and drugs. ABC transporters fulfill their functions through the ejection of molecules from the cell. Usually, this process requires energy; therefore, ABC transporters have the ability to bind the ATP and to hydrolyze it to ADP and phosphate (Pi) with energy generation [4]. This is necessary for the translocation of molecules across the cell membrane, contrary to its concentration gradient, this being possible due to the specific structure of ABC transporters. These transmembrane proteins are fairly conserved in composition. Their structure includes the ATP-binding domain (NBD), which exhibits ATPase activity and is responsible for ATP hydrolysis. As a result of this reaction, the second important component of ABC transporter, i.e., the transmembrane domain (TMD), can change in conformation [5]. This is important due to the fact that TMD is the domain that recognizes substrates and marks the paths of their translocation across the cell membrane. Moreover, the motifs Walker A and Walker B are present within the NBD domain, which are characteristic of all ATP-binding proteins, as well as motif C (ABC Signature Motif), with the sequence “LSGGQ,” which is only specific for ABC proteins (Figure 1). In the construction of ABC transporters, other regions can be distinguished such as loops A, Q, D, H, and X, which affect the classification of these proteins’ subfamilies [6]. However, due to the amino acid sequence in the NBD region and its structural organization, all ABC transporters have been grouped into seven subfamilies, from ABCA to ABCG (Table 1). In addition to the systematic name, some of these transporters are known by different names, including MDR1/3 (multi-drug resistance transporter; ABCB1/4), TAP1/2 (transporter associated with antigen processing; ABCB2/3), MRP1-6 (multidrug resistance-associated protein; ABCC1-6), MRP7-9 (ABCC10-12), CFTR (cystic fibrosis transmembrane conductance regulator; ABCC7), SUR1/2 (sulfonylurea receptor; ABCC8/9), and BCRP (breast cancer resistance protein; ABCG2) [7][8].
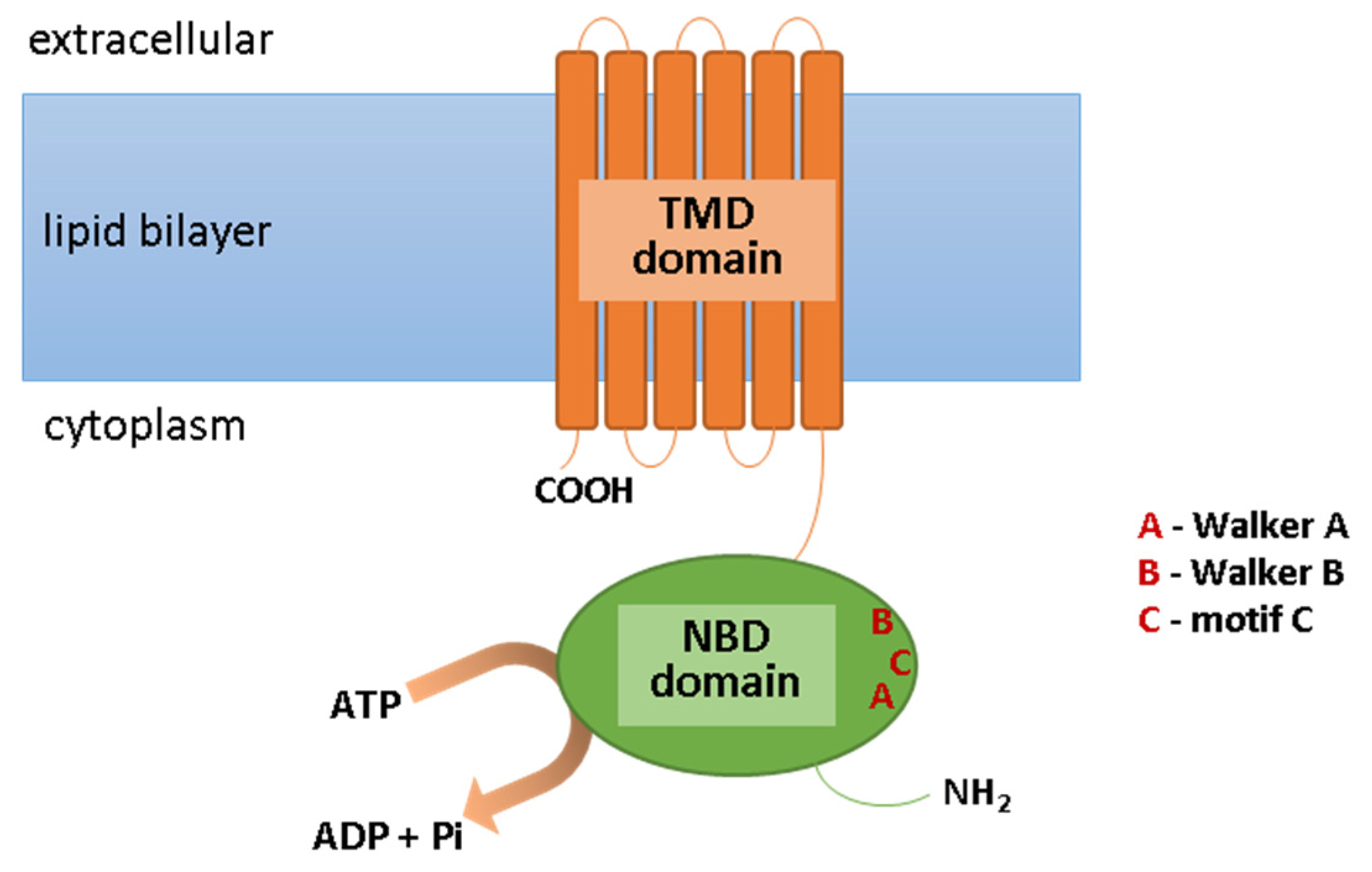
Figure 1. Scheme of the ABC transporter structure. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; NBD, ATP-binding domain; Pi, phosphate; TMD, transmembrane domain.
Table 1. The list of ABC transporters with their main functions. Abbreviations: ABC, ATP-binding cassette transporter; BCRP, breast cancer resistance protein; CFTR, cystic fibrosis transmembrane conductance regulator; MDR, multi-drug resistance transporter; MRP, multidrug resistance-associated protein; SUR, sulfonylurea receptor; TAP, transporter associated with antigen processing.
| Subfamily | Transporters | Main Function |
|---|---|---|
| ABCA | ABCA 1-9, 12 | transport of cholesterol and lipids |
| ABCB | ABCB 1 (MDR1), ABCB 2-3 (TAP1-2), ABCB 4 (MDR3), ABCB 5-11 |
transport of peptides and metabolites |
| ABCC | ABCC 1-6 (MRP1-6), ABCC 7 (CFTR), ABCC 8-9 (SUR1-2), ABCC 10-12 (MRP7-9) |
transport of ions, cell-surface receptors |
| ABCD | ABCD 1-4 | participate in peroxisome activation |
| ABCE | ABCE 1 | multidrug resistance |
| ABCF | ABCF 1-3 | regulation of innate immune response |
| ABCG | ABCG 1, ABCG 2 (BCRP), ABCG 4,5,8 |
transport of drugs, toxins, lipids, cholesterol and other steroids |
2. ABC Transporters in the Skin
The expression and activity of ABC transporters in skin cells is indisputably linked to their role in skin protection against harmful xenobiotics and the oxidative stress that they induce [9]. However, the current knowledge concerning these proteins allows for the conclusion that the activity of ABC transporters is dependent on numerous factors and that they have a much wider range of action in relation to skin cells (Figure 2).

Figure 2. Roles and functions of ABC transporters in skin cells under physiological conditions (green), in pathological states (red), and during therapy (blue). Abbreviation: ABC, ATP-binding cassette transporter.
2.1. Activation and Suppression According to Oxidative Conditions
It is generally assumed that the appearance of an agonistic xenobiotic in the cytoplasm of the cell activates the ABC transporters and induces the efflux of this potentially harmful compound outside the cell [9]. Exposure to these compounds is very often accompanied by oxidative stress (the oxidative effect of these compounds or a side effect of their metabolism). It has not been shown that free radicals formed at the time of exposure directly affect the functioning of ABC transporters; however, there are many pathways linking oxidative stress with these transporters [10][11]. Reactive oxygen species (ROS) conjugated with GSH, glucuronide, and sulphate only are agonistic molecules for ABC transporters [12]. However, in the case of lung cancer cells, it was found that low doses of anticancer drugs, by inducing a moderate increase in ROS levels (approximately a 3-4-fold increase of the control levels), promote a defense response which results in an increase in the expression of ABC proteins, thus providing these cells with drug resistance [11]. This might be connected with an ROS-induced activity of transcription factors, such as nuclear factor-κB (NFκB), responsible for the formation of inflammation, and nuclear factor E2-related factor-2 (Nrf2), responsible for the biosynthesis of antioxidant proteins [13]. Therefore, NFκB increases the expression of ABC transporters during inflammation [14][15], while Nrf2 initiates ABC transporters in response to oxidative stress [16][17].
Oxidative stress arising from, e.g., exposure of cells/organism to pathogenic factors (exogenous and endogenous), often leads to the activation of kinases involved in intracellular signal transduction, including mitogen-activated protein kinases (MAPKs) [18]. As a result, numerous proteins are phosphorylated, including ABC transporters. The data in the literature indicate that the phosphorylation of ABC proteins is often a constitutive element of the functioning of transporters, and is necessary for their full activity, especially under oxidative conditions [19]. Moreover, MAPKs activation by ROS additionally induces NFκB and Nrf2 activity, thus favoring the expression of ABC transporters [17][20].
It is known that, while oxidative stress activates most ABC transporters, antioxidants such as vitamin C, flavonoids, or phytocannabinoids are able to suppress their activity [21][22][23][24]. Due to the recent increased public interest in aging and disease prevention, the use of herbal preparations, especially those containing high doses of natural antioxidants, has become very popular, raising the potential for interactions with the implemented drug therapies. In relation to the influence of antioxidants on ABC transporters, their action is not only based on ROS scavenging, but they are also able to inhibit drug interaction with ABC transporters during therapy, as well as prevent nucleotide hydrolysis, thus limiting the access of transporters to the energy from ATP hydrolysis [23]. Therefore, antioxidants could be considered as potential modulators of multidrug resistance and as therapeutic agents to suppress ABC transporter activity under drug-induced oxidative conditions.
2.2. Main Functions in the Skin
It has been reported that ABC transporters in the skin have different intensities of distribution in the epidermis compared to the dermis. For example, MRP1 has a strong expression in whole skin specimens and the dermis, and a weak expression in the epidermis [25]. This leads to the uptake of compounds from the epidermal compartment and their secretion into the deeper layers of the skin. Moreover, by coordinating the efflux of steroid hormones from normal human epidermal keratinocytes, ABC transporters ensure proper hormonal balance in the skin [26]. ABC transporters’ expression in human skin biopsies has been correlated with sweat metabolites, which indicates their role in sweat secretion and, thus, an indirect effect on body thermoregulation [27]. By removing contact allergens and exogenous compounds, such as fragments of pathogens, outside the cell, ABC transporters also play an important role in the migration of Langerhans cells and help maintain a healthy immune response in the skin [28][29]. ABC transporters also translocate lipid metabolites between cell organelles in order to regulate lipid homeostasis and prevent disease development [30]. It has been found that a dysfunction of ABCA12, which is responsible for the translocation of glucosylceramides (GlcCer) into lamellar granules, leads to a disturbance of the skin’s barrier functions and is even co-responsible for the development of a rare skin disease called harlequin ichthyosis [31].
ABC transporters also have play a significant role in melanoma, as well non-melanoma skin cancers, where the expression of these molecules is always present in a high level compared to non-cancerous human skin cells [32][33][34][35]. The exact mechanism of the ABC proteins expression in skin cancer cells is not known; however, ABC-dependent drug efflux in these cells leads to cancer multidrug resistance by decreasing intracellular drug accumulation [34][36][37][38]. Moreover, ABC transporters additionally protect the mitochondrial genome of melanoma cells against drug-induced DNA damage [36]. It has also been observed that high levels of ABC transporters in melanoma cells favors their migration and invasion, being a prognosis of numerous metastases and failure of anticancer therapy [39][40].
The presented examples are only a fragment of ABC transporters’ role in the skin that is currently known. However, it can already be seen at this stage how important they are in the functioning of cells both in normal physiology and in pathological states (Figure 2).
References
- Oldham, M.L.; Davidson, A.L.; Chen, J. Structural insights into ABC transporter mechanism. Curr. Opin. Struct. Biol. 2008, 18, 726–733.
- Kobuchi, H.; Moriya, K.; Ogino, T.; Fujita, H.; Inoue, K.; Shuin, T.; Yasuda, T.; Utsumi, K.; Utsumi, T. Mitochondrial Localization of ABC Transporter ABCG2 and Its Function in 5-Aminolevulinic Acid-Mediated Protoporphyrin IX Accumulation. PLoS ONE 2012, 7, e50082.
- Mahringer, A.; Fricker, G. ABC transporters at the blood-brain barrier. Expert Opin. Drug Metab. Toxicol. 2016, 12, 499–508.
- Wilkens, S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015, 7, 14.
- Jones, P.M.; George, A.M. Mechanism of ABC transporters: A molecular dynamics simulation of a well characterized nucleotide-binding subunit. Proc. Natl. Acad. Sci. USA 2002, 99, 12639–12644.
- Beis, K. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 2015, 43, 889–893.
- Dean, M.; Moitra, K.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Hum. Mutat. 2022, 43, 1162–1182.
- Bates, S.E.; Robey, R.; Knutsen, T.; Honjo, Y.; Litman, T.; Dean, M. New ABC transporters in multi-drug resistance. Expert Opin. Ther. Targets 2000, 4, 561–580.
- Osman-Ponchet, H.; Boulai, A.; Kouidhi, M.; Sevin, K.; Alriquet, M.; Gaborit, A.; Bertino, B.; Comby, P.; Ruty, B. Characterization of ABC transporters in human skin. Drug Metabol. Drug Interact. 2014, 29, 91–100.
- Grewal, G.K.; Kukal, S.; Kanojia, N.; Saso, L.; Kukreti, S.; Kukreti, R. Effect of oxidative stress on ABC transporters: Contribution to epilepsy pharmacoresistance. Molecules 2017, 22, 365.
- Yuan, T.; Hu, J.; Zhu, X.; Yin, H.; Yin, J. Oxidative stress-mediated up-regulation of ABC transporters in lung cancer cells. J. Biochem. Mol. Toxicol. 2022, 36, e23095.
- Järvinen, E.; Deng, F.; Kiander, W.; Sinokki, A.; Kidron, H.; Sjöstedt, N. The Role of Uptake and Efflux Transporters in the Disposition of Glucuronide and Sulfate Conjugates. Front. Pharmacol. 2022, 12, 802539.
- Rubio, V.; García-Pérez, A.I.; Herráez, A.; Diez, J.C. Different roles of Nrf2 and NFKB in the antioxidant imbalance produced by esculetin or quercetin on NB4 leukemia cells. Chem. Biol. Interact. 2018, 294, 158–166.
- Miller, D.S. Regulation of ABC Transporters Blood-Brain Barrier. The Good, the Bad, and the Ugly. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 125, pp. 43–70.
- Di, Q.; Yu, N.; Liu, H.; Hu, Y.; Jiang, Y.; Yan, Y.K.; Zhang, Y.F.; Zhang, Y.D. Nuclear factor-kappa B activity regulates brain expression of P-glycoprotein in the kainic acid-induced seizure rats. Mediators Inflamm. 2011, 2011, 670613.
- Wang, X.; Campos, C.R.; Peart, J.C.; Smith, L.K.; Boni, J.L.; Cannon, R.E.; Miller, D.S. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J. Neurosci. 2014, 34, 8585–8593.
- Maher, J.M.; Dieter, M.Z.; Aleksunes, L.M.; Slitt, A.L.; Guo, G.; Tanaka, Y.; Scheffer, G.L.; Chan, J.Y.; Manautou, J.E.; Chen, Y.; et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology 2007, 46, 1597–1610.
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639.
- Stolarczyk, E.I.; Reiling, C.J.; Paumi, C.M. Regulation of ABC Transporter Function via Phosphorylation by Protein Kinases. Curr. Pharm. Biotechnol. 2011, 12, 621–635.
- Li, L.; Cataisson, C.; Flowers, B.; Fraser, E.; Sanchez, V.; Day, C.P.; Yuspa, S.H. Topical Application of a Dual ABC Transporter Substrate and NF-κB Inhibitor Blocks Multiple Sources of Cutaneous Inflammation in Mouse Skin. J. Invest. Dermatol. 2019, 139, 1506–1515.e7.
- Zecchinati, F.; Barranco, M.M.; Arana, M.R.; Tocchetti, G.N.; Domínguez, C.J.; Perdomo, V.G.; Ruiz, M.L.; Mottino, A.D.; García, F.; Villanueva, S.S.M. Reversion of down-regulation of intestinal multidrug resistance-associated protein 2 in fructose-fed rats by geraniol and vitamin C: Potential role of inflammatory response and oxidative stress. J. Nutr. Biochem. 2019, 68, 7–15.
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 101613.
- Di Pietro, A.; Conseil, G.; Pérez-Victoria, J.M.; Dayan, G.; Baubichon-Cortay, H.; Trompier, D.; Steinfels, E.; Jault, J.M.; De Wet, H.; Maitrejean, M.; et al. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell. Mol. Life Sci. 2002, 59, 307–322.
- Morris, M.E.; Zhang, S. Flavonoid-drug interactions: Effects of flavonoids on ABC transporters. Life Sci. 2006, 78, 2116–2130.
- Skazik, C.; Wenzel, J.; Marquardt, Y.; Kim, A.; Merk, H.F.; Bickers, D.R.; Baron, J.M. P-Glycoprotein (ABCB1) expression in human skin is mainly restricted to dermal components. Exp. Dermatol. 2011, 20, 450–452.
- Heise, R.; Skazik, C.; Rodriguez, F.; Stanzel, S.; Marquardt, Y.; Joussen, S.; Wendel, A.F.; Wosnitza, M.; Merk, H.F.; Baron, J.M. Active transport of contact allergens and steroid hormones in epidermal keratinocytes is mediated by multidrug resistance related proteins. J. Invest. Dermatol. 2010, 130, 305–308.
- Nielsen, M.M.K.; Aryal, E.; Safari, E.; Mojsoska, B.; Jenssen, H.; Prabhala, B.K. Current state of slc and abc transporters in the skin and their relation to sweat metabolites and skin diseases. Proteomes 2021, 9, 23.
- van de Ven, R.; de Jong, M.C.; Reurs, A.W.; Schoonderwoerd, A.J.N.; Jansen, G.; Hooijberg, J.H.; Scheffer, G.L.; de Gruijl, T.D.; Scheper, R.J. Dendritic Cells Require Multidrug Resistance Protein 1 (ABCC1) Transporter Activity for Differentiation. J. Immunol. 2006, 176, 5191–5198.
- Randolph, G.J.; Beaulieu, S.; Pope, M.; Sugawara, I.; Hoffman, L.; Steinman, R.M.; Muller, W.A. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc. Natl. Acad. Sci. USA 1998, 95, 6924–6929.
- Tarling, E.J.; Vallim, T.Q.d.A.; Edwards, P.A. Role of ABC transporters in lipid transport and human disease. Trends Endocrinol. Metab. 2013, 24, 342–350.
- Scott, C.A.; Rajpopat, S.; Di, W.L. Harlequin ichthyosis: ABCA12 mutations underlie defective lipid transport, reduced protease regulation and skin-barrier dysfunction. Cell Tissue Res. 2013, 351, 281–288.
- Heimerl, S.; Bosserhoff, A.K.; Langmann, T.; Ecker, J.; Schmitz, G. Mapping ATP-binding cassette transporter gene expression profiles in melanocytes and melanoma cells. Melanoma Res. 2007, 17, 265–273.
- Keshet, G.I.; Goldstein, I.; Itzhaki, O.; Cesarkas, K.; Shenhav, L.; Yakirevitch, A.; Treves, A.J.; Schachter, J.; Amariglio, N.; Rechavi, G. MDR1 expression identifies human melanoma stem cells. Biochem. Biophys. Res. Commun. 2008, 368, 930–936.
- Fukunaga-Kalabis, M.; Herlyn, M. Beyond ABC: Another mechanism of drug resistance in melanoma side population. J. Invest. Dermatol. 2012, 132, 2317–2319.
- Thinnes, F.P. Nonmelanoma skin cancer is associated with reduced alzheimer disease risk. Neurology 2013, 81, 2056.
- Elliott, A.M.; Al-Hajj, M.A. ABCB8 mediates doxorubicin resistance in melanoma cells by protecting the mitochondrial genome. Mol. Cancer Res. 2009, 7, 79–87.
- Chen, K.G.; Valencia, J.C.; Gillet, J.-P.; Hearing, V.J.; Gottesman, M.M. Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma. Pigment Cell Melanoma Res. 2009, 22, 740–749.
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy resistance mechanisms in advanced skin cancer. Oncol. Rev. 2017, 11, 19–25.
- Colone, M.; Calcabrini, A.; Toccacieli, L.; Bozzuto, G.; Stringaro, A.; Gentile, M.; Cianfriglia, M.; Ciervo, A.; Caraglia, M.; Budillon, A.; et al. The multidrug transporter P-glycoprotein: A mediator of melanoma invasion? J. Invest. Dermatol. 2008, 128, 957–971.
- Setia, N.; Abbas, O.; Sousa, Y.; Garb, J.L.; Mahalingam, M. Profiling of ABC transporters ABCB5, ABCF2 and nestin-positive stem cells in nevi, in situ and invasive melanoma. Mod. Pathol. 2012, 25, 1169–1175.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
03 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




