
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohsen Sheykhhasan | -- | 3108 | 2022-12-30 11:32:12 | | | |
| 2 | Amina Yu | + 4 word(s) | 3112 | 2023-01-04 02:46:19 | | |
Video Upload Options
LncRNAs can operate as decoys by binding to microRNAs or transcription factors to sequester them away from their target locations and obstruct transcription and translation. The role of lncRNA as competing endogenous RNAs (ceRNAs) in the development of cancer has been demonstrated. Through their regulatory effects on DNA sequences in cis-acting and trans-acting lncRNAs, the lncRNAs can modulate many biological processes such as cell growth, proliferation, differentiation, invasion, progression, apoptosis, epithelial–mesenchymal transition (EMT), and tumorigenesis. There is growing proof that lncRNAs interact with DNA in sequence-specific ways by forming triple helix (triplex) structures. The transcriptional factors bound on a specific DNA sequence that take control of the gene expression frequently interact with LncRNA. On the other hand, LncRNA co-transcriptionally form RNA-DNA hybrids such as R-loops recognized by chromatin modifiers or by transcription factors to activate or inhibit target gene transcription.
1. The Effect of FLVCR1-AS1 on Several Cancers
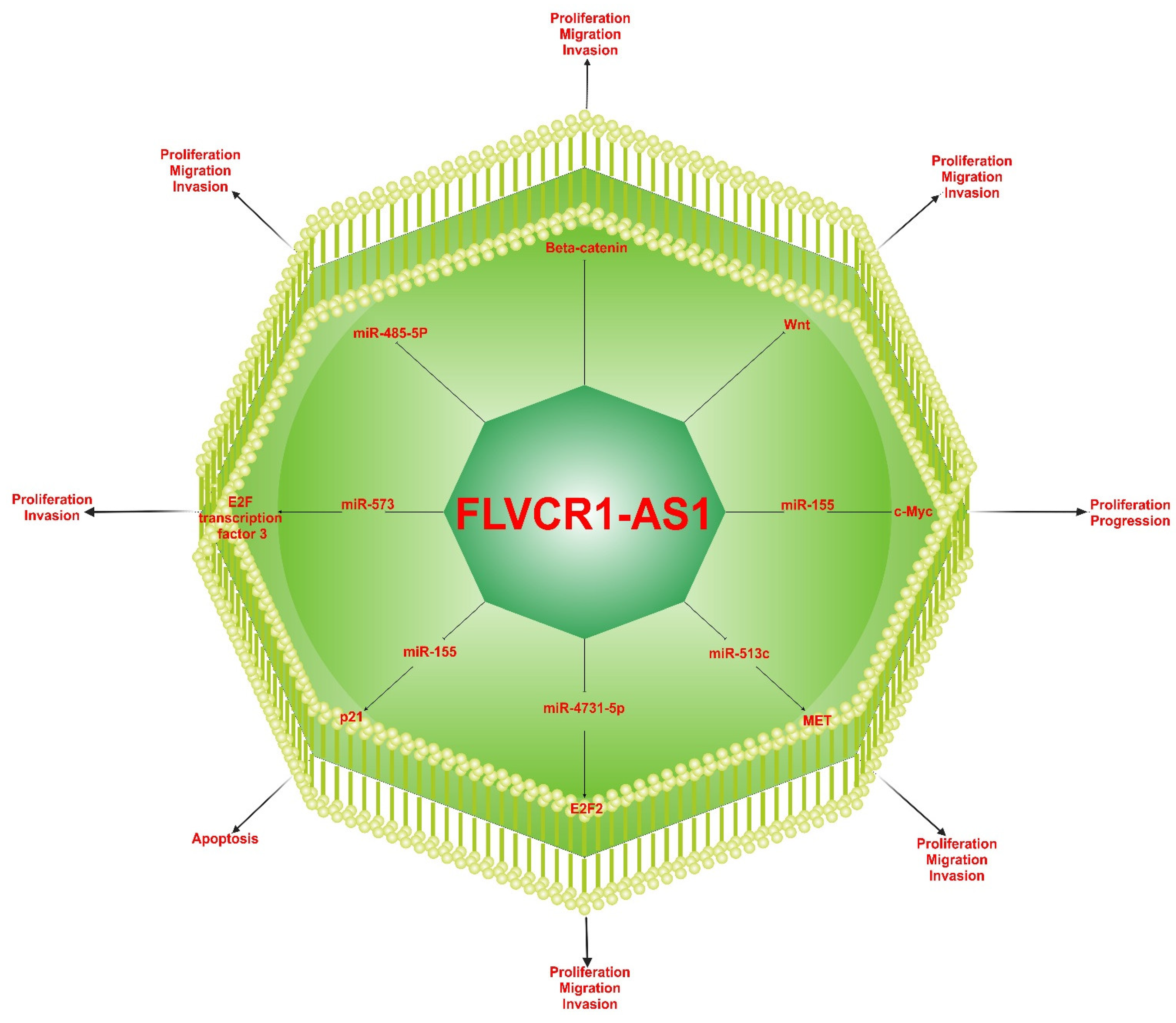
1.1. Cholangiocarcinoma
1.2. Hepatocellular Carcinoma
1.3. Gastric Cancer
1.4. Colorectal Cancer
1.5. Glioma and Glioblastoma
1.6. Non-Small Cell Lung Cancer
1.7. Ovarian Cancer
1.8. Breast Cancer
1.9. Osteosarcoma
1.10. Pancreatic Cancer
2. The Effect of FBXL19-AS1 on Several Cancers
2.1. Hepatocellular Carcinoma
2.2. Gastric Cancer
2.3. Colorectal Cancer
2.4. Glioma
2.5. Lung Cancer
2.6. Cervical Cancer
2.7. Breast Cancer
2.8. Osteosarcoma
2.9. Nasopharyngeal Carcinoma
2.10. Acute Myeloid Leukemia
3. Molecular Mechanisms of FBXL19-AS1
References
- Zheng, B.; Jeong, S.; Zhu, Y.; Chen, L.; Xia, Q. miRNA and lncRNA as biomarkers in cholangiocarcinoma(CCA). Oncotarget 2017, 8, 100819–100830.
- Bao, W.; Cao, F.; Ni, S.; Yang, J.; Li, H.; Su, Z.; Zhao, B. lncRNA FLVCR1-AS1 regulates cell proliferation, migration and invasion by sponging miR-485-5p in human cholangiocarcinoma. Oncol. Lett. 2019, 18, 2240–2247.
- Li, G.; Shi, H.; Wang, X.; Wang, B.; Qu, Q.; Geng, H.; Sun, H. Identification of diagnostic long non-coding RNA biomarkers in patients with hepatocellular carcinoma. Mol. Med. Rep. 2019, 20, 1121–1130.
- He, Y.; Meng, X.-M.; Huang, C.; Wu, B.-M.; Zhang, L.; Lv, X.-W.; Li, J. Long noncoding RNAs: Novel insights into hepatocelluar carcinoma. Cancer Lett. 2014, 344, 20–27.
- Zhang, K.; Zhao, Z.; Yu, J.; Chen, W.; Xu, Q.; Chen, L. LncRNA FLVCR1-AS1 acts as miR-513c sponge to modulate cancer cell proliferation, migration, and invasion in hepatocellular carcinoma. J. Cell. Biochem. 2018, 119, 6045–6056.
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.
- Chiang, T.H.; Chang, W.J.; Chen, S.L.S.; Yen, A.M.F.; Fann, J.C.Y.; Chiu, S.Y.H.; Chen, Y.R.; Chuang, S.L.; Shieh, C.F.; Liu, C.Y.; et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: A long-term cohort study on Matsu Islands. Gut 2021, 70, 243–250.
- Wang, Q.-X.; Zhu, Y.-Q.; Zhang, H.; Xiao, J. Altered MiRNA Expression in Gastric Cancer: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2015, 35, 933–944.
- Shin, V.Y.; Chu, K.M. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J. Gastroenterol. 2014, 20, 10432–10439.
- Hwang, J.; Min, B.-H.; Jang, J.; Kang, S.Y.; Bae, H.; Jang, S.S.; Kim, J.-I.; Kim, K.-M. MicroRNA Expression Profiles in Gastric Carcinogenesis. Sci. Rep. 2018, 8, 14393.
- Hao, N.-B.; He, Y.-F.; Li, X.-Q.; Wang, K.; Wang, R.-L. The role of miRNA and lncRNA in gastric cancer. Oncotarget 2017, 8, 81572–81582.
- Sheykhhasan, M.; Ahmadyousefi, Y.; Seyedebrahimi, R.; Tanzadehpanah, H.; Manoochehri, H.; Dama, P.; Hosseini, N.F.; Akbari, M.; Farsani, M.E. DLX6-AS1: A putative lncRNA candidate in multiple human cancers. Expert Rev. Mol. Med. 2021, 23, E17.
- Liu, Y.; Guo, G.; Zhong, Z.; Sun, L.; Liao, L.; Wang, X.; Cao, Q.; Chen, H. Long non-coding RNA FLVCR1-AS1 sponges miR-155 to promote the tumorigenesis of gastric cancer by targeting c-Myc. Am. J. Transl. Res. 2019, 11, 793–805.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Liu, H.; Ye, D.; Chen, A.; Tan, D.; Zhang, W.; Jiang, W.; Wang, M.; Zhang, X. A pilot study of new promising non-coding RNA diagnostic biomarkers for early-stage colorectal cancers. Clin. Chem. Lab. Med. (CCLM) 2019, 57, 1073–1083.
- Han, Y.; Wang, X.; Mao, E.; Shen, B.; Huang, L. lncRNA FLVCR1-AS1 drives colorectal cancer progression via modulation of the miR-381/RAP2A axis. Mol. Med. Rep. 2020, 23, 139.
- Cui, T.; Gui, D.; Gu, C.; Yan, S.; Yue, Y.; Zhang, J.; Sun, H.; Fang, Y.; Jiang, N. Long Non-Coding RNA FLVCR1-AS1 Acts as miR-493-3p Sponge to Modulate Cancer Cell Proliferation, Invasion and Migration in Colorectal Cancer. J. Biomater. Tissue Eng. 2020, 10, 306–314.
- Mesfin, F.B.; Al-Dhahir, M.A. Gliomas. 2022 Apr 9. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Yan, Z.; Zhang, W.; Xiong, Y.; Wang, Y.; Li, Z. Long noncoding RNA FLVCR1-AS1 aggravates biological behaviors of glioma cells via targeting miR-4731-5p/E2F2 axis. Biochem. Biophys. Res. Commun. 2019, 521, 716–720.
- Gao, W.; Li, H.; Liu, Y.; Zhang, Y.; Zhao, H.; Liu, F. Long non-coding RNA FLVCR1-AS1 promotes glioma cell proliferation and invasion by negatively regulating miR-30b-3p. Mol. Med. Rep. 2020, 22, 723–732.
- Vencken, S.F.; Greene, C.M.; McKiernan, P.J. Non-coding RNA as lung disease biomarkers. Thorax 2015, 70, 501–503.
- Gao, X.; Zhao, S.; Yang, X.; Zang, S.; Yuan, X. Long non-coding RNA FLVCR1-AS1 contributes to the proliferation and invasion of lung cancer by sponging miR-573 to upregulate the expression of E2F transcription factor 3. Biochem. Biophys. Res. Commun. 2018, 505, 931–938.
- Lin, H.; Shangguan, Z.; Zhu, M.; Bao, L.; Zhang, Q.; Pan, S. lncRNA FLVCR1-AS1 silencing inhibits lung cancer cell proliferation, migration, and invasion by inhibiting the activity of the Wnt/beta-catenin signaling pathway. J. Cell. Biochem. 2019, 120, 10625–10632.
- Yan, H.; Li, H.; Silva, M.A.; Guan, Y.; Yang, L.; Zhu, L.; Zhang, Z.; Li, G.; Ren, C. LncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J. Exp. Clin. Cancer Res. 2019, 38, 356.
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA A Cancer J. Clin. 2014, 64, 52–62.
- Stuckey, A. Breast cancer: Epidemiology and risk factors. Clin. Obstet. Gynecol. 2011, 54, 96–102.
- Lin, J.; Zhai, S.; Zou, S.; Xu, Z.; Zhang, J.; Jiang, L.; Deng, X.; Chen, H.; Peng, C.; Zhang, J.; et al. Positive feedback between lncRNA FLVCR1-AS1 and KLF10 may inhibit pancreatic cancer progression via the PTEN/AKT pathway. J. Exp. Clin. Cancer Res. 2021, 40, 316.
- Xu, S.; Gong, Y.; Yin, Y.; Xing, H.; Zhang, N. The multiple function of long noncoding RNAs in osteosarcoma progression, drug resistance and prognosis. Biomed. Pharmacother. 2020, 127, 110141.
- He, D.; Zhang, X.; Zhu, X.; Maharjan, N.; Wang, Y.; Luo, P.; Liang, C.; Tu, J. Role of FBXL19-AS1 in hepatocellular carcinoma by lncRNA–miRNA–mRNA network analysis and its diagnostic and prognostic value. Res. Sq. 2020.
- Pan, Z.; Ding, J.; Yang, Z.; Li, H.; Ding, H.; Chen, Q. LncRNA FLVCR1-AS1 promotes proliferation, migration and activates Wnt/β-catenin pathway through miR-381-3p/CTNNB1 axis in breast cancer. Cancer Cell Int. 2020, 20, 214.
- Li, Z.; Hong, S.; Liu, Z. LncRNA LINC00641 predicts prognosis and inhibits bladder cancer progression through miR-197-3p/KLF10/PTEN/PI3K/AKT cascade. Biochem. Biophys. Res. Commun. 2018, 503, 1825–1829.
- Yang, N.; Chen, J.; Zhang, H.; Wang, X.; Yao, H.; Peng, Y.; Zhang, W. LncRNA OIP5-AS1 loss-induced microRNA-410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell Death Dis. 2017, 8, e2975.
- Hao, J.; Jia, Y.U.; Zi-Yu, W.; Ou, Q.; Jun, W.; Jiang, H.; Zhao-Lian, L.I. Mechanism of flavonoids of Sophorae Fructus in inhibiting proliferation, migration and invasion of hepatocellular carcinoma cells by regulating LncRNA FBXL19-AS1/miR-342-3p pathway. Zhongguo Zhong Yao Za Zhi 2020, 45, 4440–4447. (In Chinese)
- He, D.; Zhang, X.; Zhu, X.; Maharjan, N.; Wang, Y.; Luo, P.; Liang, C.; Tu, J. Identify and Validate the Transcriptomic, Functional Network, and Predictive Validity of FBXL19-AS1 in Hepatocellular Carcinoma. Front. Oncol. 2020, 10, 609601.
- Chen, Y.; Yang, L. FBXL19-AS1 aggravates the progression of hepatocellular cancer by downregulating KLF2. J. BUON 2021, 26, 1333–1339.
- Wang, X.L.; Wang, J.L.; Ma, W.; Jiang, Y.; Wan, J.H.; Jiang, S.J.; He, Z.K.; Wang, X.J.; An, Z.Y.; Liu, X.X.; et al. Long non-coding RNA FBXL19-AS1 serves as a competing endogenous RNA to regulate ZEB1 expression by sponging miR-431 in gastric cancer. J. Biol. Regul. Homeost. Agents 2020, 34, 1847–1855.
- Ji, R.X.; Ren, F.; Liu, X.Q.; Yuan, X. LncRNA FBXL19-AS1 promotes the development of gastric cancer by regulating miR-876-5p/HMGB3 axis. J. Biol. Regul. Homeost. Agents 2020, 34, 1513–1518.
- Shen, B.; Yuan, Y.; Zhang, Y.; Yu, S.; Peng, W.; Huang, X.; Feng, J. Long non-coding RNA FBXL19-AS1 plays oncogenic role in colorectal cancer by sponging miR-203. Biochem. Biophys. Res. Commun. 2017, 488, 67–73.
- Liu, X.; Wu, P.; Su, R.; Xue, Y.; Yang, C.; Wang, D.; Ruan, X.; Zheng, J.; Yang, Y.; Li, Z.; et al. IGF2BP2 stabilized FBXL19-AS1 regulates the blood-tumour barrier permeability by negatively regulating ZNF765 by STAU1-mediated mRNA decay. RNA Biol. 2020, 17, 1777–1788.
- Yu, D.-J.; Li, Y.-H.; Zhong, M. LncRNA FBXL19-AS1 promotes proliferation and metastasis via regulating epithelial-mesenchymal transition in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4800–4806.
- Jiang, Q.; Cheng, L.; Ma, D.; Zhao, Y. FBXL19-AS1 exerts oncogenic function by sponging miR-431-5p to regulate RAF1 expression in lung cancer. Biosci. Rep. 2019, 39, BSR20181804.
- Wang, L.; Zhang, X.; Liu, Y.; Xu, S. Long noncoding RNA FBXL19—AS1 induces tumor growth and metastasis by sponging miR—203a—3p in lung adenocarcinoma. J. Cell. Physiol. 2019, 235, 3612–3625.
- Huang, X.; Shi, H.; Shi, X.; Jiang, X. LncRNA FBXL19-AS1 promotes proliferation and metastasis of cervical cancer through upregulating COL1A1 as a sponge of miR-193a-5p. J. Biol. Res. 2021, 28, 20.
- Wan, S.; Ni, G.; Ding, J.; Huang, Y. Long Noncoding RNA FBXL19-AS1 Expedites Cell Growth, Migration and Invasion in Cervical Cancer by miR-193a-5p/PIN1 Signaling. Cancer Manag. Res. 2020, 12, 9741–9752.
- Xiao, Y.; Xiao, T.; Ou, W.; Wu, Z.; Wu, J.; Tang, J.; Tian, B.; Zhou, Y.; Su, M.; Wang, W. LncRNA SNHG16 as a potential biomarker and therapeutic target in human cancers. Biomark. Res. 2020, 8, 41.
- Ding, Z.; Ye, P.; Yang, X.; Cai, H. LncRNA FBXL19-AS1 promotes breast cancer cells proliferation and invasion via acting as a molecular sponge to miR-718. Biosci. Rep. 2019, 39, BSR20182018.
- Dong, G.; Pan, T.; Zhou, D.; Li, C.; Liu, J.; Zhang, J. FBXL19-AS1 promotes cell proliferation and inhibits cell apoptosis via miR-876-5p/FOXM1 axis in breast cancer. Acta Biochim. Et Biophys. Sin. 2019, 51, 1106–1113.
- Zhang, Y.; Xiao, X.; Zhou, W.; Hu, J.; Zhou, D. LIN28A-stabilized FBXL19-AS1 promotes breast cancer migration, invasion and EMT by regulating WDR66. Vitr. Cell. Dev. Biol.-Animal 2019, 55, 426–435.
- Pan, R.; He, Z.; Ruan, W.; Li, S.; Chen, H.; Chen, Z.; Liu, F.; Tian, X.; Nie, Y. lncRNA FBXL19-AS1 regulates osteosarcoma cell proliferation, migration and invasion by sponging miR-346. OncoTargets Ther. 2018, 11, 8409–8420.
- Dong, H.; Huang, C.; Huang, J. FBXL19-AS1 promotes the progression of nasopharyngeal carcinoma by acting as a competing endogenous RNA to sponge miR-431 and upregulate PBOV1. Mol. Med. Rep. 2021, 24, 647.
- Sheng, H.; Zhang, J.; Ma, Y.; Zhang, Y.; Dai, Y.; Jiang, R. lncRNA FBXL19—AS1 is a diagnosis biomarker for paediatric patients with acute myeloid leukemia. J. Gene Med. 2021, 23, e3317.




