Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Grzegorz Procyk | -- | 2036 | 2022-12-28 12:13:38 | | | |
| 2 | Vivi Li | Meta information modification | 2036 | 2022-12-29 02:07:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Grodzka, O.; Procyk, G.; Gąsecka, A. MicroRNAs in Myocarditis. Encyclopedia. Available online: https://encyclopedia.pub/entry/39497 (accessed on 16 January 2026).
Grodzka O, Procyk G, Gąsecka A. MicroRNAs in Myocarditis. Encyclopedia. Available at: https://encyclopedia.pub/entry/39497. Accessed January 16, 2026.
Grodzka, Olga, Grzegorz Procyk, Aleksandra Gąsecka. "MicroRNAs in Myocarditis" Encyclopedia, https://encyclopedia.pub/entry/39497 (accessed January 16, 2026).
Grodzka, O., Procyk, G., & Gąsecka, A. (2022, December 28). MicroRNAs in Myocarditis. In Encyclopedia. https://encyclopedia.pub/entry/39497
Grodzka, Olga, et al. "MicroRNAs in Myocarditis." Encyclopedia. Web. 28 December, 2022.
Copy Citation
Myocarditis is an inflammatory disease of the heart with a viral infection as the most common cause. It affects most commonly young adults. Although endomyocardial biopsy and cardiac magnetic resonance are used in the diagnosis, neither of them demonstrates all the required qualities. There is a clear need for a non-invasive, generally available diagnostic tool that will still remain highly specific and sensitive. These requirements could be possibly met by microribonucleic acids (miRNAs), which are small, non-coding RNA molecules that regulate many fundamental cell functions. They can be isolated from cells, tissues, or body fluids.
myocarditis
microRNA
cardiomyopathy
inflammation
clinical trials
1. Introduction
1.1. Basic Knowledge and Statistics about Myocarditis
According to the statement of the World Health Organization, myocarditis is defined as an inflammatory disease of the myocardium. It is most commonly caused by infections (primarily viral) and autoimmunization. In some cases, the etiology of the disease remains undetermined, and it is classified as an idiopathic form [1][2]. A histologic examination must be performed for the definite diagnosis of myocarditis. Viral myocarditis requires a polymerase chain reaction (PCR) detection of viruses, while autoimmune myocarditis is defined as myocarditis with a negative viral PCR test, regardless of the cardiac autoantibodies count [2][3]. Noteworthily, the disease usually affects young people, mostly aged between 20 and 50 years, not suffering from any other serious conditions [4]. These statistics are even more disturbing, taking into consideration the fact that the major long-term complication of myocarditis is dilated cardiomyopathy with chronic heart failure [5]. About 12–25% of patients die of myocarditis due to an end-stage cardiomyopathy [6]. However, in about 50% of cases, the inflammation of the heart might resolve without serious consequences, such as chronic cardiac dysfunction [7]. What is more, these different outcomes are usually seen regardless of the intensity of provided therapy. On the other hand, up to 10% of cardiomyopathies, initially unexplained, appear to be a result of myocarditis [8].
1.2. Available Diagnostic and Prognostic Tools in Myocarditis
Endomyocardial biopsy (EMB) remains the gold standard of myocarditis diagnosis, although it is an invasive procedure, and thus it is not a routine practice [9]. Concerning the experts’ consensus in the new statement, EMB is recommended for all patients with suspected myocarditis. However, many medical centers are not able to perform EMB procedures. Hence each patient with the suspicion of myocarditis should be referred to the hospital where EMB is performed [2]. The alternative method for myocarditis diagnosis is cardiac magnetic resonance (CMR), sometimes called a non-invasive gold standard [4]. The Lake Louise Criteria (LLC) are the recommended diagnostic criteria, although they may not have enough sensitivity in differentiation between recent and remote myocarditis [10][11]. New imaging techniques, such as T1 and T2 mapping, seem to be promising in terms of increasing the value of CMR [12]. Nevertheless, some limitations preclude implementing this method into the clinical routine, amongst them: the unavailability of advanced techniques, lack of validation in myocarditis, and some inconsistency with EMB findings [3][6].
The intensity of the treatment is not always positively correlated with the outcome of myocarditis [8]. The prognosis may be established based on EMB, which shows the underlying etiology and inflammation type [13]. Interestingly, fulminant myocarditis usually resolves with a better outcome than the chronic form of this disease [14]. Nonetheless, EMB remains an invasive procedure and is not always performed. Thus prognosis, in the case of many patients, stays unclear.
All the above implies the need for an efficient, easily accessible, and low-priced non-invasive diagnostic tool. Recently, much research has been focusing on investigating small particles, such as extracellular vesicles (EVs) or microribonucleic acids (miRNAs/miRs), circulating in the blood or found in tissues [15]. In terms of myocarditis diagnosis, miRNAs could meet the abovementioned expectations [16] (Figure 1).
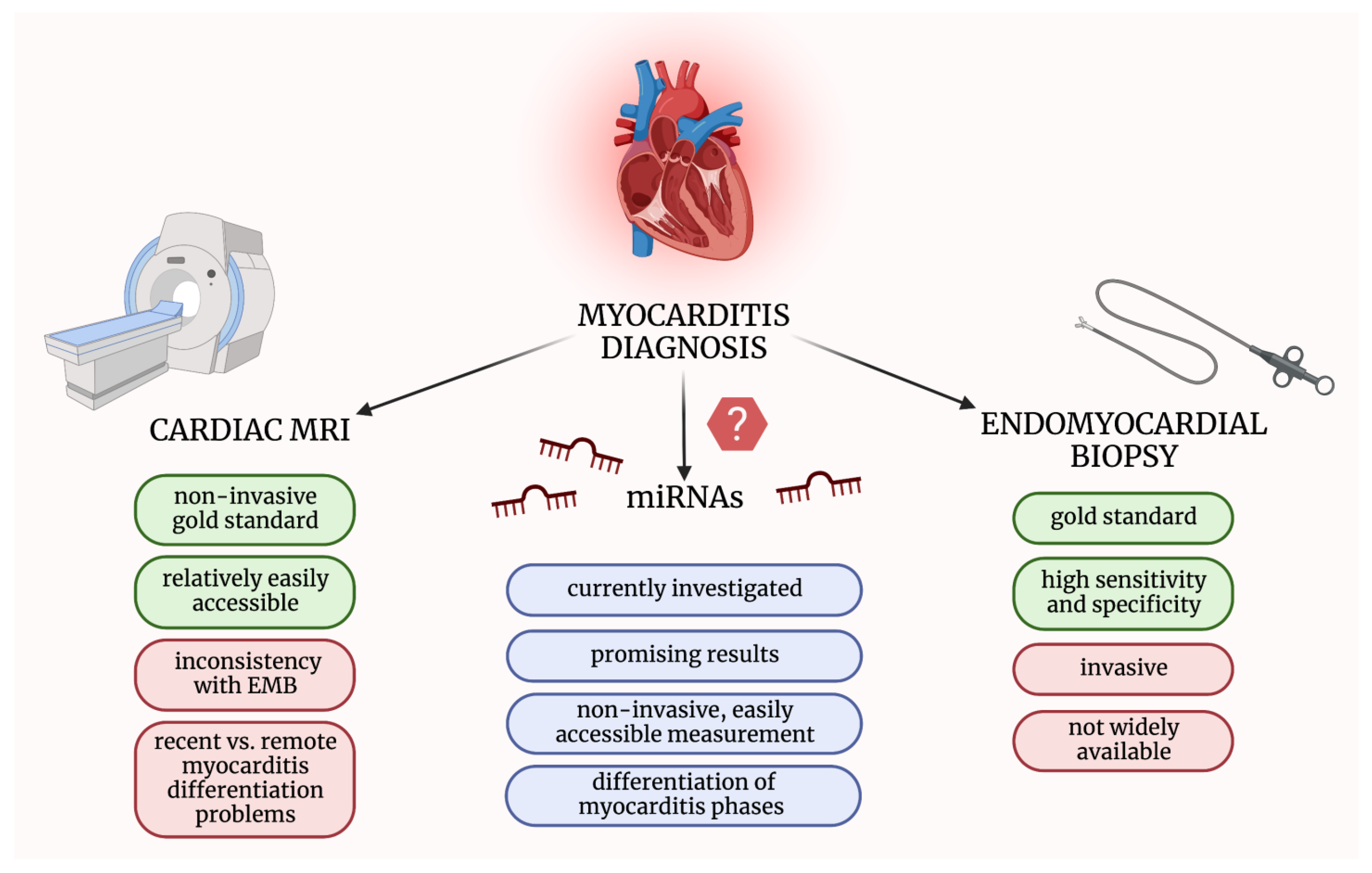
Figure 1. Comparison of methods used in myocarditis diagnosis and microRNAs’ advantages as potential diagnostic biomarkers. EMB—endomyocardial biopsy; miRNAs—micro-ribonucleic acids; MRI—magnetic resonance imaging. The figure was created with BioRender.com, licensed version by A.G.
1.3. Biosynthesis and Function of miRNAs
MiRNAs are a class of small ribonucleic acids (RNAs) containing from 19 to 25 nucleotides [17]. Although they are non-coding molecules, they play a crucial role in regulating gene expression [18]. A single miRNA molecule can target hundreds of different mRNAs associated with fundamental cell functions [19]. Approximately one-third of the regulation of the protein-coding genes is related to the miRNAs [20][21].
The initial step of the miRNA synthesis is the miRNA gene transcription by RNA polymerase II, forming a large primary miRNA (pri-miRNA) [22]. This molecule is composed of one or a few stem-loop structures and is subsequently cleaved by the Drosha enzyme into a single stem-loop structure called precursor miRNA (pre-miRNA) containing about 70 nucleotides [23]. Afterward, pre-miRNA is transported from the nucleus to the cytoplasm, where it is cleaved by a nuclease Dicer into a double-stranded miRNA consisting of approximately 22 nucleotides [24]. This miRNA is loaded into an argonaut protein to compose the RNA-induced silencing complex (RISC) [25]. The expression of the target mRNA is regulated by binding its 3′ end to the complementary 5′ end of the mature miRNA. Depending on the complementarity degree, mRNA can be repressed or degraded [21][24]. Furthermore, there are other mechanisms, independent of Drosha and Dicer, able to generate miRNAs. In addition, noncanonical binding sites have been identified [24][26].
It has been estimated that about 18% of miRNA-mRNA complexes are created with the 3′ end of miRNA instead of the 5′ end [27]. The information about involved miRNA’s ends was pointed out only by some of the authors of the analyzed literature, and therefore researchers have featured it only in some of the referred studies.
1.4. The Putative Role of miRNAs in Myocarditis
MiRNAs have already been proven to play a crucial role in the pathogenesis of different cardiovascular diseases, such as myocardial infarction, arrhythmias, heart failure, or coronary heart disease [28]. They have also been evidenced to be involved in the development of neoplastic diseases [29]. Therefore, current studies are investigating these small molecules with great interest [30][31][32][33][34]. MiRNAs can be isolated not only from cells and tissues but also from body fluids, such as cerebrospinal fluid or serum. Quantitative PCR (qPCR) is a method widely used to measure the level of miRNAs [35]. However, it can also be utilized to determine potential targets for studied miRNAs, which can be very useful in resolving different pathogenic pathways [17]. Several miRNA profiling studies have demonstrated that the blood and tissue levels of different miRNAs are significantly deregulated in myocarditis [36][37]. They might be used to differentiate from other cardiac disorders, to distinguish different stages of myocarditis, or to prognose the degree of cardiac damage and possible outcome.
2. MicroRNAs in Patients Suffering from Myocarditis
So far, few clinical studies investigating the role of microRNAs in myocarditis have been conducted. Researchers searched PubMed Database and eventually included 21 original clinical research relevant to the discussed field. Researchers have divided these studies into the following parts: (i) microRNAs in pediatric myocarditis patients, (ii) tissue microRNAs in adult myocarditis patients, and (iii) circulating microRNAs in adult myocarditis patients (Figure 2).
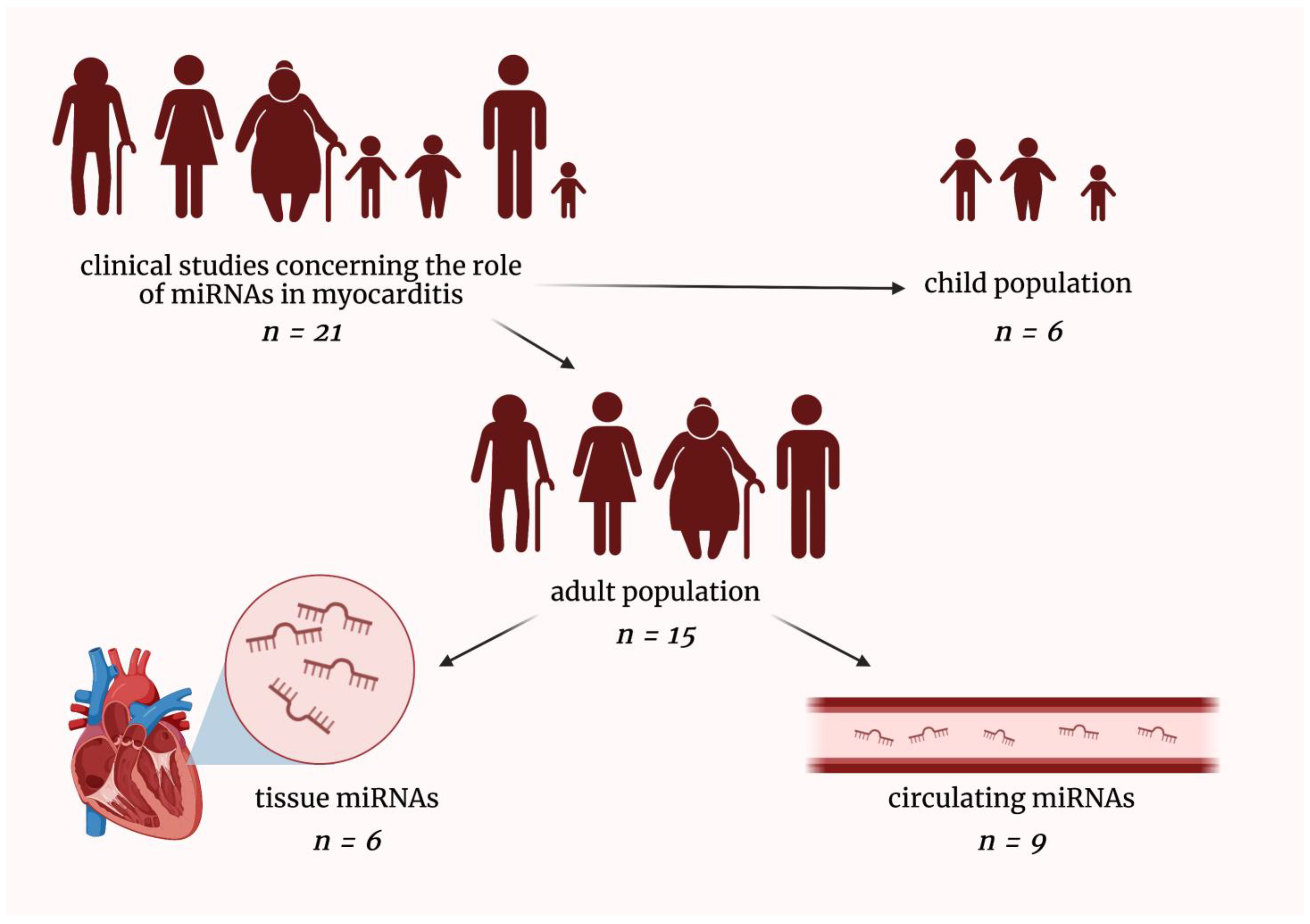
Figure 2. Graphical presentation of dividing clinical studies included in this entry. miRNAs—micro-ribonucleic acids; n—a number of included clinical studies in a given field. The figure was created with BioRender.com, licensed version by A.G.
2.1. MicroRNAs in Pediatric Myocarditis Patients
Yan et al. studied a group of children suffering from viral myocarditis and compared them to healthy controls. The level of miR-146b was significantly higher in the first group as compared to the latter. The increase was positively correlated with the level of myocardial injury [38]. Another research group demonstrated that miR-217 and miR-543 levels were also significantly higher in children suffering from viral myocarditis compared to healthy controls. The results of this research suggest that the measurement of miRNAs level might be a novel diagnostic tool for myocarditis which is usually underdiagnosed in the pediatric population [39].
Goldberg et al. compared the levels of different miRNAs in children suffering from viral myocarditis in different phases of this disease. Two miRNAs demonstrated significant changes. The level of miR-208a was elevated in the acute phase, and it was subsequently diminishing. The decrease in the miR-21 level was observed during the resolution or chronic phase, while in the acute and subacute phases, it was increased. Additionally, another miR-208b expression level during the subacute phase was negatively correlated with systolic left ventricle function during the chronic phase, which suggests a prognostic value of the miR-208a measurements. Both miRNAs can be considered diagnostic biomarkers for myocarditis [40].
Zhang et al. conducted a study investigating children suffering from viral myocarditis. The level of miR-381 was measured in these patients, and it appeared to be decreased compared to its level in children that had recovered from myocarditis. Moreover, it was negatively correlated with the levels of cyclooxygenase-2, which is a substantial enzyme for inflammatory cytokines production [41]. Moreover, there was another study that included children suffering from viral myocarditis and compared them to healthy individuals. It was observed that the level of miR-133b was lower in the group of children with myocarditis. The severity of myocardial lesions was also assessed, and a negative correlation between miR-133b expression and heart damage in viral myocarditis was observed [42].
2.2. Tissue MicroRNAs in Adult Myocarditis Patients
Besler et al. investigated patients suffering from viral myocarditis. The elevated levels of miR-133a and miR-155 were observed in these patients as compared to patients with dilated cardiomyopathy. Noteworthily, miR-133a and miR-155 levels were positively correlated with the inflammatory cell count in patients with viral myocarditis. Moreover, an increased level of miR-133a was associated with reduced fibrosis and myocytes’ necrosis, as well as the improved function of the left ventricle. Patients presenting with higher levels of miR-133a had increased overall survival and decreased occurrence of malignant arrhythmias. Moreover, they were less often hospitalized due to heart failure [43]. Slightly opposite results concerning miR-133a were obtained by Ferreira et al. They studied myocardial samples derived from patients suffering from Chagas disease (manifesting mainly with myocarditis). They discovered that miR-1, miR-133a, miR-133b, miR-208a, and miR-208b were downregulated in samples from patients with Chagas disease as compared to samples from healthy hearts of organ donors. Moreover, three of them: miR-1, miR-133a, and miR-208b, were significantly less expressed in the study group as compared not only to organ donors but also to patients with DCM. Some targets for these miRNAs were identified, and they were found to be responsible for cardiac hypertrophy and dysfunction or normal proliferation of cardiomyocytes [44].
Bao et al. investigated patients suffering from acute myocarditis and compared them to healthy controls. Eight different miRNAs were assessed, and it was found that miR-155 and miR-148a presented higher levels in heart tissue derived from patients with myocarditis [45]. Another research group investigated patients suffering from viral myocarditis, and they observed a downregulation of miR-214 and miR-146b in patients with myocarditis as compared to healthy controls [46].
2.3. Circulating MicroRNAs in Adult Myocarditis Patients
Blanco-Domínguez et al. investigated patients suffering from acute myocarditis, comparing them to patients with myocardial infarction (MI) and healthy controls. The first group of patients was shown to present increased levels of hsa-miR-Chr8:96 as compared to other groups. Moreover, the results remained significant after adjusting factors such as age, sex, ejection fraction, and troponin concentration in serum [47]. A similar study was performed by Nie et al., who investigated patients affected by fulminant myocarditis and compared them to patients suffering from MI and healthy controls. The level of miR-4281 was elevated in the group of patients with myocarditis and MI as compared to healthy controls, while the level of miR-4763-3p was significantly higher only in myocarditis patients. Interestingly the extent of demonstrated increase in miRNA levels was negatively correlated with the severity of fulminant myocarditis. Therefore, miR-4763-3p appears as a promising biomarker to differentiate myocarditis from MI [36].
In another study, Aleshcheva et al. included three groups: (i) myocarditis patients, (ii) dilated cardiomyopathy (DCM) patients, and (iii) healthy donors. After evaluation of different miRNA levels in serum within these groups, it was shown that Let-7f, miR-93, miR-197, miR-223, and miR-379 were significantly deregulated in patients suffering from myocarditis. The expression of miR-93, miR-197, and miR-379 was upregulated compared to both remaining groups, while let-7f and miR-223 levels were downregulated compared to healthy controls. The specificity of myocarditis identification with the use of the aforementioned biomarkers in a single serum sample was within the range of 93–95% [48].
References
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842.
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648.
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193.
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute myocarditis: Aetiology, diagnosis and management. Clin. Med. 2021, 21, e505–e510.
- Cooper, L.T., Jr. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538.
- Tymińska, A.; Ozierański, K.; Skwarek, A.; Kapłon-Cieślicka, A.; Baritussio, A.; Grabowski, M.; Marcolongo, R.; Caforio, A.L. Personalized Management of Myocarditis and Inflammatory Cardiomyopathy in Clinical Practice. J. Pers. Med. 2022, 12, 183.
- Schultheiss, H.P.; Kühl, U.; Cooper, L.T. The management of myocarditis. Eur. Heart J. 2011, 32, 2616–2625.
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327.
- Pilati, M.; Rebonato, M.; Formigari, R.; Butera, G. Endomyocardial Biopsy in Pediatric Myocarditis and Dilated Cardiomyopathy: A Tool in Search for a Role. J. Cardiovasc. Dev. Dis. 2022, 9, 24.
- Puntmann, V.O.; Zeiher, A.M.; Nagel, E. T1 and T2 mapping in myocarditis: Seeing beyond the horizon of Lake Louise criteria and histopathology. Expert Rev. Cardiovasc. Ther. 2018, 16, 319–330.
- Gannon, M.P.; Schaub, E.; Grines, C.L.; Saba, S.G. State of the art: Evaluation and prognostication of myocarditis using cardiac MRI. J. Magn. Reson. Imaging 2019, 49, e122–e131.
- Liguori, C.; Farina, D.; Vaccher, F.; Ferrandino, G.; Bellini, D.; Carbone, I. Myocarditis: Imaging up to date. Radiol. Med. 2020, 125, 1124–1134.
- Ammirati, E.; Veronese, G.; Bottiroli, M.; Wang, D.W.; Cipriani, M.; Garascia, A.; Pedrotti, P.; Adler, E.D.; Frigerio, M. Update on acute myocarditis. Trends Cardiovasc. Med. 2021, 31, 370–379.
- McCarthy, R.E., 3rd; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Hare, J.M.; Baughman, K.L. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N. Engl. J. Med. 2000, 342, 690–695.
- Procyk, G.; Bilicki, D.; Balsam, P.; Lodziński, P.; Grabowski, M.; Gąsecka, A. Extracellular Vesicles in Atrial Fibrillation-State of the Art. Int. J. Mol. Sci. 2022, 23, 7591.
- Gartshteyn, Y.; Tamargo, M.; Fleischer, S.; Kapoor, T.; Li, J.; Askanase, A.; Winchester, R.; Geraldino-Pardilla, L. Endomyocardial biopsies in the diagnosis of myocardial involvement in systemic lupus erythematosus. Lupus 2020, 29, 199–204.
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207.
- Lu, J.; Clark, A.G. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012, 22, 1243–1254.
- Hoefig, K.P.; Heissmeyer, V. MicroRNAs grow up in the immune system. Curr. Opin. Immunol. 2008, 20, 281–287.
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584.
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465.
- Creugny, A.; Fender, A.; Pfeffer, S. Regulation of primary microRNA processing. FEBS Lett. 2018, 592, 1980–1996.
- Michlewski, G.; Cáceres, J.F. Post-transcriptional control of miRNA biogenesis. RNA 2019, 25, 1–16.
- Bernardo, B.C.; Ooi, J.Y.; Lin, R.C.; McMullen, J.R. miRNA therapeutics: A new class of drugs with potential therapeutic applications in the heart. Future Med. Chem. 2015, 7, 1771–1792.
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43.
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741.
- Helwak, A.; Kudla, G.; Dudnakova, T.; Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 2013, 153, 654–665.
- Colpaert, R.M.W.; Calore, M. Epigenetics and microRNAs in cardiovascular diseases. Genomics 2021, 113, 540–551.
- Inoue, J.; Inazawa, J. Cancer-associated miRNAs and their therapeutic potential. J. Hum. Genet. 2021, 66, 937–945.
- Halushka, P.V.; Goodwin, A.J.; Halushka, M.K. Opportunities for microRNAs in the Crowded Field of Cardiovascular Biomarkers. Annu. Rev. Pathol. 2019, 14, 211–238.
- Oh, J.H.; Kim, G.B.; Seok, H. Implication of microRNA as a potential biomarker of myocarditis. Clin. Exp. Pediatr. 2022, 65, 230–238.
- Procyk, G.; Klimczak-Tomaniak, D.; Sygitowicz, G.; Tomaniak, M. Circulating and Platelet MicroRNAs in Cardiovascular Risk Assessment and Antiplatelet Therapy Monitoring. J. Clin. Med. 2022, 11, 1763.
- Neri, M.; Fabbri, M.; D’Errico, S.; Di Paolo, M.; Frati, P.; Gaudio, R.M.; La Russa, R.; Maiese, A.; Marti, M.; Pinchi, E.; et al. Regulation of miRNAs as new tool for cutaneous vitality lesions demonstration in ligature marks in deaths by hanging. Sci. Rep. 2019, 9, 20011.
- Rocchi, A.; Chiti, E.; Maiese, A.; Turillazzi, E.; Spinetti, I. MicroRNAs: An Update of Applications in Forensic Science. Diagnostics 2020, 11, 32.
- Chen, C.; Tan, R.; Wong, L.; Fekete, R.; Halsey, J. Quantitation of microRNAs by real-time RT-qPCR. Methods Mol. Biol. 2011, 687, 113–134.
- Nie, X.; He, M.; Wang, J.; Chen, P.; Wang, F.; Lai, J.; Li, C.; Yu, T.; Zuo, H.; Cui, G.; et al. Circulating miR-4763-3p Is a Novel Potential Biomarker Candidate for Human Adult Fulminant Myocarditis. Mol. Ther. Methods Clin. Dev. 2020, 17, 1079–1087.
- Corsten, M.F.; Papageorgiou, A.; Verhesen, W.; Carai, P.; Lindow, M.; Obad, S.; Summer, G.; Coort, S.L.; Hazebroek, M.; van Leeuwen, R.; et al. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ. Res. 2012, 111, 415–425.
- Yan, M.; Wang, J.; Wang, S.; Zhang, Y.; Liu, L.; Zhao, H. Expression Levels of MicroRNA-146b and Anti-Cardiac Troponin I in Serum of Children with Viral Myocarditis and Their Clinical Significance. Iran. J. Public Health 2021, 50, 510–519.
- Xia, K.; Zhang, Y.; Sun, D. miR-217 and miR-543 downregulation mitigates inflammatory response and myocardial injury in children with viral myocarditis by regulating the SIRT1/AMPK/NF-κB signaling pathway. Int. J. Mol. Med. 2020, 45, 634–646.
- Goldberg, L.; Tirosh-Wagner, T.; Vardi, A.; Abbas, H.; Pillar, N.; Shomron, N.; Nevo-Caspi, Y.; Paret, G. Circulating MicroRNAs: A Potential Biomarker for Cardiac Damage, Inflammatory Response, and Left Ventricular Function Recovery in Pediatric Viral Myocarditis. J. Cardiovasc. Transl. Res. 2018, 11, 319–328.
- Zhang, Y.; Sun, L.; Sun, H.; Yu, Z.; Liu, X.; Luo, X.; Li, C.; Sun, D.; Li, T. MicroRNA-381 protects myocardial cell function in children and mice with viral myocarditis via targeting cyclooxygenase-2 expression. Exp. Ther. Med. 2018, 15, 5510–5516.
- Zhang, Y.; Sun, L.; Sun, H.; Liu, X.; Luo, X.; Li, C.; Sun, D.; Li, T. Overexpression of microRNA-133b reduces myocardial injuries in children with viral myocarditis by targeting Rab27B gene. Cell. Mol. Biol. 2017, 63, 80–86.
- Besler, C.; Urban, D.; Watzka, S.; Lang, D.; Rommel, K.P.; Kandolf, R.; Klingel, K.; Thiele, H.; Linke, A.; Schuler, G.; et al. Endomyocardial miR-133a levels correlate with myocardial inflammation, improved left ventricular function, and clinical outcome in patients with inflammatory cardiomyopathy. Eur. J. Heart Fail. 2016, 18, 1442–1451.
- Ferreira, L.R.; Frade, A.F.; Santos, R.H.; Teixeira, P.C.; Baron, M.A.; Navarro, I.C.; Benvenuti, L.A.; Fiorelli, A.I.; Bocchi, E.A.; Stolf, N.A.; et al. MicroRNAs miR-1, miR-133a, miR-133b, miR-208a and miR-208b are dysregulated in Chronic Chagas disease Cardiomyopathy. Int. J. Cardiol. 2014, 175, 409–417.
- Bao, J.L.; Lin, L. MiR-155 and miR-148a reduce cardiac injury by inhibiting NF-κB pathway during acute viral myocarditis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2349–2356.
- Chen, Z.G.; Liu, H.; Zhang, J.B.; Zhang, S.L.; Zhao, L.H.; Liang, W.Q. Upregulated microRNA-214 enhances cardiac injury by targeting ITCH during coxsackievirus infection. Mol. Med. Rep. 2015, 12, 1258–1264.
- Blanco-Domínguez, R.; Sánchez-Díaz, R.; de la Fuente, H.; Jiménez-Borreguero, L.J.; Matesanz-Marín, A.; Relaño, M.; Jiménez-Alejandre, R.; Linillos-Pradillo, B.; Tsilingiri, K.; Martín-Mariscal, M.L.; et al. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. N. Engl. J. Med. 2021, 384, 2014–2027.
- Aleshcheva, G.; Pietsch, H.; Escher, F.; Schultheiss, H.P. MicroRNA profiling as a novel diagnostic tool for identification of patients with inflammatory and/or virally induced cardiomyopathies. ESC Heart Fail. 2021, 8, 408–422.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
29 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




