
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muthaiah Shellaiah | -- | 17557 | 2022-12-27 09:07:52 | | | |
| 2 | Muthaiah Shellaiah | -4839 word(s) | 12718 | 2022-12-28 05:04:56 | | | | |
| 3 | Vivi Li | -3851 word(s) | 8867 | 2022-12-29 07:43:20 | | |
Video Upload Options
Gold- and silver nanoparticles (Au NPs and Ag NPs)-based colorimetric detection of specific analytes has attracted intense research interest and is still in great demand. The majority of Au NPs- and Ag NPs-based sensory reports have revealed that, during the analyte recognition, dispersed NPs typically aggregated and displayed color changes from wine-red to blue/purple and yellow to orange/brown, respectively. On the other hand, only a few reports demonstrated that the aggregated Au NPs and Ag NPs underwent anti-aggregation in the presence of certain analytes, which displayed reversed color changes from blue/purple to wine-red and orange/brown to yellow, correspondingly. There are some examples of anti-aggregation phenomena mentioned in a vast number of studies on Au NPs- and Ag NPs-based colorimetric sensors via NP aggregation.
1. Introduction
In this entry, utilization of the anti-aggregation strategy in the Au NPs/Ag NPs-based colorimetric detection of metal ions, anions, bio-analytes, pesticides, and herbicides is illustrated with mechanistic and binding details. Uniqueness of its design, linear range, limit of detection (LOD), and real-time applicability are discussed with future scopes. The schematic shown in Figure 1 illustrates the anti-aggregation strategy applied in Au NPs/Ag NPs-based colorimetric sensors toward metal ions, anions, bio-analytes, pesticides, and herbicides.
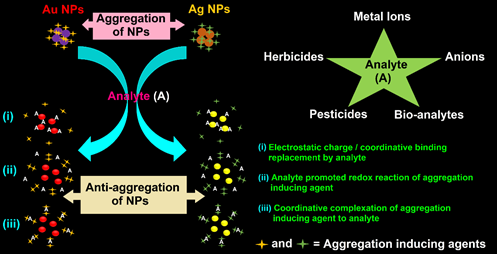
Figure 1. Schematic of the anti-aggregation strategy applied in Au NPs/Ag NPs-based colorimetric sensors toward metal ions, anions, bio-analytes, pesticides, and herbicides.
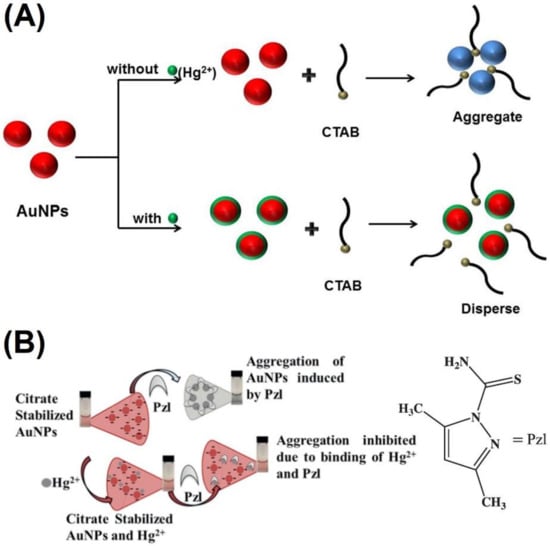
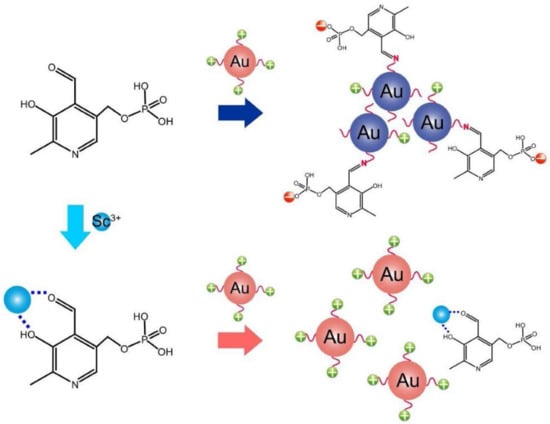
2. Au NPs-Based Colorimetric Assay of Metal Ions via Anti-Aggregation
3. Au NPs-Based Colorimetric Quantification of Anions via Anti-Aggregation
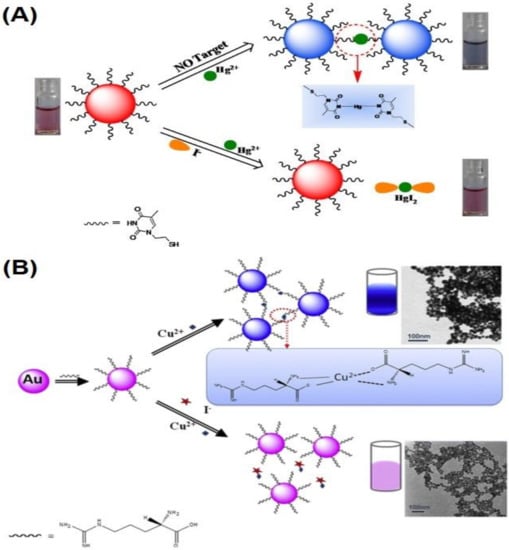
4. Ag NPs-Based Colorimetric Assay of Diverse Analytes via Anti-Aggregation
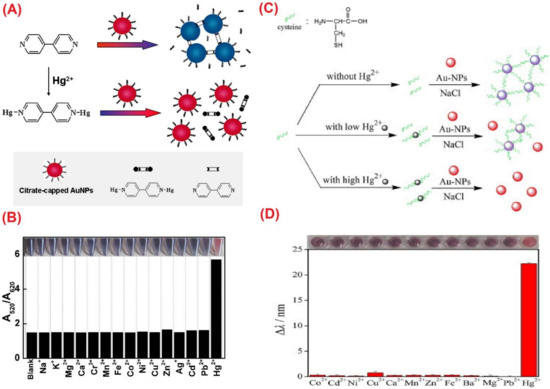
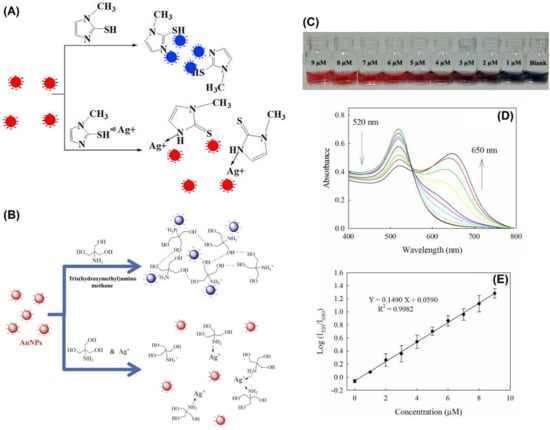
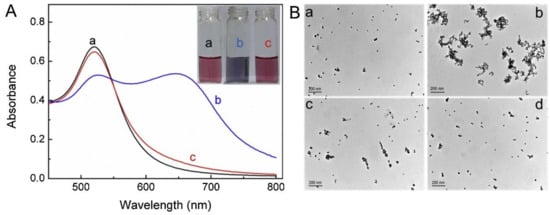
Silver nanoparticles (Ag NPs) were also reported toward distinct analyte quantification via the anti-aggregation strategy similar to Au NPs, as discussed in this section. Duan et al. described the use of citrate-capped Ag NPs (CI-Ag NPs) toward the detection of Hg2+ via anti-aggregation in the presence of the aggregation-inducing agent 6-thioguanine (0.83 µM) [75], in which 6-thioguanine induced aggregation of Ag NPs to produce a color change from yellow to brown. However, the brown color was reversed to yellow via anti-aggregation by pre-mixing Hg2+ (at different concentrations). The underlying mechanism was that 6-thioguanine induced aggregation due to its strong electrostatic interaction toward CI-Ag NPs. However, the aggregation was inhibited by the presence of Hg2+ (via strong binding of 6-thioguanine with Hg2+) and resulted in the anti-aggregation of CI-Ag NPs. The best colorimetric response was achieved at pH 4 (100 mM HAc-NaAc) after 30 min incubation. The linear range of Hg2+ was between 0 and 333 nM (at A530/A394), with an LOD of 4 nM. SPR, TEM, interference, and spring water studies (recoveries of between 103 and 105%) validated this work as a major innovation. Figure 12A–C display the colorimetric response, SPR variations, and linearity of Hg2+ induced anti-aggregation of 6-thioguanine-CI-Ag NPs system, respectively.
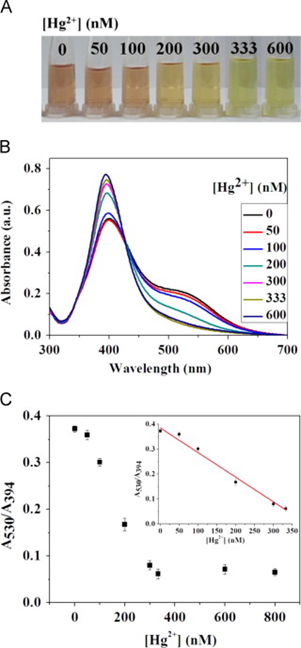
Figure 12. (A) The photographic images of the 6-thioguanine–Ag NPs solutions in the presence of different concentrations of Hg2+ (0–600 nM). (B) The corresponding UV–vis spectra of 6-thioguanine–Ag NPs. (C) The absorbance ratio (A530/A394) of 6-thioguanine–Ag NPs versus Hg2+ concentration. Inset to C: plot of A530/A394 versus the Hg2+ concentration. The concentration of 6-thioguanine was 0.83 μM ((A–C) are reproduced with the permission from [75]).
Anti-aggregation-enabled colorimetric assay of Cu2+ was demonstrated by employing CI-Ag NPs and engaging 1,4-dithiothreitol (DTT; 10 µM) as the aggregation-inducing agent [76], wherein DTT induced the aggregation of Ag NPs via a strong Ag-S bonding and displayed a color change from yellow to deep green. Upon pre-mixing Cu2+ in 2,6-pyridinedicarboxylic acid (PDCA, 5 mM), the DTT oxidized to form an intramolecular-S-S-bond, which inhibited aggregation and produced a reversed color change from deep green to yellow. The best result was attained at pH 2–10 (buffer: HCl-NaOH) after 20 min incubation. The linearity of Cu2+ was between 0.1 and 2 µM (at A408), with an LOD of 0.1 µM. SPR, TEM, interference, and real samples (tap water and ground water; recoveries of ≥95%, with an RSD of <8%) interrogations validated this method. Hence, it can be regarded as a remarkable research, but details on particle size variations are still missing.
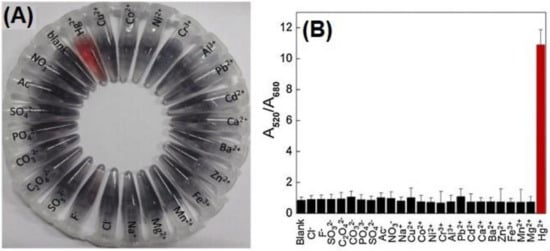
He and co-workers reported the anti-aggregation-enabled colorimetric detection of Mn2+ by employing CI-Ag NPs (conc. = 0.28 nM) and L-Arginine (L-Arg; 0.7 mM) as the aggregation-inducing agent [77]. The Ag-N covalent interaction between Ag NPs and L-Arg caused aggregation, with the color of the solution changing from yellow to colorless. The color of Ag NPs changed back from colorless to yellow via anti-aggregation when the above solution was pre-treated with different concentrations of Mn2+. In fact, the anti-aggregation was attributed to the stronger attraction of L-Arg toward Mn2+ than that of the Ag NPs. The best result was obtained at pH 9.4 (buffer; BR) after 40 min incubation. Two linear ranges for Mn2+ were established between 0 and 700 nM and 5 and 70 µM (both at A390), with an LOD of 20 nM. As illustrated in Figure 13A,B, none of the competing species shows interference to Mn2+ selectivity. By this method, real water (lake, tap, and river) recoveries were >90%, with an RSD < 3%. This work is regarded as an exceptional innovation, but it still lacks particle size variations details.
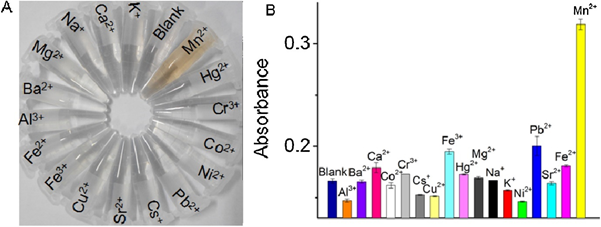
Figure 13. (A) The photographs and (B) the corresponding absorbance of the Ag NPs-l-arginine dispersions in the presence of different metal ions. The concentrations of both Mn2+ ions and other metal ions were 10 µM ((A,B) are reproduced with the permission from [77]).
Basiri et al. developed the Ag NPs–decorated graphene nanocomposites (rGO@Ag NPs; particle size = 3.7 ± 0.8 nm) for anti-aggregation-enabled colorimetric detection of Cu2+ using dopamine (DA; 25 µM) as the aggregation-inducing agent. The Ag NPs and 5 mg/mL of rGO was boiled at pH 10 and at 100 °C for 10 min to afford rGO@Ag NPs. The rGO@Ag NPs were well characterized by SPR, TEM, FTIR, XPS, and XRD studies (XRD = X-ray diffraction). Due to the H-bonding and π–π interactions between DA and rGO@Ag NPs, aggregation occurred (witnessed by TEM) with a color change from yellow to brown. In the presence of Cu2+, the interaction of DA to rGO@Ag NPs was hindered due to selective chelation of Cu2+ with DA and resulted in anti-aggregation (seen by TEM) with a color change from brown to yellow. Note that none of the competing species induces anti-aggregation, as shown in Figure 14. The best colorimetric response was observed at pH 7 (buffer: 10 mM PBS) after 15 min incubation. The linearity of Cu2+ ranged between 0.02 and 1.5 µM (at A405/A515), with an LOD of 9.8 nM. Moreover, real samples (human urine for DA and tomato for Cu2+) investigations demonstrated recoveries of >98%, with an RSD of <3%; thus, it can be noted as an excellent method.
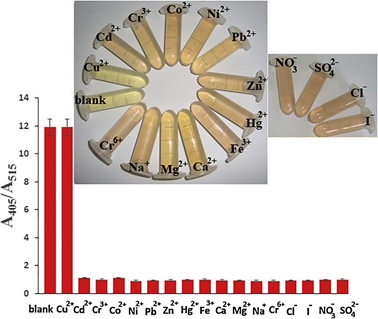
Figure 14. Colorimetric signals in the presence of different metal ions and anions (1.5 µM) as the interferences of Cu2+. Inset: the photographic images of the corresponding solutions (reproduced with the permission from [78]).
An anti-aggregation-enabled colorimetric assay of Br− and I− was demonstrated using CI-Ag NPs (particle size = 10–15 nm; conc. = 69.4 µM) and Cr3+ (125 µM) as the aggregation-inducing agent [79]. In the presence of Cr3+, the Ag NPs aggregated to produce a color change from yellow to brown. Due to the complexation of Cr3+ with Br− and I−, anti-aggregation occurred to reverse the color from brown to yellow. The linearity of Br– and I– was between 0.99 and 5.66 µM (at A500) and 0.99 and 4.16 µM (at A500), with LODs of 1.67 µM and 1.32 µM, respectively. Though this report was validated by SPR, DLS, and interference studies, data in TEM, pH, incubation time, and real-time investigations were insufficient; therefore, it can be only regarded as a preliminary work. To this track, the sulfanilic acid (SA) and catechol (CAT)–functionalized Ag NPs aggregate (SA-CAT-Ag NPs) was utilized for anti-aggregation-enabled colorimetric detection of F– [80]. The CA was firstly oxidized by silver ions followed by SA nucleophilic attack to deliver aggregated SA-CAT-Ag NPs with red color. By adding F– to the above mixture, it adsorbed on the Ag surface and stripped the SA-CAT stabilizer from -SO3− moiety, hence the aggregation was disturbed with a color change from red to yellow. The best color response was attained at pH 4–9 after 1 min incubation. The linear range of F– was between 1 and 40 µM (at A397/A508), with an LOD of 0.2 µM. This work was authenticated by SPR, TEM, interference (negligible), and spring water investigations. Thus, it can be noted as a nice approach for F– quantification.
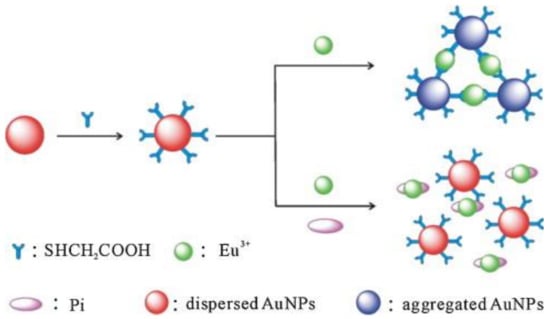
Dong et al. demonstrated using poly-vinyl pyridine (PVP)–modified Ag NPs (PVP-Ag NPs; particle size = 6.24 nm) the colorimetric assay pyrophosphate ((P2O74−; PPi) via the anti-aggregation strategy [81]. Herein, the Pb2+ (5 µM) was employed as the aggregation-inducing agent to induce the PVP-Ag NPs (due to its crosslink effect with PVP) aggregation and to produce a color change from yellow to blue. Anti-aggregation was induced by pre-mixing PPi, which resulted in a colorimetric response from blue to yellow due to the chelating interaction between PPi and Pb2+. The best result was obtained at pH 5–9 at 20 °C after 30 min incubation. The linear ranges of PPi were between 0.2 and 2.0 μM and 2.0 and 10.0 μM (at A396), with an LOD of 0.2 µM. This work was authorized by smartphone device, SPR, TEM, statistical, theoretical, interference, real samples (tap water (recoveries between 79.6 and 107.6%) and canned meat (66.7–118.4%)) interrogations. Therefore, it can be regarded as an exceptional invention in Ag NPs-based colorimetric assay. Following a similar mechanistic approach, the 2-Mercapto-ethane sulfonate–modified silver nanoplates (MS-Ag NPls) were employed in the colorimetric detection of PO43− (Pi) on PADs in the presence of the aggregation-inducing agent Eu3+ (65.81 µM) [82]. In the presence of Eu3+, the color of MS-Ag NPls changed from pink to purple, which was reversed (from purple to pink) during the quantification of Pi. All the sensory experiments were conducted at pH 7 (buffer: 25 mM Tris) at an optimum incubation time of 3 min. The linear regression of Pi was between 10.42 and 313 µM (at A520), with an LOD of 3.44 µM. This work was also authenticated by water and soil investigations. Moreover, this is the only report available on the Ag NPls-based colorimetric assay. Therefore, it is regarded as a remarkable method.
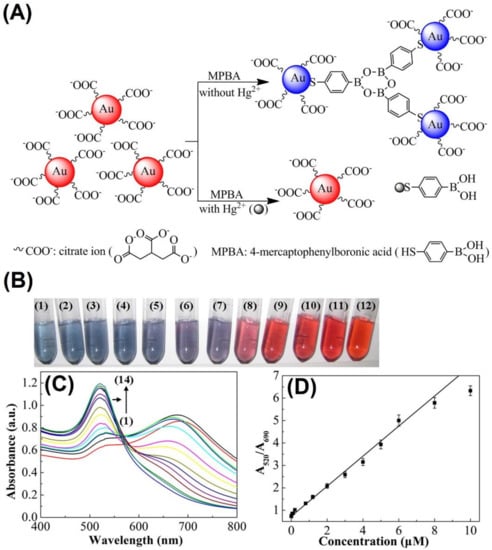
The surface-enhanced Raman scattering (SERS) technique was employed with 4-mercaptopyridine (4-MPY)-modified Ag NPs (4-MPY-Ag NPs) for anti-aggregation-enabled detection of trypsin (an enzyme that aids with digestion) in the presence of the aggregation-inducing agent protamine (0.3 µg/mL (10 µL)) [83]. The presence of +vely charged protamine over 4-MPY-Ag NPs induced aggregation via strong electrostatic interaction with a color change from yellow to deep green. However, trypsin enhanced the enzymatic fragmentation of protamine, which resulted in anti-aggregation with a reversed color change from deep green to yellow. The SERS linear range was between 0.14 nM and 13.8 μM (at 1096 cm−1), with an LOD of 0.14 nM. All the SERS experiments were conducted at pH 7.4 (buffer: HEPES) within 1 min. Based on SERS, TEM, and interference studies, this work can be regarded as a unique one. An anti-aggregation-enabled colorimetric assay of poly diallyl-dimemethyl-ammonium chloride (PDADMAC; a coagulant in water and wastewater treatment) was proposed by employing CI-Ag NPs (particle size = 6 ± 2 nm) and 0.2 M PBS buffer (pH 7.4) as the aggregation-inducing agent [84]. The phosphate buffer induced aggregation of Ag NPs, which resulted in a color change from yellow to colorless. However, in the presence of PDADMAC, the Ag NPs were stabilized by electrostatic attraction and steric hindrance, which resulted in being dispersed NPs with a reversed color change (from colorless to yellow). Note that the colorimetric responses occurred within 3 min. The linear range of PDADMAC was between 1 and 100 mg. L−1 (at A396), with an LOD of 0.7 mg. L−1. This tactic was well authorized by SPR, TEM, interference, and tap water interrogations. Thus, it can be noted as a nice innovation. However, further optimization is mandatory toward the LOD reduction.
Though anti-aggregation-enabled Ag NPs-based colorimetric assays seem to be effective in analytes quantification, available reports are much less sufficient than that of aggregated Ag NPs-based colorimetric sensors [85][86][87][88][89][90]. Therefore, further research is required as suggested in perspectives.
5. Design/Sensory Requirements, Advantages, and Limitations
5.1. Probe Design and Sensory Requirements
The development of exceptional Au NPs- and Ag NPs-based colorimetric probes and their anti-aggregation-enabled sensory performances must follow the requirements as stated below:
- The selected label-free/modified Au NPs- or Ag NPs-based probes must possess dispersion stability in color and firm electrostatic charges to be able to interact with aggregation-inducing agents and to afford a certain degree of aggregation and a distinct colorimetric response.
- Selection of a suitable aggregation-inducing agent is essential to attain the primary colorimetric changes. An aggregation-inducing agent should have any of the following three characteristics: (i) analyte replaceable electrostatic interactions, (ii) analyte disposable interactive binding (such as Au-S, Ag-S, Au-N, Ag-N, etc.) over the NPs’ surface, and (iii) analyte coordinative units (such as thymine, -SH, -NH2, -COOH, -OCH3, and -OC2H5, etc.) that are able to form coordination complexes or undergo catalytic reactions to be released from the NPs’ surface. Moreover, it is essential to fix the concentration of the aggregation-inducing agent before taking further sensory interrogations.
- Optimizations of the effective pH conditions, suitable pH buffer solution, concentration, and maintenance circumstances are necessary for anti-aggregation-based colorimetric analyte detection.
- It is essential to fix the exact incubation time to achieve stable colorimetric response and to help to clarify the underlying mechanism and kinetics of the anti-aggregation.
- Though most of the anti-aggregation-enabled colorimetric sensory are conducted at room temperature, it is also important to fix the operative temperature toward real-time applications.
- To avoid complications in real water investigations, it is necessary to identify the interfering effect, allowed concentration, required masking agent, etc.
5.2. Advantages
Anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric assays have following advantages, as stated below:
- In general, anti-aggregation-enabled Au NPs- and Ag NPs-based analyte quantifications are conducted in an aqueous environment; therefore, they are tactics comparable to the available luminescent inorganic and organic nanomaterials-based sensors performed in diverse solvent conditions [122–130].
- The anti-aggregation-enabled Au NPs- and Ag NPs-based analyte detection can be observed with naked eye, thereby becoming a competing strategy to the reported organic dyes-based sensors [91][92][93][94][95][96][97].
- In the anti-aggregation-enabled sensory studies, sequential colorimetric detection of the aggregation-inducing agent and analyte can be carried out with reliable linearity and LODs down to nano- and pico-molar levels. Thus, it can be regarded as an advantage over other methods.
- By using the anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric assay, diverse analytes can be detected with specificity under a fixed pH value, incubation time, and operative temperature.
- Anti-aggregation of Au NPs in the presence of certain peptide sequences can be employed to avoid amyloid aggregation and Alzheimer’s disease occurrence.
- The anti-aggregation strategy in Au NPs- and Ag NPs-based colorimetric sensors can be effectively applied in real samples, such as water, soil, plasma, urine, etc.; thus, it can be noted as a great advantage toward development of the “state of the art” method.
5.3. Limitations
Anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric sensors also have a few limitations, as discussed below:
- Anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric sensory responses are aquatic environment-dependent; therefore, the detection of analytes in other solvent systems is not possible with this method.
- There are many optimization conditions (such as the aggregation-inducing agent, pH (buffer), NaCl effect, incubation time, temperature, etc.) required in order to attain the sensory responses, thereby limiting the frequent use of the anti-aggregation strategy.
- Due to the co-existence of the aggregation-inducing agent and analyte in the same mixture, the re-use of the mixture is not possible at all. Similarly, many reports used complicated assay protocols, which may have led to unknown human errors and restricted the use of this method.
- Elucidation of precise sensory mechanisms requires supportive evidence, such as FTIR, TEM, XPS, XRD, etc. Thus, this method was limited by available instruments and cost-effectiveness.
- The best result of anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric sensors can only be attained at a fixed interfering specie concentration, which is not possible with real samples (may contain large amounts of interference); therefore, real-time use of the strategy is still questionable.
- Real water/soil samples from different places contain various complicated matrixes. Thus, applying the anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric assay to all the real samples is not possible or may not deliver expected results.
6. Conclusions and Perspectives
In this entry, discussions on anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric sensing of metal ions, anions, bio-analytes, pesticides, and herbicides are delivered in great details. The assay protocols, optimized concentrations of aggregation-inducing agents, pH (buffer solution), incubation times, and operating temperatures are provided and tabulated for readers. The exact underlying mechanisms of each probe leading to the colorimetric response are explained with clear evidence. Real-time applications are illustrated, and comments are given on the performance of each individual report. Finally, researchers suggest the probe design/sensory requirements together with the merits and limitations of the anti-aggregation strategy. Though the anti-aggregation strategy-based colorimetric sensors are noted as being great innovations, there are still a few perspective points that need to be focused on, as noted below:
- Most reports on anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric sensors used complicated assay protocols, which should be simplified toward “state of the art”.
- Performance of anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric sensors depends on the pH values and buffer used. The use of unified pH buffer solution must be justified in the future to achieve a unique anti-aggregation strategy.
- By means of anti-aggregation, numerous reports are available on Au NPs-based colorimetric assay of Hg2+; however, none of them were commercialized toward practicality. This requires more efforts in future.
- The majority of anti-aggregation-based colorimetric sensors by Au NPs and Ag NPs focused on single-analyte quantification so far, which must be upgraded to multi-analyte detection. For example, the aggregation-inducing agent Hg2+ also has the coordination tendency for biothiols and I−, hence it can be employed in the quantification of both analytes.
- In many reports, anti-aggregation due to coordination of aggregation agents with analytes was not supported by valid evidence, which requires further clarification.
- Currently, anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric sensors are only available for the detection of limited metal analytes (such as Hg2+, Cu2+, Ag+, Mn2+, and Sc3+), thereby requiring more attention toward developing discrimination of more metal ion species.
- Anti-aggregation proposed for oxidative catalytic reactions falls short of evidence, and it should be addressed with more experimental data.
- Concerning the social impact, anti-aggregation-enabled in vitro colorimetric assay of amyloid β peptide toward the inhibition of Alzheimer disease requires great attention in future research work.
- Reports on anti-aggregation-enabled Au-NPs colorimetric assay of anions, pesticides, and herbicides are insufficient to justify the effectiveness of the strategy toward those analytes. This issue should be addressed in future.
- Only a few reports are available on the anti-aggregation-stimulated Ag NPs-based colorimetric sensors toward diverse analytes detection. Therefore, research on anti-aggregation of Ag NPs toward the discrimination of distinct analytes requires great attention.
- Compositions of Au NPs and Ag NPs with other established luminescent nanomaterials can be the focus to afford anti-aggregation-based colorimetric and luminescent responses toward specific analytes.
- Many anti-aggregation-based sensory reports lack theoretical supports, which should be addressed using density functional theory (DFT) investigations in future.
Though there are still many unclearly issues requiring further clarification, colorimetric recognition of specified analytes via anti-aggregation is becoming an important research field. Currently, there are many groups working on the development of new anti-aggregation-enabled Au NPs- and Ag NPs-based colorimetric sensory probes to remedy the aforementioned issues. However, the anti-aggregation strategy can be regarded as exceptional for analyte quantification in terms of naked eye detection ability and real-time applicability.
References
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865.
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612.
- Hua, Y.; Ma, J.; Li, D.; Wang, R. DNA-Based Biosensors for the Biochemical Analysis: A Review. Biosensors 2022, 12, 183.
- Aktar, W.M.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdisc Toxicol. 2009, 2, 1–12.
- Gianessi, L.P. The Increasing Importance of Herbicides in Worldwide Crop Production. Pest Manag. Sci. 2013, 69, 1099–1105.
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications. Chemosensors 2021, 9, 305.
- Prosposito, P.; Burratti, L.; Venditti, I. Silver Nanoparticles as Colorimetric Sensors for Water Pollutants. Chemosensors 2020, 8, 26.
- Walekar, L.; Dutta, T.; Kumar, P.; Ok, Y.S.; Pawar, S.; Deep, A.; Kim, K.-H. Functionalized Fluorescent Nanomaterials for Sensing Pollutants in the Environment: A Critical Review. TrAC Trends Anal. Chem. 2017, 97, 458–467.
- Bulska, E.; Ruszczyńska, A. Analytical Techniques for Trace Element Determination. Phys. Sci. Rev. 2017, 2, 20178002.
- Vervoort, N.; Goossens, K.; Baeten, M.; Chen, Q. Recent Advances in Analytical Techniques for High Throughput Experimentation. Anal. Sci. Adv. 2021, 2, 109–127.
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125.
- Shellaiah, M.; Sun, K.-W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors 2021, 9, 101.
- Auwalu, M.A.; Cheng, S. Diketopyrrolopyrrole Fluorescent Probes, Photophysical and Biological Applications. Chemosensors 2021, 9, 44.
- Shellaiah, M.; Chen, Y.-T.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Pyrene-Based AIEE Active Nanoprobe for Zn2+ and Tyrosine Detection Demonstrated by DFT, Bioimaging, and Organic Thin-Film Transistor. ACS Appl. Mater. Interfaces 2021, 13, 28610–28626.
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-Based Sensors: A Review. Poly. Test. 2018, 67, 342–348.
- Shellaiah, M.; Simon, T.; Thirumalaivasan, N.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-Capped Gold-Copper Nanoclusters for Fluorometric Determination and Imaging of Chromium(VI) and Dopamine. Microchim. Acta 2019, 186, 788.
- Terai, T.; Nagano, T. Fluorescent Probes for Bioimaging Applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521.
- Sahoo, S.K.; Crisponi, G. Recent Advances on Iron(III) Selective Fluorescent Probes with Possible Applications in Bioimaging. Molecules 2019, 24, 3267.
- Shellaiah, M.; Awasthi, K.; Chandran, S.; Aazaad, B.; Sun, K.W.; Ohta, N.; Wu, S.-P.; Lin, M.-C. Methylammonium Tin Tribromide Quantum Dots for Heavy Metal Ion Detection and Cellular Imaging. ACS Appl. Nano Mater. 2022, 5, 2859–2874.
- Shellaiah, M.; Sun, K.-W. Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications. Biosensors 2022, 12, 550.
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861.
- Hussain, M.; Nafady, A.; Sirajuddin; Avcı, A.; Pehlivan, E.; Nisar, J.; Sherazi, S.T.H.; Balouch, A.; Shah, M.R.; Almaghrabi, O.A.; et al. Biogenic Silver Nanoparticles for Trace Colorimetric Sensing of Enzyme Disrupter Fungicide Vinclozolin. Nanomaterials 2019, 9, 1604.
- Shellaiah, M.; Simon, T.; Sun, K.W.; Ko, F.-H. Simple Bare Gold Nanoparticles for Rapid Colorimetric Detection of Cr3+ Ions in Aqueous Medium with Real Sample Applications. Sens. Actuators B 2016, 226, 44–51.
- Zhao, L.; Zhao, L.; Miao, Y.; Liu, C.; Zhang, C. A Colorimetric Sensor for the Highly Selective Detection of Sulfide and 1,4-Dithiothreitol Based on the In-Situ Formation of Silver Nanoparticles Using Dopamine. Sensors 2017, 17, 626.
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290.
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Nanodiamonds Conjugated to Gold Nanoparticles for Colorimetric Detection of Clenbuterol and Chromium(III) in Urine. Microchim. Acta 2017, 185, 74.
- Sanskriti, I.; Upadhyay, K.K. Twinning as a Guiding Factor in Morphological Anisotropy of Silver Nanoparticles Stabilized Over L–DOPA: A Colorimetric Probe for Sulfide in Aqueous Medium. ChemistrySelect 2019, 4, 3803–3810.
- Shellaiah, M.; Thirumalaivasan, N.; Sun, K.W.; Wu, S.-P. A pH Cooperative Strategy for Enhanced Colorimetric Sensing of Cr(III) Ions Using Biocompatible L-Glutamic Acid Stabilized Gold Nanoparticles. Microchem. J. 2021, 160, 105754.
- Singh, R.; Mehra, R.; Walia, A.; Gupta, S.; Chawla, P.; Kumar, H.; Thakur, A.; Kaushik, R.; Kumar, N. Colorimetric Sensing Approaches Based on Silver Nanoparticles Aggregation for Determination of Toxic Metal Ions in Water Sample: A Review. Inter. J. Environ. Anal. Chem. 2021, 1–16.
- Sabela, M.; Balme, S.; Bechelany, M.; Janot, J.-M.; Bisetty, K. A Review of Gold and Silver Nanoparticle-Based Colorimetric Sensing Assays. Adv. Eng. Mater. 2017, 19, 1700270.
- Guo, L.; Xu, Y.; Ferhan, A.R.; Chen, G.; Kim, D.-H. Oriented Gold Nanoparticle Aggregation for Colorimetric Sensors with Surprisingly High Analytical Figures of Merit. J. Am. Chem. Soc. 2013, 135, 12338–12345.
- Zhang, L.; Yuan, Y.; Wen, X.; Li, Y.; Cao, C.; Xiong, Q. A Coordination and Ligand Replacement Based Three-Input Colorimetric Logic Gate Sensing Platform for Melamine, Mercury Ions, and Cysteine. RSC Adv. 2015, 5, 59106–59113.
- Wang, N.; Yang, H.-F.; Zhu, X.; Zhang, R.; Wang, Y.; Huang, G.-F.; Zhang, Z.-R. Synthesis of Anti-aggregation Silver Nanoparticles Based on Inositol Hexakisphosphoric Micelles for A Stable Surface Enhanced Raman Scattering Substrate. Nanotechnology 2009, 20, 315603.
- Yang, L.; Wang, N.; Zheng, G. Enhanced Effect of Combining Chlorogenic Acid on Selenium Nanoparticles in Inhibiting Amyloid β Aggregation and Reactive Oxygen Species Formation In Vitro. Nanoscale Res. Lett. 2018, 13, 303.
- Yang, L.; Wang, W.; Chen, J.; Wang, N.; Zheng, G. A Comparative Study of Resveratrol and Resveratrol-Functional Selenium Nanoparticles: Inhibiting Amyloid β Aggregation and Reactive Oxygen Species Formation Properties. J. Biomed. Mater. Res. A 2018, 106, 3034–3041.
- Li, M.; Shi, P.; Xu, C.; Ren, J.; Qu, X. Cerium Oxide Caged Metal Chelator: Anti-aggregation and Anti-oxidation Integrated H2O2-responsive Controlled Drug Release for Potential Alzheimer’s Disease Treatment. Chem. Sci. 2013, 4, 2536–2542.
- Ishtikhar, M.; Rahisuddin; Khan, M.V.; Khan, R.H. Anti-aggregation Property of Thymoquinone Induced by Copper-Nanoparticles: A Biophysical Approach. Inter. J. Biol. Macromol. 2016, 93, 1174–1182.
- Kong, L.; Zhou, X.; Shi, G.; Yu, Y. Molybdenum Disulfide Nanosheets-Based Fluorescent “Off-to-On” Probe for Targeted Monitoring and Inhibition of β-Amyloid Oligomers. Analyst 2020, 145, 6369–6377.
- Duangdeewong, C.; Choengchan, N.; Wattanasin, P.; Teerasong, S. Direct Determination of Ethanol in Alcoholic Beverages Based on Its Anti-aggregation of Melamine-Silver Nanoparticle Assembly. Talanta 2022, 250, 123751.
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972.
- Lechuga, M.; Fernández-Serrano, M.; Jurado, E.; Núñez-Olea, J.; Ríos, F. Acute Toxicity of Anionic and Non-ionic Surfactants to Aquatic Organisms. Ecotoxicol. Environ. Safety 2016, 125, 1–8.
- Wang, T.; Ramnarayanan, A.; Cheng, H. Real Time Analysis of Bioanalytes in Healthcare, Food, Zoology and Botany. Sensors 2018, 18, 5.
- Dai, J.; Ma, C.; Zhang, P.; Fu, Y.; Shen, B. Recent Progress in the Development of Fuorescent Probes for Detection of Biothiols. Dyes Pig. 2020, 177, 108321.
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of Formulants and Heavy Metals in Glyphosate-Based Herbicides and Other Pesticides. Toxicol. Rep. 2018, 5, 156–163.
- DeLorenzo, M.E.; Scott, G.I.; Ross, P.E. Toxicity of Pesticides to Aquatic Microorganisms: A Review. Environ. Toxicol. Chem. 2001, 20, 84–98.
- Najafzadeh, F.; Ghasemi, F.; Hormozi-Nezhad, M.R. Anti-aggregation of Gold Nanoparticles for Metal Ion Discrimination: A Promising Strategy to Design Colorimetric Sensor Arrays. Sens. Actuators B 2018, 270, 545–551.
- Li, Y.; Wu, P.; Xu, H.; Zhang, Z.; Zhong, X. Highly Selective and Sensitive Visualizable Detection of Hg2+ Based on Anti-aggregation of Gold Nanoparticles. Talanta 2011, 84, 508–512.
- Ding, N.; Zhao, H.; Peng, W.; He, Y.; Zhou, Y.; Yuan, L.; Zhang, Y. A Simple Colorimetric Sensor Based on Anti-aggregation of Gold Nanoparticles for Hg2+ Detection. Colloids Surf. A 2012, 395, 161–167.
- Lou, T.; Chen, L.; Zhang, C.; Kang, Q.; You, H.; Shen, D.; Chen, L. A Simple and Sensitive Colorimetric Method for Detection of Mercury Ions Based on Anti-aggregation of Gold Nanoparticles. Anal. Methods 2012, 4, 488–491.
- Li, Y.-L.; Leng, Y.-M.; Zhang, Y.-J.; Li, T.-H.; Shen, Z.-Y.; Wu, A.-G. A New Simple and Reliable Hg2+ Detection System Based on Anti-aggregation of Unmodified Gold Nanoparticles in the Presence of O-phenylenediamine. Sens. Actuators B 2014, 200, 140–146.
- Huang, G.G.; Chen, Y.-T.; Lin, Y.-R. Development of A Gold Nanoparticle Based Anti-aggregation Method for Rapid Detection of Mercury(ii) in Aqueous Solutions. Anal. Methods 2014, 6, 5690–5696.
- Zhou, Y.; Dong, H.; Liu, L.; Li, M.; Xiao, K.; Xu, M. Selective and Sensitive Colorimetric Sensor of Mercury (II) Based on Gold Nanoparticles and 4-Mercaptophenylboronic Acid. Sens. Actuators B 2014, 196, 106–111.
- Tang, J.; Wu, P.; Hou, X.; Xu, K. Modification-free and N-Acetyl-L-cysteine-Induced Colorimetric Response of AuNPs: A Mechanistic Study and Sensitive Hg2+ Detection. Talanta 2016, 159, 87–92.
- Jin, W.; Huang, P.; Wei, G.; Cao, Y.; Wu, F. Visualization and Quantification of Hg2+ Based on Anti-aggregation of Label-free Gold Nanoparticles in the Presence of 2-Mercaptobenzothiazole. Sens. Actuators B 2016, 233, 223–229.
- Rajeshwari, A.; Karthiga, D.; Chandrasekaran, N.; Mukherjee, A. Anti-aggregation-Based Spectrometric Detection of Hg(II) at Physiological pH Using Gold Nanorods. Mater. Sci. Eng. C 2016, 67, 711–716.
- Sun, X.; Liu, R.; Liu, Q.; Fei, Q.; Feng, G.; Shan, H.; Huan, Y. Colorimetric Sensing of Mercury (II) Ion Based on Anti-aggregation of Gold Nanoparticles in the Presence of Hexadecyl Trimethyl Ammonium Bromide. Sens. Actuators B 2018, 260, 998–1003.
- Kataria, R.; Sethuraman, K.; Vashisht, D.; Vashisht, A.; Mehta, S.K.; Gupta, A. Colorimetric Detection of Mercury Ions Based on Anti-aggregation of Gold Nanoparticles Using 3, 5-Dimethyl-1-thiocarboxamidepyrazole. Microchem. J. 2019, 148, 299–305.
- Mao, L.; Wang, Q.; Luo, Y.; Gao, Y. Detection of Ag+ Ions Via an Anti-aggregation Mechanism Using Unmodified Gold nanoparticles in the Presence of Thiamazole. Talanta 2021, 222, 121506.
- Safavi, A.; Ahmadi, R.; Mohammadpour, Z. Colorimetric Sensing of Silver ion Based on Anti Aggregation of Gold Nanoparticles. Sens. Actuators B 2017, 242, 609–615.
- Selva Sharma, A.; SasiKumar, T.; Ilanchelian, M. A Rapid and Sensitive Colorimetric Sensor for Detection of Silver Ions Based on the Non-aggregation of Gold Nanoparticles in the Presence of Ascorbic Acid. J. Cluster Sci. 2018, 29, 655–662.
- Gao, Q.; Zheng, Y.; Song, C.; Lu, L.-Q.; Tian, X.-K.; Xu, A.-W. Selective and Sensitive Colorimetric Detection of Copper Ions Based on Anti-aggregation of the Glutathione-Induced Aggregated Gold Nanoparticles and Its Application for Determining Sulfide Anions. RSC Adv. 2013, 3, 21424–21430.
- Hormozi-Nezhad, M.R.; Abbasi-Moayed, S. A Sensitive and Selective Colorimetric Method for Detection of Copper Ions Based on Anti-aggregation of Unmodified Gold Nanoparticles. Talanta 2014, 129, 227–232.
- Deng, H.-H.; Huang, K.-Y.; Fang, Q.-H.; Lv, Y.-P.; He, S.-B.; Peng, H.-P.; Xia, X.-H.; Chen, W. Schiff Base and Lewis Acid-Base Interaction-Regulated Aggregation/Dispersion of Gold Nanoparticles for Colorimetric Recognition of Rare-Earth Sc3+ Ions. Sens. Actuators B 2020, 311, 127925.
- Plaisen, S.; Cheewasedtham, W.; Rujiralai, T. Robust Colorimetric Detection Based on the Anti-aggregation of Gold Nanoparticles for Bromide in Rice Samples. RSC Adv. 2018, 8, 21566–21576.
- Chen, L.; Lu, W.; Wang, X.; Chen, L. A Highly Selective and Sensitive Colorimetric Sensor for Iodide Detection Based on Anti-aggregation of Gold Nanoparticles. Sens. Actuators B 2013, 182, 482–488.
- Zhou, G.; Zhao, C.; Pan, C.; Li, F. Highly Sensitive and Selective Colorimetric Detection of Iodide Based on Anti-Aggregation of Gold Nanoparticles. Anal. Methods 2013, 5, 2188–2192.
- Rasouli, Z.; Ghavami, R. Colorimetric Sensing of Iodide by the Competitive Interactions in the Surface of Gold Nanoparticles with the Simultaneous Aggregation/ Anti-Aggregation Mechanisms in Edible Salts. Current Anal. Chem. 2018, 14, 539–547.
- Peng, R.; He, H.; Wang, Q.; Yan, X.; Yu, Q.; Qin, H.; Lei, Y.; Luo, L.; Feng, Y. Cu(Ⅱ) Triggering Redox-regulated Anti-aggregation of Gold Nanoparticles for Ultrasensitive Visual Sensing of Iodide. Anal. Chim. Acta 2018, 1036, 147–152.
- Pournaghi, A.; Keshvari, F.; Bahram, M. Colorimetric Determination of Iodine Based on Highly Selective and Sensitive Anti-aggregation Assay. J. Iran. Chem. Soc. 2019, 16, 143–149.
- Deng, H.-H.; Wu, C.-L.; Liu, A.-L.; Li, G.-W.; Chen, W.; Lin, X.-H. Colorimetric Sensor for Thiocyanate Based on Anti-aggregation of Citrate-Capped Gold Nanoparticles. Sens. Actuators B 2014, 191, 479–484.
- Song, J.; Huang, P.-C.; Wan, Y.-Q.; Wu, F.-Y. Colorimetric Detection of Thiocyanate Based on Anti-aggregation of Gold Nanoparticles in the Presence of Cetyltrimethyl Ammonium Bromide. Sens. Actuators B 2016, 222, 790–796.
- Zhao, Y.; Liu, R.; Cui, X.; Fu, Q.; Yu, M.; Fei, Q.; Feng, G.; Shan, H.; Huan, Y. Colorimetric Sensor for Thiocyanate Based on Anti-aggregation of Gold Nanoparticles in the Presence of 2-Aminopyridine. Anal. Sci. 2020, 36, 1165–1169.
- Lu, L.; Zhang, J.; Yang, X. Simple and Selective Colorimetric Detection of Hypochlorite Based on Anti-aggregation of Gold Nanoparticles. Sens. Actuators B 2013, 184, 189–195.
- Liu, W.; Du, Z.; Qian, Y.; Li, F. A Specific Colorimetric Probe for Phosphate Detection Based on Anti-aggregation of Gold Nanoparticles. Sens. Actuators B 2013, 176, 927–931.
- Duan, J.; Yin, H.; Wei, R.; Wang, W. Facile Colorimetric Detection of Hg2+ Based on Anti-aggregation of Silver Nanoparticles. Biosens. Bioelectron. 2014, 57, 139–142.
- Ye, Y.; Guo, Y.; Yue, Y.; Huang, H.; Zhao, L.; Gao, Y.; Zhang, Y. Colorimetric Sensing of Copper Ions Based on the Anti-aggregation of Unmodified Silver Nanoparticles in the Presence of 1,4-Dithiothreitol. Anal. Methods 2015, 7, 566–572.
- He, Y.; Zhang, X. Ultrasensitive Colorimetric Detection of Manganese(II) Ions Based on Anti-aggregation of Unmodified Silver Nanoparticles. Sens. Actuators B 2016, 222, 320–324.
- Basiri, S.; Mehdinia, A.; Jabbari, A. Green Synthesis of Reduced Graphene Oxide-Ag Nanoparticles as A Dual-Responsive Colorimetric Platform for Detection of Dopamine and Cu2+. Sens. Actuators B 2018, 262, 499–507.
- Bothra, S.; Kumar, R.; Pati, R.K.; Kuwar, A.; Choi, H.-J.; Sahoo, S.K. Virgin Silver Nanoparticles as Colorimetric Nanoprobe for Simultaneous Detection of Iodide and Bromide Ion in Aqueous Medium. Spectrochim. Acta A 2015, 149, 122–126.
- Motahhari, A.; Abdolmohammad-Zadeh, H.; Farhadi, K. Development of A New Fluoride Colorimetric Sensor Based on Anti-aggregation of Modified Silver Nanoparticles. Anal. Bioanal. Chem. Res. 2021, 8, 79–89.
- Dong, C.; Ma, X.; Qiu, N.; Zhang, Y.; Wu, A. An Ultra-Sensitive Colorimetric Sensor Based on Smartphone for Pyrophosphate Determination. Sens. Actuators B 2021, 329, 129066.
- Pinyorospathum, C.; Rattanarat, P.; Chaiyo, S.; Siangproh, W.; Chailapakul, O. Colorimetric Sensor for Determination of Phosphate Ions Using Anti-aggregation of 2-Mercaptoethanesulfonate-Modified Silver Nanoplates and Europium Ions. Sens. Actuators B 2019, 290, 226–232.
- Chen, L.; Fu, X.; Li, J. Ultrasensitive Surface-Enhanced Raman Scattering Detection of Trypsin Based on Anti-aggregation of 4-Mercaptopyridine-Functionalized Silver Nnanoparticles: An Optical Sensing Platform Toward Proteases. Nanoscale 2013, 5, 5905–5911.
- Trisaranakul, W.; Chompoosor, A.; Maneeprakorn, W.; Nacapricha, D.; Choengchan, N.; Teerasong, S. A Simple and Rapid Method Based on Anti-aggregation of Silver Nanoparticles for Detection of Poly(diallyldimethylammonium chloride) in Tap Water. Anal. Sci. 2016, 32, 769–773.
- Shellaiah, M.; Sun, K.W. Review on Nanomaterial-Based Melamine Detection. Chemosensors 2019, 7, 9.
- Abdel-Lateef, M.A.; Almahri, A.; Alzahrani, E.; Pashameah, R.A.; Abu-Hassan, A.A.; El Hamd, M.A. Sustainable PVP-Capped Silver Nanoparticles as a Free-Standing Nanozyme Sensor for Visual and Spectrophotometric Detection of Hg2+ in Water Samples: A Green Analytical Method. Chemosensors 2022, 10, 358.
- Mochi, F.; Burratti, L.; Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Iucci, G.; Casalboni, M.; De Matteis, F.; Casciardi, S.; et al. Plasmonic Sensor Based on Interaction between Silver Nanoparticles and Ni2+ or Co2+ in Water. Nanomaterials 2018, 8, 488.
- Rossi, A.; Zannotti, M.; Cuccioloni, M.; Minicucci, M.; Petetta, L.; Angeletti, M.; Giovannetti, R. Silver Nanoparticle-Based Sensor for the Selective Detection of Nickel Ions. Nanomaterials 2021, 11, 1733.
- Liu, X.; Wang, J.; Wang, Y.; Huang, C.; Wang, Z.; Liu, L. In Situ Functionalization of Silver Nanoparticles by Gallic Acid as a Colorimetric Sensor for Simple Sensitive Determination of Melamine in Milk. ACS Omega 2021, 6, 23630–23635.
- Sharma, R.; Dhillon, A.; Kumar, D. Mentha-Stabilized Silver Nanoparticles for High-Performance Colorimetric Detection of Al(III) in Aqueous Systems. Sci. Rep. 2018, 8, 5189.
- Nguyen, V.-N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent Developments of BODIPY-Based Colorimetric and Fluorescent Probes for the Detection of Reactive Oxygen/Nitrogen Species and Cancer diagnosis. Coord. Chem. Rev. 2021, 439, 213936.
- Arumugaperumal, R.; Srinivasadesikan, V.; Lin, M.-C.; Shellaiah, M.; Shukla, T.; Lin, H.-C. Facile Rhodamine-Based Colorimetric Sensors for Sequential Detections of Cu(ii) Ions and Pyrophosphate (P2O74−) Anions. RSC Adv. 2016, 6, 106631–106640.
- Liu, B.; Zhuang, J.; Wei, G. Recent Advances in the Design of Colorimetric Sensors for Environmental Monitoring. Environ. Sci. Nano 2020, 7, 2195–2213.
- Fang, H.-P.; Shellaiah, M.; Singh, A.; Raju, M.V.R.; Wu, Y.-H.; Lin, H.-C. Naked Eye and Fluorescent Detections of Hg2+ Ions and Cysteine via J-Aggregation and Deaggregation of a Perylene Bisimide Derivative. Sens. Actuators B 2014, 194, 229–237.
- Shellaiah, M.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Novel Rhodamine Probe for Colorimetric and Fluorescent Detection of Fe3+ Ions in Aqueous Media with Cellular Imaging. Spectrochim. Acta A 2020, 242, 118757.
- Chen, S.; Xue, Z.; Gao, N.; Yang, X.; Zang, L. Perylene Diimide-Based Fluorescent and Colorimetric Sensors for Environmental Detection. Sensors 2020, 20, 917.
- Ericson, M.N.; Shankar, S.K.; Chahine, L.M.; Omary, M.A.; Herbing, I.H.v.; Marpu, S.B. Development of Neutral Red as a pH/pCO2 Luminescent Sensor for Biological Systems. Chemosensors 2021, 9, 210.





