1. Development of Muco-Adhesives and Muco-Adhesive Drug Delivery Systems
1.1. Evaluating Muco-Adhesion
The process of muco-adhesion and the development of muco-adhesives using a wide variety of methods and techniques, has been widely studied. Such themes are influenced by experiment design, the applicability and properties of various bio-polymers, drug dosage forms, and instrumental variables. The three main tests of muco-adhesion are tensile, shear strength, and peel strength; in vitro or ex vivo tests for efficacy of muco-adhesion
[1][2][3][4]. further, in vivo imaging is used to evaluate muco-adhesion and delivery efficacy
[1][3][4]. However, there is no standard apparatus for testing muco-adhesion, which results in numerous tests for muco-adhesion and a lack of uniformity and cohesion between test methods, and thereby renders comparing the reported findings/results rather difficult
[1][3][4].
Generally, tensile strength is the most commonly used method or test to assess muco-adhesion
[1][5][6]. Usually, in this technique, force is applied perpendicularly to the polymer-substrate interface, creating tensile stress, which equals pressure over the contact area, to determine the amount of force required to separate the two
[1]. Another common test is the wash-off test
[4]. herein, the polymer is attached to a slide in a USP tablet disintegration apparatus, which replicates the mucosa and moves the polymer up and down until complete detachment of the polymer from the mucosa replicates
[4][7].
Furthermore, shear stress is similar to the tensile test in that stress is uniformly distributed over the adhesive joint and is intended to measure the mechanical properties of the entire system
[1]. Pressure is equally distributed over the whole system, and force is required to detach the whole system, although shear stress redirects the forces along the joint interface
[1]. After all, the peel strength stress focuses the force at the edge of the joint and calculates the energy or force resisting peeling
[1]. For most muco-adhesive systems, the peel strength is not relevant, though it is very useful for patch-based systems
[1]. Finally, in vivo tests for muco-adhesion are often limited by ethical and feasibility constraints
[1]. nonetheless, one common in vivo technique is to monitor gastro-intestinal transit and residence times of muco-adhesive polymers, or drug release (tracking/bio-distribution) from in situ, pre-implanted devices
[4]. Using radioactive labeling on the delivery system and regular measurement of radioactivity in the target area, the muco-adhesive capacity of the delivery system can be hence, approximated
[3][4].
1.2. Delivery Sites and Routes for Muco-Adhesive Bio-Polymer Drug Delivery Systems
To prolong drug action, muco-adhesive dosage forms need to be in close contact with the absorbing surface to increase residence time
[4]. Muco-adhesive polymers are typically delivered in sites such as the eye conjunctiva, oral cavity, nasal cavity, vagina, and the gastro-intestinal (GI) tract, since the mucosa lines these areas of the body
[3][4]. Each route has different benefits based on the properties of the dosage form at the site. While buccal and sub-lingual sites have more rapid onsets by by-passing first-pass metabolism, they can be inconvenient for the person due to taste and food intake
[8]. Microvilli in the GI tract may enhance absorption but may result in first-pass metabolism and requires consideration of stability
[8]. Rectal and vaginal routes have excellent local drug absorption but are difficult to administer
[8].
1.2.1. Buccal/Oral Cavity (Intra-Oral)
Even though the buccal cavity has a smaller surface of around 50 cm
[1], its ease of access renders it a preferred location for drug delivery by by-passing hepatic first-pass metabolism and local oral lesion treatments
[8][9]. Both, buccal and sub-lingual routes can provide direct access to the systemic circulation via bypassing first-pass metabolism; however, due to its smooth, immobile surface, the buccal mucosa is less permeable than the sub-lingual mucosa
[3][4][8]. Consequently, whereas sub-lingual delivery tends to release the drug quickly, the muco-adhesive formulation is more controlled in the buccal mucosa and, consequently, better for such drug delivery (better/higher bio-availability)
[8]. To avoid the saliva washing away the drug, the delivery system typically includes a film impermeable to water
[8]. Given that this region has non-keratinized epithelia with more permeable tissue relative to the skin, drugs that have short half-lives, poor permeability, need for a sustained release effect, sensitive enzymatic degradation, or poor solubility, would benefit from being delivered via the oral cavity
[4]. Buccal and sub-lingual drug delivery systems come in tablet-, film- or spray-formats, amongst others.
1.2.2. Ocular Cavity (Eye Conjunctiva)
Generally, while the conjunctiva, cornea, anterior chamber, and iris usually respond well to topical treatment, the eyelids tend to more frequently require systemic treatment. Goblet cells secrete mucin in the eye conjunctiva but not in the cornea; therefore, muco-adhesive polymers would attach well to the conjunctival but not the corneal mucus (static vs. dynamic barriers)
[4]. Ophthalmic dosage forms improve with prolonged contact with eye tissues
[4]. Yet, due to tearing formation (and tear dilution), and eyelid blinking, the medication or loaded/released drug can be rapidly removed from the ocular cavity, resulting in poorer bio-availability, that can be then reduced using ocular inserts or patches
[9].
1.2.3. Reproductive Lumen (Vaginal and Rectal)
Vaginal and rectal lumen delivery has been considered for topical and systemic treatment
[4][9]. Systemic vaginal delivery bypasses hepatic first-pass metabolism and can lower dosing frequencies by remaining in the vagina for prolonged periods during the day and night
[3][4][9]. The surface area of the vagina is enhanced by many epithelial folds and micro-ridges that cover the epithelial cell surface
[4]. One challenge in this route is the possibility of migration within the vagina or rectal lumen that would affect the specific location of delivery
[9]. Muco-adhesive polymers help minimize this migration, thereby, improving the therapeutic efficacy
[9]. Yet, this route can be deemed subpar and inefficient due to the prompt transit of the drug-containing delivery system past the absorption site
[9].
1.2.4. Nasal Cavity
The nasal cavity is an excellent histologic route for drug delivery. Similar to the buccal cavity, the nasal cavity enables muco-adhesive formulation development, and has gained attention and interest to promote dosage form residence time as well as improving intimacy of contact with absorptive membranes of the biological system
[8]. Indeed, the surface area of the nasal mucosal layer ranges from 150 to 200 cm
[1] and has enhanced muco-ciliary activity with foreign particles
[4][8]. As mentioned earlier, the mucus (and membrane) is composed of a glycoprotein, mucin, with negative charges due to the presence of sialic acid residues. In addition, the nasal cavity has dense vascular networks, permeable membranes, and an absorption capability, by-passing the first-pass hepatic metabolism; rendering the rate and extent of absorption comparable with intravenous (I.V.) drug administration
[4].
1.2.5. The GI Tract
Besides that, drug delivery across the mucosa bypasses the first-pass hepatic metabolism, herein, this route facilitates avoiding the degradation of
gastro-intestinal enzymes. Hence, localizing a drug in parts of the GI propelled the development of muco-adhesive systems
[4][9]. The main goal of oral use would be to significantly increase the residence time of the drug for a localized effect and to enable dosing once a day
[4]. Modulating the delivery transit time has been investigated extensively in the GI
[9].
2. Muco-Adhesive Bio-Polymers: From Basics to Applications
2.1. Characteristics of Bio-Polymers
Generally, bio-polymers are held together by primary bonds (covalent bonds) and secondary bonds (van der Waals and hydrogen bonds). Co-polymers are polymers composed of two or more different types of monomers. Three types of polymers can adhere to biological surfaces: (1) polymers using non-covalent and non-specific electrostatic interactions; (2) polymers with hydrophilic functional groups that enable hydrogen bonding with the substrate; and (3) polymers that bind to receptor sites on the mucus surface
[1]. Further, polymers may also be classified as hydrophilic, hydrogels, or thermoplastic
[2]. Hydrophilic polymers, such as chitosan, methylcellulose, and plant gums, swell when in contact with water and undergo complete dissolution
[2]. Hydrogels, such as sodium alginate and guar gum, are typically cross-linked polymers and have limited swelling capabilities
[2]. Thermoplastic polymers include polymers that generate carboxylic acid groups while degrading, such as polyanhydrides
[2]. Some polymers are utilized in muco-adhesive formulations, including synthetic, biocompatible, biodegradable, polyorthoesters, polyphosphoesters, polyanhydrides, and polyphosphazenes
[2]. Because physical and chemical interactions can affect the adhesion between the polymer and the mucosal substrate, several properties should be considered when determining the optimal polymer, particularly for a bioadhesive (muco-adhesive) drug delivery system
[3]. For example, the polymer (and any degradation by-product) should be non-irritant, non-toxic, and non-absorbable (biodegradable)
[1]. Ideally, the polymer can form a strong non-covalent bond with the mucosal cell surface and adhere quickly to moist tissue with some specificity to the site
[1]. The polymer should not decompose while in storage or on the shelf using the specific drug dosage form
[1]. The polymer should be easily incorporated into the drug with no hindrance once released
[1]. last but not least, for the product to remain competitive, the cost of the polymer should not be too expensive
[1].
Briefly, muco-adhesive bio-polymers play a role in facilitating the desirable mucosal interactions (the mucosal layer is made up of mucus which is secreted by the goblet cells or glandular columnar epithelial cells and is visco-elastic), hence, there are certain characteristics of polymers (whether natural, synthetic or composite/hybrid) that affect the resulting muco-adhesion. Designing and developing muco-adhesive polymers may be affected by different biochemical and structural polymer-to-mucosa interactions, such as hydrophilicity, molecular weight, charge ± and the pH. Indeed, whilst muco-adhesive bio-polymers are mainly water-soluble in nature, some can also be water-insoluble, for example
[1][3][9]. A summary of the properties of muco-adhesive polymers follows.
2.2. Factors Affecting Muco-Adhesion
A muco-adhesive bio-polymer ideally has the capability to become sticky and has chain flexibility at the mucosal pH and ionic strength, small enough to favor inter-penetration yet considerable enough for entanglement, with optimal molecular weight, and relevant degree and rate of polymer swelling
[10].
2.2.1. Hydrophilicity
Bio-adhesive polymers contain hydrophilic functional groups that enable hydrogen bonding with the substrate, expand (swell) in water, and enhance exposure to anchor sites
[1]. Swollen bio-polymers are efficient at maximizing the distance between the chains, resulting in chain flexibility and efficient substrate penetration
[1].
2.2.2. Molecular Weight
Briefly, bio-polymers with low molecular weight allow for better inter-penetration while if with high molecular weight would allow for entanglements
[1]. thereby, increasing the molecular weight (of the chosen bio-polymer or bio-polymers) improves muco-adhesion, because the polymers diffuse, inter-penetrate, and/or interlock with the mucosa at the site of application
[9]. Although the optimal molecular weight to maximize muco-adhesion depends and varies on/with each type of polymer and its inherent adhesive character alongside the nature of the target tissue (and route), bio-adhesive forces increase with the molecular weight (up to 100 kDa); above which there is no further benefit
[1][9].
2.2.3. Cross-Linking and Swelling Factor
Swelling, which is needed to induce mobility, plays a key role in the desired inter-penetration between the bio-polymer and the mucosal substrate, since it exposes the muco-adhesive sites to hydrogen bonding
[11]. herein, bio-polymer concentration, ionic strength, and water concentration can influence swelling
[11]. Although loose cross-linking is preferred for a higher degree of swelling, over-hydration can decrease adhesion and clear the muco-adhesive system from the mucosa
[1][11]. Hence, determining the rate of swelling (at design and development stage) can be important for an effective muco-adhesive drug delivery system with an
optimized time of clearance and rate of drug release
[11]. Further, a higher initial force of application increases inter-penetration and muco-adhesive strength. Higher initial contact time between the muco-adhesive and the substrate increases swelling and inter-penetration of the polymer chains
[11]. This
initial applied strength and contact time correlates with muco-adhesion, because controlled contact improves regulation, inter-penetration, and degree of swelling, as follows:
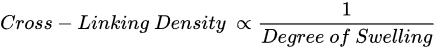
Therefore, there is an indirect relationship between the cross-link density and the degree of swelling, also known as hydration
[1][11]. Basically, a decrease in the cross-link density increases flexibility and the hydration rate
[1]. Besides, the polymer surface area is directly proportional to muco-adhesion
[1]. Furthermore, formulation of the adhesion promoters can enrich the muco-adhesion of cross-linked polymers
[1]. The theories and approaches of the muco-adhesion process, discussed above, apply herein.
2.2.4. pH at the Muco-Adhesive Bio-Polymer-Substrate Interface
The pH level at the interface of the bio-polymer and the substrate can impact the adhesion of the bio-adhesives with ionizable groups and polyanions such as the carboxylic acids
[1][3]. pH can cause changes in dissociation and hydration based on the polymer pH and ± charge
[9]. Local pH levels that are higher than the polymer pK will result in large ionization and lower than the pK in unionization
[1].
2.2.5. Concentration of the Active Bio-Polymer
An optimum concentration of active bio-polymer can be used to maximize liquid muco-adhesive formulations
[1]. As polymer concentration increases, access to the polymer chain decreases, so adhesion decreases due to the limited available chain length for inter-penetration
[9]. Generally, the ideal polymer concentration is 1–2.5 percent molecular mass
[9]. However, for solid dosages such as tablets, polymer concentration and muco-adhesion strength are directly related
[1]. In addition, spatial conformation impacts polymer concentration
[9]. Furthermore, an increase in the polymer chain length results in a greater polymer flexibility, penetration, entanglement, and so, muco-adhesion strength
[9].
2.2.6. Drug/Excipient Concentration
Drug concentration can impact muco-adhesion. Indeed, a study exploring the effect of propranolol hydrochloride on Carbopol found that limiting water in a system do increase elasticity and adhesion
[1]. Further, an increase in the toluidine blue O concentration in Gantrez, has increased muco-adhesion to the cheek due to the electro-static interactions between the cationic drug and the anionic co-polymer
[1].
2.2.7. Mucin Turnover Rate
Physiological factors, such as the rate of mucus turnover, can also impact muco-adhesion in systems
[1][3]. This turnover rate can be influenced by the disease state and the presence of a bio-adhesive device
[1][12]. The mucin clearance system will decrease the residence time on the mucosal layer
[12]. Both, cell renewal and mucin turnover rates depend on the type and location of the mucosa
[12].
3. Bio-Inspired Polymers and Application in Drug Delivery
3.1. Chitosan
Chitosan is a natural, biocompatible, and biodegradable polysaccharide, derived from shell-fish, that has been extensively used for wound closure, hemostatic, and other applications and that is soluble in aqueous solutions with pH less than 6.5
[13][14]. Lehr et al. evaluated the muco-adhesive properties of chitosan in vitro by measuring detachment forces for swollen pig intestinal mucosa polymer films in saline media
[15]. Whereas natural polymers hydroxypropyl- and carboxymethylcellulose exhibited almost no muco-adhesion, the cationic polymer chitosan was muco-adhesive relative to Polycarbophil
[15]. Further, He et al. evaluated the muco-adhesive properties of chitosan microspheres in vitro by measuring the mucin absorbed on microspheres and using turbidimetric measurements
[16]. Adsorption studies between mucin and chitosan microspheres with varying crosslinking studies indicated strong interactions
[16]. Chitosan microspheres were also retained in biological tissue
[16]. In another study, Sogias et al. investigated the interactions between chitosan and gastric mucin to understand why this polymer is muco-adhesive
[14]. Decreasing the number of amino groups enhanced the pH solubility window of chitosan yet decreased its ability to aggregate mucin
[14]. Although the electrostatic attraction interactions between chitosan and gastric mucin can be inhibited with 0.2 M sodium chloride, these forces do not suppress aggregation of mucin particles when chitosan is present.
[14] The impact of mucin aggregation in the presence of chitosan in solutions containing 8M urea or 10%
v/
v ethanol suggests that hydrogen bonding and hydrophobic effects, respectively impact muco-adhesion
[14]. Previously, Xu et al. used chitosan to create adhesive hydrogels
[17][18][19]. Bio-adhesives based on chitosan exhibited strong adhesive properties to mucosal tissues and minimal cytotoxicity
[13]. Xu et al. found that mixing chitosan with catechol-containing compounds, 3,4-dihydroxy-L-phenylalanine (DOPA), hydrocaffeic acid (HCA), or dopamine has been shown to increase muco-adhesive properties and swelling
[18]. The hydrogel factor was highest in the presence of DOPA and dopamine but lower in HCA
[18]. The HCA-chitosan hydrogel had slow catechol release, perhaps due to electrostatic interactions between chitosan and HCA
[18]. Additionally, a decrease in hydrogel swelling and HCA release increased muco-adhesion in HCA-chitosan hydrogen when compared to chitosan hydrogels only, in rabbit small intestine
[18]. In addition, oxidation of HCA during contact with the mucosa enhanced muco-adhesion of HCA-chitosan hydrogels
[18]. This approach is bio-inspired, because muco-adhesion of chitosan hydrogels in wet conditions was increased by adding catechol-containing compounds
[18][19]. In addition, Kim et al. reacted chitosan with 3,4-dihydroxy hydrocinnamic acid-mediated and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride to synthesize a chitosan-catechol conjugate
[20]. This method was inspired by mussel adhesion to surfaces
[20]. Mucin-particle interaction, turbidimetry, surface plasmon resonance spectroscopy, rheological characterizations, and in vivo fluorescence imaging techniques were used to measure the muco-adhesive properties in mice
[20]. Compared to chitosan and polyacrylic acid separately, the chitosan-catechol conjugate yielded better muco-adhesion
[20]. It is noteworthy that chitosan-catechol, typically, is prepared via chemical, electrochemical, or enzymatic syntheses
[21]. The notable properties of chitosan-catechol include solubility, tissue adhesion, mechanical strength, and biocompatibility
[21]. In addition, this conjugate can be prepared in different forms, such as hydrogels, films, sponges, microparticles, or even nanoparticles
[21]. The development of mimics (biomimetics/biomimicry) such as polyethyleneimine–catechol, chitosan–catechol, and catecholic polymers was inspired by the abundance of catecholamine in mussel adhesive proteins
[21]. For example, Yamada et al. synthesized chitosan-catechol adhesives that are resistant to water and inspired by insect cuticular sclerotization
[21][22]. Rheologically, tyrosinase-catalyzed reactions and subsequent un-catalyzed reactions increased the viscosity of the chitosan solutions with adhesive shear strengths over 400 kPa
[22]. Moreover, chitosan is limited in its poor load-bearing capability in hydrated conditions and dissolvability in pH conditions lower than 6
[23]. Zvarec et al. synthesized and prepared nanoparticle composites inspired by and mimicking the mechanical strength of squid beaks and mussel thread coatings using chitosan
[23]. Finally, the inorganic γ-Fe
2O
3 and catechol organic interfaces enhanced the coordination between bonding and stability, at high temperatures and physiological pH conditions
[23].
3.2. Mussel Adhesive Protein (MAP)
MAP, or mussel adhesive protein, is a 130-kDa protein that adheres to underwater surfaces
[24]. MAP is likely muco-adhesive due to it containing DOPA, which has a hydrogen bond capability that has been attributed to its ability to interact with mucosal surfaces
[25]. Indeed, MAP was shown to exhibit strong muco-adhesive properties in a thin film by Schnurrer et al. and in soluble form by Deacon et al.
[24][25][26]. MAP is produced and inspired by blue mussel
Mytilus edulis and perhaps worthy of further research.
3.3. Alginate-PEGAc
Previously, Davidovich-Pinhas et al. synthesized a muco-adhesive polymer labeled Alginate-PEGAc whereby the alginate backbone holds acrylate polyethylenglycol
[27]. This polymer demonstrates the strength, simplicity, and gelation useful for muco-adhesion
[27]. briefly, alginate (from algae) is biocompatible and bioactive with low toxicity
[28]. The combination of PEG inter-penetration with the mucosal surface layer and the Michael-type addition reaction between the polymer acrylate end group and mucosal glycoprotein sulfide end group, strong bonding between the polymer and mucosal layer occurred/resulted
[27]. The formation of this polymer was demonstrated through nuclear magnetic resonance, lack of cytotoxicity was tested through cell viability assays in vitro, and its muco-adhesive properties were evaluated through adhesion assays
[27]. further, scanning electron microscopy was used to characterize the alginate-PEGAc; a synthetic polymer inspired by protein PEGylation, today, is used for controlled drug release and has since transformed the food and the biomedical industries
[27][28].
3.4. Pectin-Sodium Carboxymethyl Cellulose System
Gupta et al. demonstrated the ability to use polypeptides to orally deliver salmon calcitonin using muco-adhesive polymers salmon calcitonin in vivo
[29]. This system was inspired by the design of transdermal patches and was prepared by creating a matrix of carbopol, pectin, and sodium carboxymethylcellulose (1:1:2) and coating the matrix on three of four sides with impermeable, flexible ethyl cellulose backing layer
[29]. The polymer matrix system had strong muco-adhesion to porcine small intestine in vitro, withstanding forces up to 100 times the overall weight of the system
[29]. This system is used to deliver drugs orally, which was previously rendered challenging due to poor stability in the stomach and permeation across the intestine
[29]. both, pectin and sodium carboxymethylcellulose are natural compounds, that are also to be held or classified as ‘
bio-inspired’.
3.5. Carbopol 934P
Carbopol gels in water have also been evaluated for their muco-adhesive properties
[30]. Tamburic et al. evaluated Carbopol 934, rheologically, with continuous shear, creep, and oscillatory measurements
[31]. When compared to other gel systems, Carbopol 934 had the highest degree of gel network elasticity and viscosity, with low thixotropy
[31]. Tamburic et al. also investigated the muco-adhesive properties of different polyacrylic acid gel systems
[32]. When compared to EX-214 and Noveon AA-1, Carbopols 934P and 974P had the greatest muco-adhesive strength and had small differences between these two systems due to the neutralizing agents
[32]. In addition, there was a correlation between muco-adhesive strength and rheological tan δ (phase lag) values
[32]. Furthermore, Carbopol has been combined with other polymers to develop a drug delivery system with a mucoadhesive-controlled release
[30]. Bera et al. utilized the mucoadhesive properties of the carboxypolymethylene (CP934P) polymer to synthesize glipizide microbeads regulating blood sugar in patients with diabetes
[30]. Herein, CP934P is a biodegradable, biocompatible carbopol polymer that is useful for its swellability (swelling capability), biosafety, low cost, and lack of absorption by tissues
[30]. Indeed, CP934P was able to reduce the fasting blood glucose levels in rats and guinea pigs and had high muco-adhesivity
[30]. On the other hand, Blanco-Fuente et al. studied the bio-adhesive properties of carbopol hydrogels intended for buccal administration of propranolol HC1 in vitro with a tensile tester in/under different hydration conditions
[33]. Briefly, the limited water in the system resulted in an increased adhesion
[33]. Additionally, an increase in polymer molecular weight and crosslinking percentage decreased adhesion capacity
[33]. When propranolol HC1 was added to the hydrogens, adhesion increased in the limited water system, since the polymer-drug complex formation enhanced elasticity
[33]. When propranolol HC1 was added to the hydrogens, adhesion increased in the limited water system, since the polymer-drug complex formation enhanced elasticity, and decreased in the system with no water limitation due to precipitation of the carbopol-propranolol HC1 complex
[33]. In another study, Takeuchi et al. evaluated the effectiveness of the muco-adhesive properties of carbopol-coated liposomes in orally administering calcitonin to rats
[34]. Muco-adhesive liposomes coated with carbopol polymers and with chitosan polymers were prepared in rat intestines
[34]. The adhesive property of liposomes coated with carbopol polymers was inversely related to pH levels of the dispersing medium, perhaps due to electric repulsion between the carbopol liposomes and the mucosal layer
[34]. Administering carbopol-coated liposomes with calcitonin prolonged the reduction of calcium concentration in the blood
[34]. Dodou et al. designed a synthetic muco-adhesive polymer that can move down the colon
[35]. Safe propagation requires generating friction with the colonic surface
[35]. Micro-patterning muco-adhesive films were able to generate high static friction in vitro and prevented damaging the colonic surface
[35]. This was inspired by adhesives secreted by sea stars in the form of sponges released by tubular feet
[35].
3.6. Spider Silk
As a strong polymer that is biocompatible, biodegradable, non-toxic, and lightweight, spider silk has great potential to be used as an adhesive material
[36]. Spider silks have inspired several polymer blends, including 4RepCT variants, aggregate silk, and pyriform silk, yet require further research to explore the potential of each polymer as a mucoadhesive
[36]. For example, Peng et al. adopted water-soluble recombinant spider dragline silk protein to create spinning dope
[37]. The artificial spider silk was spun via a bio-inspired microfluidic chip mimicking natural spinning
[37]. The tensile strength was 510 MPa, and elongation of the fibers was 15%
[37]. Future advances have the potential to result in use.
3.7. Spider Silk 4RepCT Variants
Petrou et al. proved spider silk material as muco-adhesive by genetically engineering two variants of spider silk protein 4RepCT that exhibited functional muco-adhesive properties, including mucin binding properties and electrostatic interactions
[36]. Petrou et al. speculate that these variants may be used individually or together with bio-functional silk proteins to build protein-based materials in mucosal treatments
[36]. As a newly synthesized polymer inspired by spider silk, further research is required to characterize it, including the strength of the interactions and the ability to deliver drugs
[36].
3.8. Aggregate Silk Glue
Visco-elastic aggregate silk glue from orb-weaver spiders has the potential to be used as muco-adhesive polymers due to their adhesive properties, for instance in high humidity conditions or situations
[17]. The primary sequence of aggregate silk proteins lacks DOPA that strengthens adhesion
[17]. Yet, the Dahlquist criteria for adhesives define robust adhesion to have Young’s modulus lower than 100 kPa; atomic force microscopy measurements suggest an average Young’s modulus of 70 ± 47 kPa for aggregate silk glue
[17]. The strong adhesion and elasticity of this silk-based glue might lie in the structural hierarchy, a high percentage of charged amino acid, and motif structure enabling mobility needed for mucosal swelling and interaction
[17]. The composition, structure, and environment-dependent behavior of aggregate silk could be useful as a “silk-based
bio-mimetic glue”.
3.9. Pyriform Silk
Similar to aggregate silk, pyriform silk is a natural polymer that is inspired by spiders
[38]. Although not specifically studied in the context of muco-adhesion, its properties render pyriform silk a potential muco-adhesive polymer. Spider threads have diverse mechanical properties that are useful biologically
[38]. According to Wolff et al., pyriform spins into attachment discs, which are utilized to anchor silken threads to substrates, and is a biodegradable, biocompatible, and versatile polymer
[39]. In addition, spiders produce silk as solid fiber with high polar content and fibrous cement components with charged amino acids similar to muco-adhesives
[17]. For example, Blasingame et al. reported that the Pyriform Spidroin 1 of black widow spiders as a gene, enabled anchoring silk fibers in attachment discs to solid substrates
[38]. Another gene—Pyriform Spidroin 2—was noted in orb-weaving attachment discs by Geurts et al. to spin synthetic spider silk fibers into a viscous liquid that rapidly solidifies and glues these fibers to substrates
[40]. The cement part that spiders yield can serve as a visco-elastic fluid that can be used to provide the high contact area needed for hydrogen bonding and adhesive strength
[17][39]. Indeed, pyriform is a versatile natural polymer that is biodegradable and biocompatible
[39]. Wolff et al. found that the anisotropy and “hierarchical organization” of pyriform silk enabled the silk bio-adhesives to uniquely enhance adhesion strength
[39]. In addition, the adhesion strength of the attachment discs can be modulated by spinneret movements macroscopically
[39]. Pyriform polymerizes under ambient conditions that are functional in <1 s, and is stable for years
[39].
3.10. Silkworm
Silkworms are more accessible to commercialize, because they can be formed, unlike spiders
[17]. Silkworms produce a fiber with a fibroin core and sericin coating
[17]. Silkworm fibroin was shown to be adhesive as a pH-sensitive hydrogel through electro-gelation, or e-gels
[17]. The strength of the e-gels, inspired by silkworms, gives their potential to be mucoadhesive
[17]. Serban et al. synthesized solubilized silkworm fibroin with polyethylene glycol, which had strong adhesive properties
[41]. This new class of blend with silk fibroin and polyethylene glycol is cytocompatible, crosslinks within seconds, and can potentially stabilize through β-sheet silk formation
[41]. Silk-polyethylene glycol sealants have comparable or better adhesive strength, lower swelling, and longer degradation times compared to polyethylene glycol sealants alone
[41]. Kundu et al. designed a muco-adhesive film by combining silk fibroin with hydroxy propyl methyl cellulose and with poly ethylene glycol 400 for transmucosal drug delivery
[42]. These fabricated stable muco-adhesive films were used as a vehicle for trans-mucosal delivery
[42]. Increase in fibroin content of the films enhanced mechanical properties, ex vivo bio-adhesive strength, water stability, degree of swelling, and stability of films in simulated saliva needed for fast muco-adhesion
[42]. Wei et al. bio-mimicked the silkworm spinning process by applying bio-inspired dry spinning equipment to spin regenerated silk fibroin fibers from aqueous solutions in air
[43]. Spinning dopes with pH levels of 5.2 to 6.9 exhibited high spinnability
[43]. Dry spinning experiments resulted in a breaking strength of 46 MPa under optimal conditions and could be increased to 359 MPa after being drawn into 80 vol.% ethanol aqueous solution
[43]. In another study, Lou et al. fabricated bio-inspired microfluidic concentrators based on the photolithography process
[44]. Silk glands and spinning ducts of silkworms were bio-mimicked to design microchips for regenerated silk fibroin aqueous solution
[44]. The microfluidic channel and silkworm enriched the regenerated silk fibroin concentration
[44]. Further, Kundu et al. prepared fibroin cryo-gels inspired by muga silkworm
Antheraea assamensis using 2 fabrication temperatures for liver tissue engineering. The cryo-gels could support the viability of the human hepatocarcinoma cells in Live/Dead assays
[45].
3.11. Sericin
Inspired by silkworm silk, sericin can be blended with polymers like sodium alginate and polyvinyl alcohol to contribute to drug delivery
[46][47]. Although sericin is adhesive without the fibroin core produced in the silkworm fiber, conjugation of sericin with other polymers stabilizes the structure and minimizes immunogenicity
[46][48]. Blending sericin with alginate has been used as a natural, biodegradable mucoadhesive polymer matrix for drug delivery
[46][48]. Wang et al. explored sericin in the context of tissue engineering by fabricating and characterizing covalently crosslinked 3-D natural silk protein sericin hydrogel to deliver cells and drugs
[49]. This hydrogel is injectable, enabling minimally invasive implantations, and is photoluminescent, hence, useful for bio-imaging and in vivo tracking
[49]. Its cell-adhesiveness makes it effective for promoting cell attachment and proliferation
[49]. The hydrogel has high porosity, elasticity, and pH-dependent dynamics which are useful in drug delivery
[49]. Moreover, Jin et al. designed 3-D silk fibroin hydrogel via a bio-inspired mineralization approach of hydroxyapatite for bone tissue engineering
[50]. Incorporation of calcium within the hydrogel regulated orientation growth, and the concentration of calcium modulated mineralization
[50]. The compressive strength of the mineralized hydrogel correlated with the hydrogel mineral content
[50].
3.12. Caddisfly Silk
Caddisfly silk is a strong adhesive with force stemming from post-translational phosphorylation of serine
[51][52]. Inspired by aquatic caddisworm silk, the viscous natural polymer serves as an underwater cement that adheres to hard surfaces
[17][52]. Caddisfly silk has the potential to be muco-adhesive due to its strong force underwater
[17][52]. Stewart et al. adapted caddisfly larval silks to aquatic habitats by phosphorylating H-Fibroin serines
[53]. The atmosphere of silk proteins could help assemble silk fibers via electrostatic associations of arginine-rich phosphorylated blocks
[53]. The phosphorylated serine in caddisfly larval silk proteins may assist in the periodic sub-structure through calcium cross bridging
[53]. Further, Lane et al. mimicked the toughening mechanism of aquatic caddisworm silk by developing a synthetic phosphate-graft-methacrylate prepolymer
[52]. Viscous unfolding of ion crosslinks at critical stress that dissipates energy increases toughness
[52]. The toughness of the bio-inspired hydrogel was greater than that of cartilage or meniscus
[52]. In another study, Ashton et al. evaluated self-tensioning aquatic caddisfly silk
[51]. FT-IR spectroscopy demonstrated that native silk has a conformation of random coils, β-sheets, and turns
[51]. Replacing multivalent ions with sodium EDTA impacted fiber mechanics and made conformational changes
[51]. However, the effects of EDTA could be reversed by restoring calcium
[51]. Notably, molecular dynamic simulations were used to create a hypothetical structure
[51]. Wang et al. identified a new bio-adhesive silk filament protein spun by Caddisfly Larvae. The protein is 98 kDa with cysteine having the largest amino acid percentage
[54].
3.13. TAPE
Kim et al. produced a medical adhesive via inter-molecular hydrogen bonding between tannic acid and polyethylene glycol (PEG)
[55]. Beyond mixing these two compounds, TAPE does not require any other chemical synthetic procedure to be formed, interestingly
[55]. TAPE had substantially higher adhesion strength than fibrin glue, can be maintained in aqueous environments, and demonstrates effective hemostatic behavior
[55]. This natural polymer is inspired by tannic acid, which is a degradable polyphenol compound found across almost all plant species
[55]. For example, Shin et al. demonstrated that tannic acid, which is rich with pyrogallol, formulated with PEG, also known as TAPE, is muco-adhesive
[56]. Keeping TAPE on esophageal mucosal layers for several hours in vivo resulted in inter-molecular interactions between the polyphenols of tannic acids and mucin that were dependent on pH and that had higher adherence in neutral conditions compared to acidic conditions
[56].
3.14. Edible Bird’s Nest
Protein pepsin-trypsin hydrolysates found in edible bird’s nests (EBN) contain anti-oxidant peptides
[57]. Ghassem et al. identified two of these pentapeptides that are natural anti-oxidants and that have the potential to serve as nutraceutical compounds
[57]. Pentapeptide Pro-Phe-His-Pro-Tyr corresponds to f134–138 of cytochrome b of the swiftlet species
Aerodramus fuciphagus, Leu-Leu-Gly-Asp-Pro to f164–168
[57]. These peptides exhibited resistance against gastrointestinal proteases, lacked cytotoxicity in vitro in human lung cells, and prevented human liver cell damage by hydroxyl radicals
[57]. Further, Jessel et al. employed computational and experimental techniques to understand the structural biology of swiftlet nests on vertical rock walls with threaded saliva
[58]. The team generated numerical models of the nests loaded with bird and egg forces using µCT scans to evaluate stress distribution
[58]. Macro-and micro-scale structural patterns were the same across nests, indicating that the construction is managed by design principles.
[58] The response to the bird and egg loads indicated a mechanical overdesign strategy that guarantees that the stress is minimized compared to the tensile fracture strength of the material
[58]. This mechanical overdesign suggests a biological strategy to maintain resilient material constructions that protect the eggs
[58]. Moreover, a patent by Valles-Ayoub et al. makes a claim on a nutritional supplement with acetylated neuraminate and/or a compound selected from N-acetyl mannosamine, with the invention enabling serum and cellular N-acetyl mannosamine contents to increase
[59]. EBN has a high content of natural acetylated neuraminate
[59]. The patent also claims regarding controlled release from muco-adhesive polymers
[59].
Perspective
Muco-adhesion is an attractive pharmaceutical and user-friendly strategy to prolong the residency time and the contact between membranes and formulations, thereby facilitating a sustained and controlled drug delivery and reducing the frequency of administration. Indeed, the mechanisms, processes, theories and properties of muco-adhesion and, more particularly, the muco-adhesive bio-polymers, have gained increasing interest in these last decades, leading to novel solutions, devices, commercialized products, and by default, enhanced investments in research, development and innovation. Overall, muco-adhesive drug delivery systems demonstrate great potential in improving the bio-availability and increasing the therapeutic effects of drugs. The buccal (trans-buccal) delivery route, in specific, has been the most commonly-investigated to maximize drug deposition and retention time. Recent advances focus on further improving some of the limitations, including, permeability, stability at the residence site, drug loading enhancement, and muco-adhesion strength increase. Furthermore, it is noteworthy that drug delivery strategies, herein, are trending more towards the use or incorporation of nanoparticles and/or malleable hydrogels and/or multi-compartmental thin-films, for better pharmaco-kinetics and -dynamics112-114. Researchers seem to be also turning attention or preference towards the next-generation of biocompatible polymers, bio-inspired and biomimetic polymers115, that besides their muco-adhesive properties, may possess or provide novel/desired features that may further enhance the drug retention rate/bio-availability, controlled biodegradability, predictable performance and behavior in situ, or even the drug loading capacity and encapsulation efficacy, as such parameters would greatly impact the pursued release kinetic profiles and dose-response curves112-114. Furthermore, some potential muco-adhesive systems are being explored with different synthetic rate-controlling agents such as poly (acrylic acid)-based polymers. Polymer manipulation is essential for controlling the absorption rate and bio-availability of drugs, which may lead to promote advanced treatment approaches and superior therapeutic outcomes.