Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ekaterina N Baranova | -- | 2438 | 2022-12-22 16:04:47 | | | |
| 2 | Camila Xu | -2 word(s) | 2436 | 2022-12-23 04:51:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shelepova, O.V.; Baranova, E.N.; Tkacheva, E.V.; Evdokimenkova, Y.B.; Ivanovskii, A.A.; Konovalova, L.N.; Gulevich, A.A. Aromatic Plants Metabolic Engineering. Encyclopedia. Available online: https://encyclopedia.pub/entry/39107 (accessed on 07 March 2026).
Shelepova OV, Baranova EN, Tkacheva EV, Evdokimenkova YB, Ivanovskii AA, Konovalova LN, et al. Aromatic Plants Metabolic Engineering. Encyclopedia. Available at: https://encyclopedia.pub/entry/39107. Accessed March 07, 2026.
Shelepova, Olga V., Ekaterina N. Baranova, Ekaterina V. Tkacheva, Yulia B. Evdokimenkova, Aleksandr A. Ivanovskii, Ludmila N. Konovalova, Alexander A. Gulevich. "Aromatic Plants Metabolic Engineering" Encyclopedia, https://encyclopedia.pub/entry/39107 (accessed March 07, 2026).
Shelepova, O.V., Baranova, E.N., Tkacheva, E.V., Evdokimenkova, Y.B., Ivanovskii, A.A., Konovalova, L.N., & Gulevich, A.A. (2022, December 22). Aromatic Plants Metabolic Engineering. In Encyclopedia. https://encyclopedia.pub/entry/39107
Shelepova, Olga V., et al. "Aromatic Plants Metabolic Engineering." Encyclopedia. Web. 22 December, 2022.
Copy Citation
Secondary metabolites of aromatic plants are used in many health applications as drugs, pheromones, insecticides, fragrances, and antioxidants. Due to the huge commercial demand for these secondary metabolites, the need to overcome the insufficient productivity of aromatic plants has become a significant challenge. Plant breeding is a traditional, labor-intensive, and limited method to improve the ability of aromatic plants to produce secondary metabolites.
essential oil
transgenic plants
metabolic engineering
plant protection
1. Introduction
Plants produce thousands of different terpenes and terpenoids, which are the largest and most structurally diverse classes of secondary metabolites. These compounds are present in the essential oils (EOs) produced by plants. EOs are obtained from plants by steam distillation or extraction with organic solvents [1]. However, most terpene compounds are present in plant tissues in limited quantities. Plant seeds, flowers, stems, and roots most often contain 0.1–10% EOs in fresh weight [2]. Approximately 3000 different plant species are known, from which various EOs have been isolated. EOs have been widely studied in only 300 plant species, and only approximately 20 plant species have been recognized as valuable for commercial use as sources of EOs that are used regularly and in large volume [3][4]. In nature, EOs play an important role in plant protection as antibacterial, antiviral, and antifungal agents and insecticides. At the same time, they protect plants from herbivores, reducing their appetite for such plants [5][6]. They can also attract certain insects by scattering pollen and seeds, while they repel others. In addition, they are known for their antiseptic, antiviral, fungicidal, and medicinal properties. EOs isolated from plants are also used in embalming and food preservation, and as antimicrobial, analgesic, sedative, anti-inflammatory, antispasmodic, and local anesthetic agents [7].
In most cases, EO compounds are formed only in certain plant tissues, such as inflorescences or seeds, which constitute a small percentage of the entire plant biomass in one harvesting season [8]. Therefore, the extraction of useful compounds from the EOs of aromatic plants can be an expensive procedure. Although the chemical synthesis of individual organic compounds is often cheaper, and the natural product has a small market share, consumer preference for natural essential oils over synthetic compounds is becoming increasingly widespread. This is mainly due to people’s opinions that natural EOs do not contain harmful industrial impurities and often have higher natural taste quality that cannot be achieved via the chemical synthesis of these compounds [9]. The EO industry generates billions of dollars in revenue each year, as they have a wide range of applications in various fields, such as pharmaceuticals, aromatherapy, healthcare, cosmetics, food flavoring, food preservation, and perfumery [10][11]. Therefore, there is a need to reduce the costs of natural products so that they become available to a wider range of consumers. From an ecological point of view, the production of useful compounds by non-chemical environmentally friendly methods is always the preferred and necessary alternative.
Modern biotechnological techniques make it possible to facilitate production and therefore reduce the market value of natural EOs through the use of various environmentally friendly methods. The development of genetic engineering has led to the development of large-scale biosynthesis of natural products, and advances in tissue culture of aromatic plants have opened up new avenues for the large-scale and high-efficiency production of desirable bioactive compounds. Plant tissue culture (including suspension cell cultures and “hairy root” cultures) is a promising alternative for the production of rare and high-value secondary metabolites to traditional approaches (e.g., harvesting wild plants), and is more cost-effective for mass production of plant-derived substances due to a number of advantages [12][13]. Firstly, such a bioprocess is completely independent of any seasonal and geographical conditions. Second, genetic modifications including gene overexpression, RNA interference, and the gene/genome editing due to CRISPR/Cas technique can be easily applied without encountering the regulatory barriers associated with plants growing in the field [14].
2. The Specifics of Biotechnology Application in Improving the Quality of Aromatic Plants
The use of biotechnological methods, of course, can significantly increase the production of EOs and improve their quality and the predictability of a given composition in specialized cells in aromatic plants, by changing or regulating the biogenesis of related compounds, introducing missing enzymes, creating optimal conditions for cultivation, or increasing the biomass of valuable plant organs. (Figure 1).
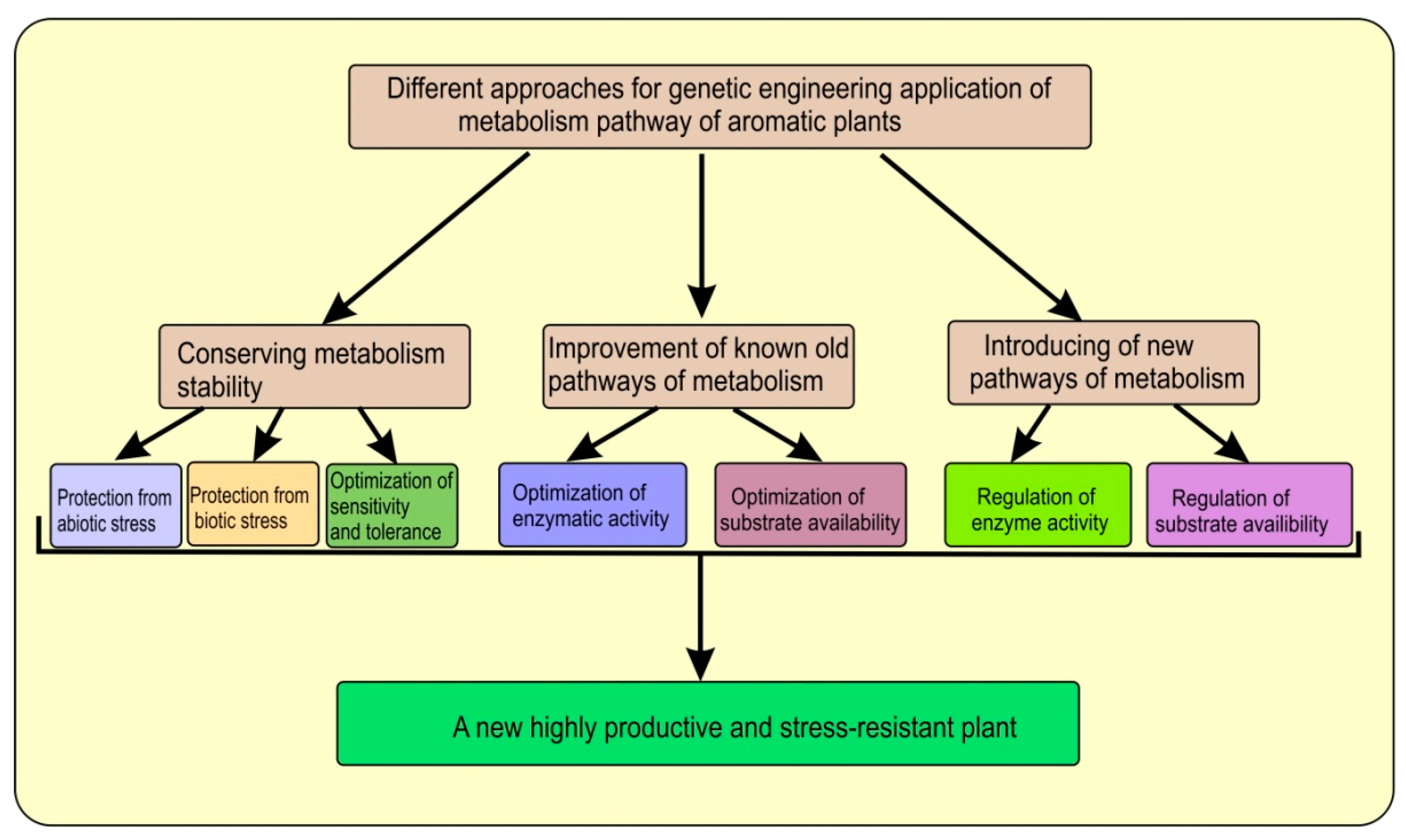
Figure 1. The scheme illustrates those physiological processes in aromatic plants that could be modified (improved) through the application of genetic engineering.
However, even those technologies that are currently well known and repeatedly utilized have significant limitations when dealing with aromatic plants. One such obstacle may be that a number of valuable aromatic plants are perennials and woody plants. This complicates the evaluation of newly modified plants, since the production of complex compounds, where various metabolic enzymes are involved in sequential stages, requires a rather long period [15]. In addition, in such plants, flowering occurs only when a certain stage of development is reached, and often flowers are the main source of EOs [16]. In this case, to assess the potential properties of such plants, the technology of grafting the obtained modified regenerants onto a rootstock of the same species can be used, followed by an assessment of the qualitative and quantitative yield of the product [17].
It is known that regenerants obtained in vitro are often tender plants, poorly adapted to ex vitro conditions, especially in the presence of difficulties with rhizogenesis; the regenerant is first grafted onto a stable root system in vitro, after which acclimatization and plant cultivation take place. Another significant problem when working with aromatic plants is their ability to accumulate a significant amount of phenols, the presence of which greatly complicates the purification of the product of interest. This creates the problem of limiting the availability of explants for transformation, since the protocols for the maceration, cultivation, and transformation of cells or tissues of such plants, produced from mature tissues, are problematic and inefficient [18]. Varietal specificity may even limit the possibility of obtaining the required modifications of especially valuable screeds of various cultivars. To solve such problems, various methods are used to achieve a higher yield of regenerated products using complex protocols for multicomponent nutrient media, a special lighting regime, drugs that reduce the rapid “aging” of cells and the excessive accumulation of harmful secondary metabolites that affect the production of regenerated shoots (roots), and the release of protoplasts, depending on the chosen method. The most commonly used are various phytohormones, synthetic regulators, osmotically active substances, altered temperature conditions (usually lower temperatures), amino acids, and peptides, as well as vitamins, activated charcoal, and antioxidants [19].
2.1. Specialized Metabolites of EOs and Their Biosynthesis
Today, terpenes or terpenoids, which are components of essential oils, represent a large and structurally diverse class of compounds, numbering more than 80,000 names [20]. The basic structures of all terpenes are synthesized as a result of two alternative and independent biosynthetic pathways located in different subcellular compartments from the universal five-carbon precursors, isopentenyl diphosphate (IPP) and its dimethylallyl diphosphate (DMAPP) all isomer [21]. The classic mevalonic acid pathway includes six enzymatic steps, starting with acetyl-CoA and ending with the formation of IPP. The MVA pathway is commonly known as the cytosolic one, where the enzymes are located in the cytosol [22]. The second pathway, which includes seven enzymatic steps, also results in the formation of IPP and DMAPP from pyruvate and glyceraldehyde-3-phosphate in plastids [21]. It is known that the enzymes of the MEP pathway are encoded by the nuclear genome and imported into plastids, where certain types of terpenoids are produced [23].
Recent studies have shown that in addition to the classical synthesis of IPP and DMAPP using enzymes in cytosol (MVA pathway) and plastids (MEP pathway), their synthesis is possible in both locations. Plant genomes contain the isopentenyl phosphate kinase (IPK) gene, which expresses the IPK protein found in the cytoplasm, where it converts isopentenyl phosphate (IP) and possibly dimethyl allyl phosphate (DMAP) into IPP and DMAPP via ATP-dependent phosphorylation [24].
Studies conducted in the last few years in the field of genomics and metabolomics were reflected in a series of review papers [25][26][27][28][29]. Some studies have shown the presence of non-canonical pathways and gene clusters involved in the formation of precursors, as well as the following terpenoid compounds in certain plant species [28]. Regulatory factors and gene clusters involved in the biosynthesis of specialized terpenoids in various plant species have been identified. It has been shown that the subcellular localization of the precursors pool and introduced enzymes are the crucial factors for increasing the specific terpenoid production in plants [29]. Additionally, if at present, the enhanced synthesis of a particular terpene compound was due to the overexpression of a single introduced gene, then future studies may focus on the delivery of several genes comprising several stages in the metabolic pathway of the biosynthesis of the target compound (Figure 2). Further understanding of the fine regulation of already known and lesser understood biosynthetic pathways, identification of new genes or gene clusters in selected species of industrially important aromatic plants, may lead to the production of commercially significant amounts of valuable terpene compounds in host plants.
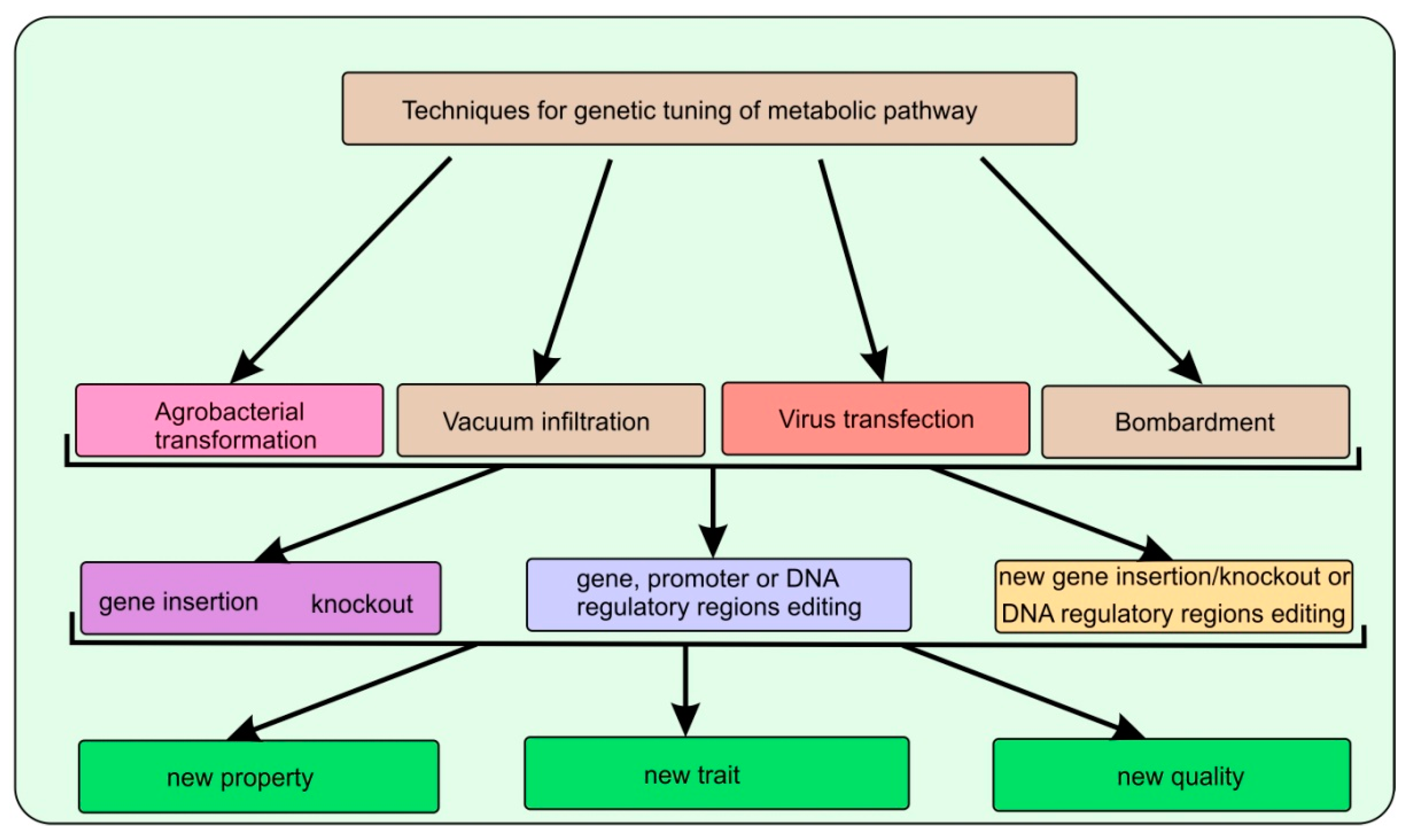
Figure 2. The scheme illustrates the genetic engineering methods used to modify aromatic plants: methods for delivering genetic material into the germplasm of plant cells; molecular genetic targets exposed to directed action; and the end result to be achieved.
Currently, there are only a few examples of the production of specialized terpenoids in host plants due to advances in metabolic engineering. Thus, the development of a pathway for the biosynthesis of menthol in peppermint (Mentha x piperita L.) made it possible, as a result of the suppression of the menthofuran synthase gene, to increase the yield of EO with a reduced amount of undesirable (+)-menthofuran in the MFS7A transgenic line [30]. Moreover, the overexpression of DXR led to an increase in the yield of EO in transgenic plants grown in a greenhouse by more than 50% at a low level of (+)-menthofuran (≤1.9% in EO) and (+)-pulegone (approximately 0.2% in essential oil) [31]. Field trials of these transgenic peppermint lines showed similar results in terms of EO yield and composition to those observed in greenhouse-grown lines [30]. Another example of the successful metabolic engineering of specialized terpenoids is the genetic engineering of sweet wormwood (Artemisia annua L.), in which the combined expression of the HMGR, FPPS, and DBR2 genes resulted in the more than three-fold higher accumulation of artemisinin [32].
However, so far, engineering with the introduction of foreign genes for the biosynthesis of specialized terpenoids using a transgenic approach has been successful in producing commercially advantageous amounts of specialized terpenoids in only a few plant species. It involves manipulation via the relevant pathways for the synthesis of many metabolites; although it requires great effort, it would allow us, in the future, to better understand both already known and less studied pathways and regulatory mechanisms, and to identify new genes or gene clusters involved in the biosynthesis of specialized terpenoids. The understanding of biosynthesis and its regulation can be used to develop homologous or heterologous transgenic systems to obtain higher amounts of commercially valuable terpenoids, as well as to increase plant yields and improve the quality of their EOs in industrial production.
2.2. Diseases of Aromatic Plants
Thus far, the focus has been on diseases affecting agricultural plants, and diseases affecting aromatic plants have been largely ignored. The cultivation of aromatic plants faces serious threats from various groups of phytopathogens (bacteria, fungi, viruses, phytoplasmas, nematodes), which reduce the yields of these crops and the quality of the crude materials obtained [33].
The main pests that cause serious damage to aromatic plants are various types of nematodes, they damage the roots of the host plant and reduce their productivity and yield [34][35][36][37]. Some of them are carriers of viruses for these plants, such as Arabis mosaic virus, Strawberry latent ring spot virus and Tobacco ring spot virus). Other pests also carry viruses. So, aphids are intermediate hosts of such viruses as Alfalfa mosaic virus, Cucumber mosaic virus and Mint vein banding associated virus, thrips carry Impatiens necrotic spot virus and Tomato spotted wilt virus, whitefly-Tomato leaf curl Pakistan virus, and unknown vectors-Tobacco mosaic virus and Lychnis ringspot virus [38][39]. Various fungal pathogenic invasions of these species are also observed in aromatic plants: Puccinia menthae (rust), Rhizoctonia solani (air rot), Rhizoctonia solani/bataticola (root and stolon rot), Verticillium dahliae (wilt), Phoma stasseri (stem rot), Alternaria alternata (leaf rot or leaf spot) and Erysiphe cischoracearum (powdery mildew) [40][41].
At present, the use of the possibilities of bioengineering of aromatic plants makes it possible to increase plant resistance to diseases [42][43]. For example, genes encoding proteins capable of degrading mycotoxins can be introduced into plants [44]. Plant protein baits that serve to capture pathogens can be modified to avoid the specificity of pathogen recognition [45][46]. The mechanism of RNA interference to provide robust viral immunity by targeting the degradation of viral RNA can also be used [47]. Natural or engineered immune receptors that recognize different pathogen strains can be introduced singly or in combination to provide reliable resistance to broad-spectrum diseases [48].
Phytoplasmas are a fairly large group of pathogens of aromatic plants. They cause changes in the amount and composition of secondary metabolites in diseased plants, which greatly affects the concentration of valuable phytochemicals [49][50]. Thus, in St. John’s wort (Hypericum perforatum) infected with phytoplasma 16SrVII, the EO yield significantly decreased (0.11 vs. 0.75% in healthy plants), and the content of sesquiterpenes increased in their composition, while the content of monoterpene hydrocarbons (and aliphatic compounds) decreased [50]. As one of the mechanisms by which phytoplasma spreads, several proteins secreted by phytoplasma in host plants have been identified and named as effector molecules, namely SAP54, SAP11, TENGU, SAP21, etc., which ensure its colonization and survival in the host plant [51]. Recent molecular studies of phytoplasma effector proteins and host plant microRNAs have shed light on the complex mechanisms underlying the development of phytoplasma infection symptoms. TENGU-induced dwarfism and infertility in plants are associated with the altered biosynthesis of auxin and jasmonic acid. SAP 54 plays a significant negative role in the degradation of transcription factors involved in flower development. The role of another effector molecule, SAP11, is manifested in the proliferation of axillary shoots and symptoms of hairy root [51]. Although the mechanisms by which phytoplasma infections spread remain a complex issue, studying the processes involved will provide a platform for developing measures to control phytoplasma-related diseases.
Despite the fact that biotic stress entails an increase in the production of secondary metabolic products, since they perform protective functions in plant organisms, the penetration of pathogens into the host plant is nevertheless a major obstacle to obtaining high-quality products. The development of the resistance of the main plants to unfavorable conditions of cultivation, and to diseases and pests, also makes it possible to increase the yield of the final product and increase their commercial value.
References
- Paul, S.; El Bethel Lalthavel Hmar, J.H.; Zothantluanga, H.K.S. Essential oils: A review on their salient biological activities and major delivery strategies. J. Mizo Acad. Sci. 2020, 20, 54–71.
- Gounaris, Y. Biotechnology for the production of essential oils, flavours and volatile isolates. A Review. Flav. Fragr. J. 2010, 25, 367–386.
- Inoue, M.; Craker, L.E. Medicinal and aromatic plants-Uses and functions. In Horticulture: Plants for People and Places; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 645–669.
- Patel, D.K. Medicinal and Aromatic Plants: Role in Human Society. Med. Aromat. Plants 2016, 5, 3.
- Gutensohn, M.; Nagegowda, D.A.; Dudareva, N. Involvement of compartmentalization in monoterpene and sesquiterpene biosynthesis in plants. In Isoprenoid Synthesis in Plants and Microorganisms: New Concepts and Experimental Approaches; Bach, T.J., Rohmer, M., Eds.; Springer: New York, NY, USA, 2013; pp. 155–169.
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571.
- Pandey, A.K.; Kumar, P.; Saxena, M.J.; Maurya, P. Distribution of aromatic plants in the world and their properties. In Feed Additives; Academic Press: Cambridge, MA, US, 2020; pp. 89–114.
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264.
- Schmidt, E. Production of essential oils. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2020; pp. 125–160.
- Aziz, Z.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Ashraf, G.M. Essential oils: Extraction techniques, pharmaceutical and therapeutic potential-a review. Curr. Drug Metab. 2018, 19, 1100–1110.
- Campêlo, M.C.S.; Medeiros, J.M.S.; Silva, J.B.A. Natural products in food preservation. Int. Food Res. J. 2019, 26, 130.
- Di Gioia, D.; Luziatelli, F.; Negroni, A.; Ficca, A.G.; Fava, F.; Ruzzi, M. Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J. Biotechnol. 2011, 156, 309–316.
- Lubbers, R.J.; Dilokpimol, A.; Visser, J.; Mäkelä, M.R.; Hildén, K.S.; de Vries, R.P. A comparison between the homocyclic aromatic metabolic pathways from plant-derived compounds by bacteria and fungi. Biotechnol. Adv. 2019, 37, 107396.
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Georgiev, M.I.; Sieniawska, E. Innovative approaches for recovery of phytoconstituents from medicinal/aromatic plants and biotechnological production. Molecules 2020, 25, 309.
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293.
- Duarte, M.C.T.; Duarte, R.M.T.; Rodrigues, R.A.F.; Rodrigues, M.V.N. Essential oils and their characteristics. Essent. Oils Food Process. Chem. Saf. Appl. 2018, 1, 1–19.
- Belide, S.; Hac, L.; Singh, S.P.; Green, A.G.; Wood, C.C. Agrobacterium-mediated transformation of safflower and the efficient recovery of transgenic plants via grafting. Plant Methods 2011, 7, 1–13.
- Ahmad, I.; Hussain, T.; Ashraf, I.; Nafees, M.; Maryam, R.M.; Iqbal, M. Lethal effects of secondary metabolites on plant tissue culture. Am. Eurasian J. Agric. Environ. Sci. 2013, 13, 539–547.
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451.
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648.
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106.
- Liao, P.; Wang, H.; Hemmerlin, A.; Nagegowda, D.A.; Bach, T.J.; Wang, M.; Chye, M.L. Past achievements, current status and future perspectives of studies on 3-hydroxy-3-methylglutaryl-CoA synthase (HMGS) in the mevalonate (MVA) pathway. Plant Cell Rep. 2014, 33, 1005–1022.
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP Pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700.
- Henry, L.K.; Gutensohn, M.; Thomas, S.T.; Noel, J.P.; Dudareva, N. Orthologs of the archaeal isopentenyl phosphate kinase regulate terpenoid production in plants. Proc. Natl. Acad. Sci. USA 2015, 112, 10050–10055.
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973.
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229.
- Bian, G.; Deng, Z.; Liu, T. Strategies for terpenoid overproduction and new terpenoid discovery. Curr. Opin. Biotechnol. 2017, 48, 234–241.
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457.
- Mani, V.; Park, S.; Kim, J.A.; Lee, S.I.; Lee, K. Metabolic perturbation and synthetic biology strategies for plant terpenoid production—An updated overview. Plants 2021, 10, 2179.
- Fuchs, L.K.; Holland, A.H.; Ludlow, R.A.; Coates, R.J.; Armstrong, H.; Pickett, J.A.; Harwood, J.L.; Scofield, S. Genetic manipulation of biosynthetic pathways in mint. Front. Plant Sci. 2022, 13, 928178.
- Lange, B.M.; Mahmoud, S.S.; Wildung, M.R.; Turner, G.W.; Davis, E.M.; Lange, I.; Baker, R.C.; Boydston, R.A. Improving peppermint essential oil yield and composition by metabolic engineering, Proc. Natl. Acad. Sci. USA 2011, 108, 16944–16949.
- Malhotra, K.; Subramaniyan, M.; Rawat, K.; Kalamuddin, M.; Qureshi, M.I.; Malhotra, P.; Mohmmed, A.; Cornish, K.; Daniell, H.; Kumar, S. Compartmentalized metabolic engineering for artemisinin biosynthesis and effective malaria treatment by oral delivery of plant cells. Mol. Plant 2016, 9, 1464–1477.
- Elad, Y.; Pertot, I. Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 2014, 28, 99–139.
- Lisei-de-Sá, M.E.; Rodrigues-Silva, P.L.; Morgante, C.V.; de Melo, B.P.; Lourenço-Tessutti, I.T.; Arraes, F.B.M.; Sousa, J.P.A.; Galbieri, R.; Amorim, R.M.S.; de Lins, C.B.J.; et al. Pyramiding dsRNAs increases phytonematode tolerance in cotton plants. Planta 2021, 254, 1–14.
- Pandey, R. Diseases of medicinal and aromatic plants: Insights in nematode biomanagement. Indian Phytopathol. 2017, 70, 12–21.
- Saikia, S.K.; Tiwari, S.; Pandey, R. Rhizospheric biological weapons for growth enhancement and Meloidogyne incognita management in Withania somnifera cv. Poshita. Biol. Control 2013, 65, 225–234.
- Gupta, R.; Tiwari, S.; Saikia, S.K.; Shukla, V.; Singh, R.; Singh, S.P.; Ajay Kumar, P.V.; Pandey, R. Exploitation of microbes for enhancing bacoside content and reduction of Meloidogyne incognita infestation in Bacopa monnieri L. Protoplasma 2015, 252, 53–61.
- Singh, A.; Gupta, R.; Saikia, S.; Pant, A.; Pandey, R. Diseases of medicinal and aromatic plants, their biological impact and management. Plant Genet. Res. 2016, 14, 370–383.
- Saeed, S.T.; Samad, A. Emerging threats of begomoviruses to the cultivation of medicinal and aromatic crops and their management strategies. Virus Dis. 2017, 28, 1–17.
- Bhandari, S.; Harsh, N.S.K.; Sharma, A.K.; Mao, L.P.; Thakur, S. A database of diseases of medicinal plants in Uttarakhand. Ind. Forester 2014, 140, 518–527.
- Gatak, S.; Polley, S.K.; Ghosh, S.K.; Chakrabarty, N. Biological Control (In Vitro) of the pathogen causing leaf blight disease of mint (Mentha arvensis L.). Plant Cell Biotechnol. Mol. Biol. 2020, 21, 57–67.
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751.
- Cook, D.E.; Mesarich, C.H.; Thomma, B.P. Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 2015, 53, 541–563.
- Karlovsky, P. Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl. Microbiol. Biotechnol. 2011, 91, 491–504.
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392.
- Kim, S.H.; Qi, D.; Ashfield, T.; Helm, M.; Innes, R.W. Using decoys to expand the recognition specificity of a plant disease resistance protein. Science 2016, 351, 684–687.
- Rosa, C.; Kuo, Y.W.; Wuriyanghan, H.; Falk, B.W. RNA interference mechanisms and applications in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610.
- Fuchs, M. Pyramiding resistance-conferring gene sequences in crops. Curr. Opin. Virol. 2017, 26, 36–42.
- Bertaccini, A.; Duduk, B. Phytoplasma and phytoplasma diseases: A review of recent research. Phytopathol. Mediter. 2010, 48, 355–378.
- Marcone, C.; Bellardi, M.G.; Bertaccini, A. Phytoplasma diseases of medicinal and aromatic plants. J. Plant Pathol. 2016, 379–404.
- Lakhanpaul, S.; Singh, V.; Kumar, S.; Singh, A. Molecular Mechanism Underlying Symptom Development in Phytoplasma Associated Diseases-The Key Players and their Role. Indian J. Entomol. 2022, 1–10.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
23 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





