Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pimduen Rungsiyakull | -- | 2049 | 2022-12-22 00:35:31 | | | |
| 2 | Camila Xu | Meta information modification | 2049 | 2022-12-22 05:11:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chokaree, P.; Poovarodom, P.; Chaijareenont, P.; Yavirach, A.; Rungsiyakull, P. Materials Used for Customized Healing Abutment. Encyclopedia. Available online: https://encyclopedia.pub/entry/39060 (accessed on 12 March 2026).
Chokaree P, Poovarodom P, Chaijareenont P, Yavirach A, Rungsiyakull P. Materials Used for Customized Healing Abutment. Encyclopedia. Available at: https://encyclopedia.pub/entry/39060. Accessed March 12, 2026.
Chokaree, Parima, Pongsakorn Poovarodom, Pisaisit Chaijareenont, Apichai Yavirach, Pimduen Rungsiyakull. "Materials Used for Customized Healing Abutment" Encyclopedia, https://encyclopedia.pub/entry/39060 (accessed March 12, 2026).
Chokaree, P., Poovarodom, P., Chaijareenont, P., Yavirach, A., & Rungsiyakull, P. (2022, December 22). Materials Used for Customized Healing Abutment. In Encyclopedia. https://encyclopedia.pub/entry/39060
Chokaree, Parima, et al. "Materials Used for Customized Healing Abutment." Encyclopedia. Web. 22 December, 2022.
Copy Citation
A customized healing abutment is designed by modifying the size and transmucosal shape of the healing abutment to mimic the natural profile of an emerging tooth.
customized healing abutment
implant abutment
dental abutment
1. Introduction
Implant treatment nowadays has become a common modality. Several factors have been reported, related to implant success; one of them is the development and maintenance of healthy peri-implant soft tissue [1]. The traditional method involves implant placement with a submerged protocol, followed by second-stage surgery after the osseointegration period. This increases the complications from additional surgery. To avoid stage-two surgery, a flapless, non-submerged protocol was proposed with either a one-piece implant or a two-piece implant with the immediate connection of a transmucosal healing abutment [2]. A two-piece implant with a transmucosal smooth hyperbolic neck present platform-switch, and a smooth transmucosal neck protruding through the peri-implant soft tissue, was shown to reduce marginal bone loss in a 3-year prospective cohort study [2]. However, with this technique, the transmucosal contour could not be altered after the implant placement. Another method to avoid stage-two surgery and create peri-implant soft tissue is with an immediate connection of a transmucosal healing abutment. In the conventional method, a standard healing abutment is usually connected to the implant fixture during the second surgery. Based on the round circular shape of a standard healing abutment, the result is a round, unnatural-looking soft tissue profile [3]. Additional appointments might be required for further tissue conditioning via multiple gradual adjustments of the provisional restoration; otherwise, difficulties upon insertion of the final prostheses could lead to the patients’ discomfort or mechanical complications, such as screw-loosening due to the rebound force from the compressed peri-implant tissue [4]. Multiple disconnections and re-insertions of the provisional restoration can potentially compromise the healing process [4][5]. Therefore, some clinicians have suggested the utilization of customized healing abutments to provide a better emergence profile of the peri-implant tissues. A customized healing abutment is designed by modifying the size and transmucosal shape of the healing abutment to mimic the natural profile of an emerging tooth. Then, it is connected to the implant on the day of surgery and left undisturbed until osseointegration and tissue maturation are achieved. Customized healing abutments can be fabricated with different materials and techniques, depending on their clinical applications. The variations in the properties of dental materials nowadays, therefore, produce different effects on the peri-implant tissues. This narrative research aims to revise the materials used for customized healing abutments with their properties related to peri-implant tissue maturation and their clinical applications.
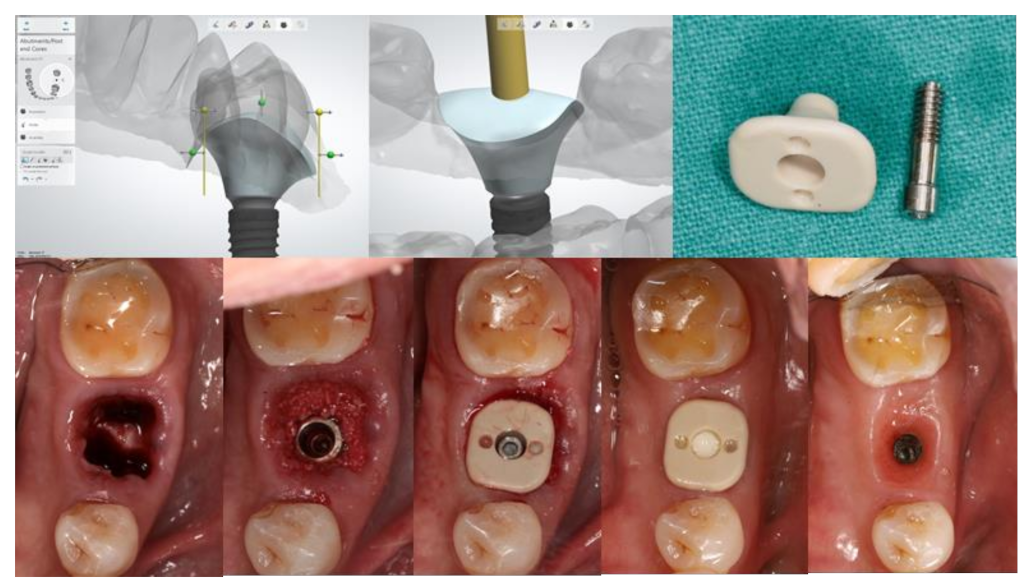
2. Customized Healing Abutment
An implant healing abutment serves two roles in dental implant treatment. The first is to promote the healing of the peri-implant soft and hard tissues during the healing phase, including the initiation of soft-tissue contouring. The second is to protect the implant site during the initial post-surgical healing stage from the accumulation of plaque or debris [6]. A healing abutment can be classified as a standard or customized healing abutment. A standard healing abutment from a manufacturer is usually prefabricated in a cylindrical, non-hex shape to allow ease of insertion in any direction [7]. The connection and tissue maturation result in a round peri-implant gingival emergence profile, which requires further gingival conditioning to shape the tissue into the desired form, unless there are difficulties upon the final prosthesis delivery, or mechanical complications such as abutment screw loosening [8][9]. In the esthetic zone, including the anterior and premolar teeth, the use of provisional restoration with a dynamic compression technique is widely used in both immediate and delayed placement [10][11][12][13][14][15]. However, immediate provisionalization must be restricted in cases where an occlusal load cannot be avoided. Immediate provisionalization, therefore, is rarely used in the posterior region, where occlusal forces may not be strictly eliminated. In such cases, a standard prefabricated healing abutment is connected and allowed peri-implant soft tissue to heal until sufficient osseointegration is achieved, which leads to a prolonged overall treatment duration. The idea of modifying the contour of the standard healing abutment was first described by Pow and McMillan [8]. With their technique, a standard healing abutment was modified by creating retentive grooves on its surface, followed by adding auto-polymerized poly (methyl methacrylate) (PMMA) resin to create a natural gingival profile for the final restoration, without the need for provisional restoration. A natural soft tissue profile was observed two weeks after the insertion. The researchers also mentioned that, with the use of this modified healing abutment, the custom abutment and final prosthesis can be easily delivered without soft tissue entrapment, and only minor discomfort without the use of local anesthesia was reported. The use of a custom-shaped healing abutment provides a proper soft tissue contour at the time of implant placement and also lacks occlusal contact (Figure 1).

Figure 1. Customized healing abutment was designed with an outline of the tooth shape with transmucosal contour to preserve soft tissue before being milled with PEEK Material.
Customized healing abutments can be fabricated from various materials commonly used in dentistry. Different types of materials contribute different properties. Since customized healing abutments are usually connected following implant placement, the differences in properties from each material can influence the peri-implant tissue healing process, as well as the tissue maturation [16].
3. Materials Used for Customized Healing Abutment
3.1. Materials Used for Customized Healing Abutment
The materials used for the fabrication of customized healing abutments are those commonly used in dentistry, including: Polyetheretherketone (PEEK) [17][18], Polymethyl methacrylate (PMMA) [19][20][21][22][23][24][25][26][27][28], zirconia [7], titanium [29], and resin composite [30]. They can be fabricated from monolithic materials or in combinations of these materials.
3.1.1. Polyetheretherketone (PEEK)
PEEK is a synthetic, tooth-colored thermoplastic polymer which belongs to the PAEK (polyaryletherketone) family [31]. PEEK presents superior physical, mechanical, and biological properties for biomedical applications, such as in orthopedics and dentistry [32]. The structure of PEEK contains repeated aromatic rings of the ether groups, which provide structural flexibility, and repeated ketone groups that provide rigidity [16]. PEEK gained popularity in dentistry as a substitution material in patients allergic to metal. PEEK can be used as a metal-free framework material for fixed and removable prostheses and various components in implant dentistry including implant fixtures, implant abutments, provisional abutments, and healing abutments; other applications include endocrowns and occlusal splints [33]. Although PEEK presents superior properties, the fabrication of customized PEEK healing abutments requires the utilization of CAD/CAM technology, thus limiting the chairside fabrication of pure PEEK customized healing abutments. Virtual designs using dental implant software allow monolithic PEEK customized healing abutments to be fabricated prior to implant surgery and be inserted after the implant placement [34]. As the chairside fabrication proceeds, a temporary PEEK cylinder can be used with a flowable composite to capture the outline of the tooth socket [35]. When customized healing abutments are fabricated from PEEK, they can be adjusted to fit the implant site by adding or reducing the contour intraorally [36]. When combined with the composite, the roughening of the PEEK surfaces increases the bond strength with the veneering resin [37].
3.1.2. Polymethyl methacrylate (PMMA)
Poly(methyl methacrylate) (PMMA) is the most commonly used polymer in dentistry. In general, PMMA polymer is prepared using a liquid methyl methacrylate (MMA) monomer along with cross-linking agents and inhibitors, and a pre-polymerized PMMA powder together with additives such as pigments and nylon or acrylic synthetic fibers [38][39]. The PMMA polymerization reaction occurs by the free radical addition and polymerization of methyl methacrylate (C5O2H8) to poly methyl methacrylate (C5O2H8)n [40]. Auto-polymerization or self-cured PMMA, is widely used in direct provisional restorations because of several advantages, including low cost, acceptable aesthetic qualities, good wear resistance, high polishability, color stability, and a good marginal fit with optimal transverse strength. Self-cured PMMA is a well-reported residual MMA monomer, which has the possibility to cause irritation in some patients [16][41]. The salivary environment can lead to the degradation of PMMA by increasing the diffusion of residual MMA monomer due to the polar properties from the immersed resin molecule [42]. Dimensional contraction and exothermic reaction during polymerization could also interfere with the oral status. Self-cured PMMA may present an ease of chairside manipulation but may limit the fabrication of customized healing abutments due to its inferior properties, including water degradation, low wear-resistance, and low fracture-resistance, which lead to possible crack formation and material fracture if an occlusal load is applied [40]. Recently, the benefit of CAD/CAM technology has allowed PMMA manufacture via rapid prototyping and milling techniques [43][44]. CAD/CAM PMMA demonstrated better mechanical properties compared to conventional heat-cured and self-cured PMMA [44][45][46][47]. Several articles reported the utilization of CAD/CAM PMMA as a customized healing abutment for socket closure following immediate and delayed implant placement [19][20][21][22][23][24][25][26][27][28].
3.1.3. Zirconia
Zirconia is a crystalline dioxide of zirconium which provides optimum properties in dentistry, including superior toughness, strength, fatigue resistance, excellent wear properties, and biocompatibility [48][49][50]. Dental zirconia generally refers to a modified yttria tetragonal zirconia polycrystal (Y-TZP). Yttria is added to stabilize the crystal structure transformation during firing at an elevated temperature, and to improve the physical properties of zirconia. The zirconia tetragonal-to-monoclinic phase transformation after exposure to stress is known as transformation toughening. During this zirconia phase transformation, the unit cell of monoclinic configuration occupies about 4% more volume than the tetragonal configuration, which is a relatively large volume change. This inhibits crack propagation, which lessens fractures and the failure of materials when used as customized healing abutments [51]. Zirconia can be used in fixed prostheses and also in implant components [52]. When zirconia was fabricated with CAD/CAM technology, the excessive machining, which can lead to tensile stresses on the material surface, was mentioned and may cause a direct impact on the material properties [53]. When zirconia customized healing abutments were fabricated, it was recommended to minimize the further adjustment of these abutments to reduce the propagation of this phenomenon [7]. Although zirconia provides several good properties, zirconia for customized healing abutments might not be popular because of its higher cost compared to other materials [7].
3.1.4. Resin Composite
Resin composite is one of the most commonly used dental materials for direct restoration. Dental resin composite typically comprises a mixture of dental resins and diverse inorganic fillers. Resins contain two or more monomers to achieve the desired mechanical properties [54]. The base monomers include bisphenol A glycidyl methacrylate (Bis-GMA), ethoxylated bisphenol A dimethacrylate (Bis-EMA), urethane dimethacrylate (UDMA), and cross-linking diluents to adjust the viscosity of the mixtures; triethylene glycol dimethacrylate (TEGDMA), decanediol dimethacrylate (D3MA), and 2-hydroxyethyl methacrylate (HEMA) are the most common compositions of dental resin composites. The reactive methacrylate groups polymerize through light-initiated curing that causes a chain-reaction polymerization followed by a cross-linking reaction. These processes are associated with the properties of resin composite, including the mechanical and physical properties. The fillers include silica-based particles, glass-ceramics, ceramics, metals, mineral particles, or polymer-based particles, which are dispersed in different concentrations in order to improve their properties [54][55]. Two types of resin composites have been used for the fabrication of customized healing abutments: flowable [35][56][57], and packable materials [30]. Studies have demonstrated the application of resin composite onto other materials, such as PEEK or titanium provisional abutments, to capture the outline of the extraction socket in immediate implant placement.
When resin composite bonds with other material, the cured composite provides a clear three-dimensional representation of the peri-implant soft tissue profile, facilitating an accurate transfer to the technical laboratory. Resin composite presents a low elastic modulus but high fracture resistance and tensile strength. Both ceramic and composite resin abutments have been shown to have a similar failure rate during in vitro accelerated fatigue testing [58][59], suggesting that resin composite could be used to fabricate healing abutments.
3.1.5. Titanium
Titanium is generally used in implant treatments. Commercially pure titanium (cp-Ti) and titanium alloy (Ti–6Al–4V) remain the most widely used materials for biomedical applications [60]. Titanium is used in alloys to fabricate dental implants due to its good mechanical properties, low density, and good bone-contact biocompatibility. Cp-Ti is available in four grades, numbered 1 to 4, according to the purity and the processing oxygen content [61]. The differences in composition demonstrate variations in corrosion resistance, ductility, and strength. The most widely used in dental implants is grade 4 cp-Ti due to its mechanical strength, and it contains the highest oxygen content (around 0.4%) [62]. Implant components such as fixtures, abutments, screws, and healing abutments can be fabricated with cp-Ti and its alloy due to their excellent biocompatibility, corrosion resistance, high strength, and low modulus of elasticity [63]. Customized healing abutments from titanium require the use of CAD/CAM implant software and have been reported in one study [29].
References
- Linkevicius, T.; Puisys, A.; Linkeviciene, L.; Peciuliene, V.; Schlee, M. Crestal Bone Stability around Implants with Horizontally Matching Connection after Soft Tissue Thickening: A Prospective Clinical Trial. Clin. Implant Dent. Relat. Res. 2015, 17, 497–508.
- Prati, C.; Zamparini, F.; Canullo, L.; Pirani, C.; Botticelli, D.; Gandolfi, M. Factors Affecting Soft and Hard Tissues Around Two-Piece Transmucosal Implants: A 3-Year Prospective Cohort Study. Int. J. Oral Maxillofac. Implant. 2020, 35, 1022–1036.
- Janakievski, J. Case report: Maintenance of gingival form following immediate implant placement—The custom-healing abutment. Adv. Esthet. Interdiscip. Dent. 2007, 3, 24–28.
- Elian, N.; Tabourian, G.; Jalbout, Z.N.; Classi, A.; Cho, S.-C.; Froum, S.; Tarnow, D.P. Accurate transfer of peri-implant soft tissue emergence profile from the provisional crown to the final prosthesis using an emergence profile cast. J. Esthet. Restor. Dent. 2007, 19, 306–314; discussion 315.
- Oh, K.C.; Kim, J.-H.; Woo, C.-W.; Moon, H.S. Accuracy of Customized Prefabricated Screw-Type Immediate Provisional Restorations after Single-Implant Placement. J. Clin. Med. 2019, 8, 490.
- Misch, C.E. Contemporary Implant Dentistry; Mosby: St. Louis, MO, USA, 1993.
- Weigl, P.; Trimpou, G.; Grizas, E.; Hess, P.; Nentwig, G.-H.; Lauer, H.-C.; Lorenz, J. All-ceramic versus titanium-based implant supported restorations: Preliminary 12-months results from a randomized controlled trial. J. Adv. Prosthodont. 2019, 11, 48–54.
- Pow, E.H.N.; McMillan, A.S. A modified implant healing abutment to optimize soft tissue contours: A case report. Implant Dent. 2004, 13, 297–300.
- Teślak, M.; Ziemlewski, A.; Foltyn, I.; Ordyniec-Kwaśnica, I.; Drogoszewska, B. Development of Custom Anatomic Healing Abutment Based on Cone-Beam Computer Tomography Measurement on Human Teeth Cross-Section. Materials 2021, 14, 4570.
- Lemongello, G.J. Customized provisional abutment and provisional restoration for an immediately-placed implant. Pract. Proced. Aesthet. Dent. 2007, 19, 419–424; quiz 426.
- Wittneben, J.-G.; Buser, D.; Brägger, U.; Belser, U.C. Peri-implant soft tissue conditioning with provisional restorations in the esthetic zone: The dynamic compression technique. Int. J. Periodontics Restor. Dent. 2013, 33, 447–455.
- Kan, J.Y.K.; Rungcharassaeng, K.; Lozada, J.L. Immediate placement and provisionalization of maxillary anterior single implants: 1-year prospective study. Int. J. Oral Maxillofac. Implant. 2003, 18, 31–39.
- Lorenzoni, M.; Pertl, C.; Zhang, K.; Wimmer, G.; Wegscheider, W.A. Immediate loading of single-tooth implants in the anterior maxilla. Preliminary results after one year. Clin. Oral Implant. Res. 2003, 14, 180–187.
- Meng, H.-W.; Chien, E.Y.; Chien, H.-H. Immediate Implant Placement and Provisionalization in the Esthetic Zone: A 6.5-Year Follow-Up and Literature Review. Case Rep. Dent. 2021, 2021, 4290193.
- Block, M.S.; Mercante, D.E.; Lirette, D.; Mohamed, W.; Ryser, M.; Castellon, P. Prospective evaluation of immediate and delayed provisional single tooth restorations. J. Oral Maxillofac. Surg. 2009, 67 (Suppl. 11), 89–107.
- Ionescu, R.N.; Totan, A.R.; Imre, M.M.; Țâncu, A.M.C.; Pantea, M.; Butucescu, M.; Farcașiu, A.T. Prosthetic Materials Used for Implant-Supported Restorations and Their Biochemical Oral Interactions: A Narrative Review. Materials 2022, 15, 1016.
- Lepidi, L.; Galli, M.; Suriano, C.; Ruggiero, G.; Calabrese, L.; Li, J.; Venezia, P. Digital planning of a customized CAD-CAM healing abutment for soft tissue conditioning at the time of implant placement: A different digital and clinical perspective in molar implant rehabilitation. J. Osseointegr. 2022, 13 (Suppl. 4), S305–S310.
- Lilet, R.; Desiron, M.; Finelle, G.; Lecloux, G.; Seidel, L.; Lambert, F. Immediate implant placement combining socket seal abutment and peri-implant socket filling: A prospective case series. Clin. Oral Implant. Res. 2022, 33, 33–44.
- Wang, L.; Wang, T.; Lu, Y.; Fan, Z. Comparing the Clinical Outcome of Peri-implant Hard and Soft Tissue Treated with Immediate Individualized CAD/CAM Healing Abutments and Conventional Healing Abutments for Single-Tooth Implants in Esthetic Areas Over 12 Months: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Implant. 2021, 36, 977–984.
- Raheem, I.M.A.; Hammad, I.A.; Kader, S.H.A.; Fahmy, R.A. Fabrication of a CAD-CAM custom healing abutment guided by a conventional dental radiograph for delayed loaded dental implants: A dental technique. J. Prosthet. Dent. 2022, 127, 49–54.
- Proussaefs, P. Use of CAD/CAM Healing Abutment Immediately After Dental Implant Placement for the Non-Esthetic Zone: A Guided Soft Tissue Healing Technique. J. Oral Implant. 2016, 42, 189–193.
- Finelle, G.; Sanz-Martín, I.; Knafo, B.; Figué, M.; Popelut, A. Digitalized CAD/CAM protocol for the fabrication of customized sealing socket healing abutments in immediate implants in molar sites. Int. J. Comput. Dent. 2019, 22, 187–204.
- Finelle, G.; Lee, S. Guided Immediate Implant Placement with Wound Closure by Computer-Aided Design/Computer-Assisted Manufacture Sealing Socket Abutment: Case Report. Int. J. Oral Maxillofac. Implant. 2017, 32, e63–e67.
- Finelle, G.; Popelut, A.; Knafo, B.; Martín, I. Sealing Socket Abutments (SSAs) in Molar Immediate Implants with a Digitalized CAD/CAM Protocol: Soft Tissue Contour Changes and Radiographic Outcomes After 2 Years. Int. J. Periodontics Restor. Dent. 2021, 41, 235–244.
- Hartman, M.J. A Workflow to Design and Fabricate a Customized Healing Abutment From a Dynamic Navigation Virtual Treatment Plan. Compend. Contin. Educ. Dent. 2021, 42, 86–92.
- Joda, T.; Ferrari, M.; Braegger, U. A digital approach for one-step formation of the supra-implant emergence profile with an individualized CAD/CAM healing abutment. J. Prosthodont. Res. 2016, 60, 220–223.
- Proussaefs, P. Custom CAD-CAM healing abutment and impression coping milled from a poly(methyl methacrylate) block and bonded to a titanium insert. J. Prosthet. Dent. 2016, 116, 657–662.
- Junior, D.; Machado, A. Rehabilitation of Anterior Tooth Loss with Immediate Implant and Preservation of Gingival Aesthetics with Use of Vertical Extraction System and Customized Healing Abutment made by CAD-CAM Technology: Case Report. SVOA Dent. 2021, 2, 232–237.
- Alshhrani, W.M.; Al Amri, M.D. Customized CAD-CAM healing abutment for delayed loaded implants. J. Prosthet. Dent. 2016, 116, 176–179.
- Akin, R. A New Concept in Maintaining the Emergence Profile in Immediate Posterior Implant Placement: The Anatomic Harmony Abutment. J. Oral Maxillofac. Surg. 2016, 74, 2385–2392.
- Suphangul, S.; Rokaya, D.; Kanchanasobhana, C.; Rungsiyakull, P.; Chaijareenont, P. PEEK Biomaterial in Long-Term Provisional Implant Restorations: A Review. J. Funct. Biomater. 2022, 13, 33.
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869.
- Papathanasiou, I.; Kamposiora, P.; Papavasiliou, G.; Ferrari, M. The use of PEEK in digital prosthodontics: A narrative review. BMC Oral Health 2020, 20, 217.
- Beretta, M.; Poli, P.P.; Pieriboni, S.; Tansella, S.; Manfredini, M.; Cicciù, M.; Maiorana, C. Peri-Implant Soft Tissue Conditioning by Means of Customized Healing Abutment: A Randomized Controlled Clinical Trial. Materials 2019, 12, 3041.
- Ruales-Carrera, E.; Pauletto, P.; Apaza-Bedoya, K.; Volpato, C.A.M.; Özcan, M.; Benfatti, C.A.M. Peri-implant tissue management after immediate implant placement using a customized healing abutment. J. Esthet. Restor. Dent. 2019, 31, 533–541.
- Bezerra, F.J.B.; Araujo, F.M.; De Oliveira, G.J.P.L.; Ghiraldini, B. Clinical application of the customizable PEEK healing abutment. A case report. J. Multidiscip. Dent. 2020, 10, 93–96.
- Sarfaraz, H.; Rasheed, M.N.; Shetty, S.S.; Prabhu, U.M.; Fernandes, K.; Mohandas, S. Comparison of the Bond Strength of Composite Resin to Zirconia and Composite Resin to Polyether Ether Ketone: An In Vitro Study. J. Pharm. Bioallied Sci. 2020, 12 (Suppl. 1), S504–S509.
- Hassan, M.; Asghar, M.; UdDin, S.; Zafar, M.S. Chapter 8—Thermoset Polymethacrylate-Based Materials for Dental Applications; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–308.
- Ronald, L.; Sakaguchi, J.L.F.; John, M. Powers, Craig’s Restorative Dental Materials, 14th ed.; Elsevier: St. Louis, MO, USA, 2019.
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299.
- Jorge, J.H.; Giampaolo, E.T.; Machado, A.L.; Vergani, C.E. Cytotoxicity of denture base acrylic resins: A literature review. J. Prosthet. Dent. 2003, 90, 190–193.
- de Sá, J.; Vieira, F.; Aroso, C.M.; Cardoso, M.; Mendes, J.M.; Silva, A.S. The Influence of Saliva pH on the Fracture Resistance of Three Complete Denture Base Acrylic Resins. Int. J. Dent. 2020, 2020, 8941876.
- Srinivasan, M.; Gjengedal, H.; Cattani-Lorente, M.; Moussa, M.; Durual, S.; Schimmel, M.; Müller, F. CAD/CAM milled complete removable dental prostheses: An in vitro evaluation of biocompatibility, mechanical properties, and surface roughness. Dent. Mater. J. 2018, 37, 526–533.
- Kalberer, N.; Mehl, A.; Schimmel, M.; Müller, F.; Srinivasan, M. CAD-CAM milled versus rapidly prototyped (3D-printed) complete dentures: An in vitro evaluation of trueness. J. Prosthet. Dent. 2019, 121, 637–643.
- Alp, G.; Murat, S.; Yilmaz, B. Comparison of Flexural Strength of Different CAD/CAM PMMA-Based Polymers. J. Prosthodont. 2018, 28, e491–e495.
- Arslan, M.; Murat, S.; Alp, G.; Zaimoglu, A. Evaluation of flexural strength and surface properties of prepolymerized CAD/CAM PMMA-based polymers used for digital 3D complete dentures. Int. J. Comput. Dent. 2018, 21, 31–40.
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J.; Özcan, M. A Comparison of the Surface Properties of CAD/CAM and Conventional Polymethylmethacrylate (PMMA). J. Prosthodont. 2019, 28, 452–457.
- Della Bona, A.; Pecho, O.E.; Alessandretti, R. Zirconia as a Dental Biomaterial. Materials 2015, 8, 4978–4991.
- Jitwirachot, K.; Rungsiyakull, P.; Holloway, J.A.; Jia-Mahasap, W. Wear Behavior of Different Generations of Zirconia: Present Literature. Int. J. Dent. 2022, 2022, 9341616.
- Jia-Mahasap, W.; Jitwirachot, K.; Holloway, J.A.; Rangsri, W.; Rungsiyakull, P. Wear of various restorative materials against 5Y-ZP zirconia. J. Prosthet. Dent. 2022, 128, e1–e814.
- Bona, A.D. Bonding to Ceramics: Scientific Evidences for Clinical Dentistry; Editoria Artes Medicas Ltda: São Paulo, Brazil, 2009.
- Nistor, L.; Grădinaru, M.; Rîcă, R.; Mărășescu, P.; Stan, M.; Manolea, H.; Ionescu, A.; Moraru, I. Zirconia Use in Dentistry-Manufacturing and Properties. Curr. Health Sci. J. 2019, 45, 28–35.
- Sundh, A.; Molin, M.; Sjögren, G. Fracture resistance of yttrium oxide partially-stabilized zirconia all-ceramic bridges after veneering and mechanical fatigue testing. Dent. Mater. 2005, 21, 476–482.
- Zhou, X.; Huang, X.; Li, M.; Peng, X.; Wang, S.; Zhou, X.; Cheng, L. Development and status of resin composite as dental restorative materials. J. Appl. Polym. Sci. 2019, 136, 48180.
- Cho, K.; Rajan, G.; Farrar, P.; Prentice, L.; Prusty, B.G. Dental resin composites: A review on materials to product realizations. Compos. Part B Eng. 2021, 230, 109495.
- Stumpel, L.J.; Wadhwani, C. Development and capture of soft tissue contours at time of implant placement. J. Prosthet. Dent. 2017, 117, 709–713.
- Stumpel, L.J.; Wadhwani, C. A Customized Healing Abutment for Immediate and Delayed Implant Cases. Compend. Contin. Educ. Dent. 2017, 38, 672–678.
- Magne, P.; Oderich, E.; Boff, L.L.; Cardoso, A.C.; Belser, U.C. Fatigue resistance and failure mode of CAD/CAM composite resin implant abutments restored with type III composite resin and porcelain veneers. Clin. Oral Implant. Res. 2011, 22, 1275–1281.
- Magne, P.; Paranhos, M.P.G.; Burnett, L.H., Jr.; Magne, M.; Belser, U.C. Fatigue resistance and failure mode of novel-design anterior single-tooth implant restorations: Influence of material selection for type III veneers bonded to zirconia abutments. Clin. Oral Implant. Res. 2010, 22, 195–200.
- Neumann, E.A.F.; Villar, C.C.; França, F.M.G. Fracture resistance of abutment screws made of titanium, polyetheretherketone, and carbon fiber-reinforced polyetheretherketone. Braz. Oral Res. 2014, 28, 1–5.
- Liu, X.; Chen, S.; Tsoi, J.K.; Matinlinna, J.P. Binary titanium alloys as dental implant materials-a review. Regen. Biomater. 2017, 4, 315–323.
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116.
- Özcan, M.; Hammerle, C. Titanium as a Reconstruction and Implant Material in Dentistry: Advantages and Pitfalls. Materials 2012, 5, 1528–1545.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.5K
Revisions:
2 times
(View History)
Update Date:
22 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





