Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sy Duong-Quy | -- | 2675 | 2022-12-21 10:58:49 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Duong-Quy, S.; Nguyen-Huu, H.; Hoang, D.C.B.; Tran-Duc, S.; Hong, L.N.T.; Nguyen-Duy, T.; Tang-Thi-Thao, T.; Phan, C.; Bui-Diem, K.; Vu-Tran-Thien, Q.; et al. Management of Obstructive Sleep Apnea with Comorbidities. Encyclopedia. Available online: https://encyclopedia.pub/entry/39035 (accessed on 04 March 2026).
Duong-Quy S, Nguyen-Huu H, Hoang DCB, Tran-Duc S, Hong LNT, Nguyen-Duy T, et al. Management of Obstructive Sleep Apnea with Comorbidities. Encyclopedia. Available at: https://encyclopedia.pub/entry/39035. Accessed March 04, 2026.
Duong-Quy, Sy, Hoang Nguyen-Huu, Dinh Chau Bao Hoang, Si Tran-Duc, Lien Nguyen Thi Hong, Thai Nguyen-Duy, Tram Tang-Thi-Thao, Chandat Phan, Khue Bui-Diem, Quan Vu-Tran-Thien, et al. "Management of Obstructive Sleep Apnea with Comorbidities" Encyclopedia, https://encyclopedia.pub/entry/39035 (accessed March 04, 2026).
Duong-Quy, S., Nguyen-Huu, H., Hoang, D.C.B., Tran-Duc, S., Hong, L.N.T., Nguyen-Duy, T., Tang-Thi-Thao, T., Phan, C., Bui-Diem, K., Vu-Tran-Thien, Q., Nguyen-Ngoc-Phuong, T., Nguyen-Nhu, V., Le-Thi-Minh, H., & Craig, T. (2022, December 21). Management of Obstructive Sleep Apnea with Comorbidities. In Encyclopedia. https://encyclopedia.pub/entry/39035
Duong-Quy, Sy, et al. "Management of Obstructive Sleep Apnea with Comorbidities." Encyclopedia. Web. 21 December, 2022.
Copy Citation
Obstructive sleep apnea (OSA) is a common disease that is often under-diagnosed and under-treated in all ages. Personalized medicine in OSA should focus on the management of patients’ comorbidities. Comorbidities of OSA are more common in adult or elderly patients than children. These include chronic respiratory diseases, cardiovascular diseases, and endocrinological or neurological disorders. The optimal management of OSA, using a personalized approach, should target comorbidities which may improve patient outcomes.
OSA
CPAP
personalized management
Comorbidities
1. Personalized Approaches for Patients with Obstructive Sleep Apnea (OSA) and Airway Diseases
1.1. OSA and Allergic Rhinitis
The first upper airway disease which is comorbid with OSA is allergic rhinitis (AR), called AROSA (allergic rhinitis and OSA) [1]. A meta-analysis of 44 studies involving 6086 participants found that the proportion of OSA adults with AR was 35.2% (95% CI, 25.6–44.7) and the percentage of OSA children with AR was 45.2% (95% CI, 25.4–65.0) [2]. The prevalence of OSA in AR patients may even be up to 79.7% in young people [3]. The results of the previous research showed that OSA in patients with persistent allergic rhinitis was more severe than in healthy subjects (AHI = 17 ± 12 vs. 6 ± 3; p < 0.01); in particular, treatment with an antihistamine combined with a leukotriene receptor antagonist, or with intranasal steroid alone, made a significant reduction in the severity of OSA, measured by AHI (8 ± 4 vs 17 ± 12; p < 0.01) [4].
Thus, personalized approaches for patients with AROSA should be based on the pathophysiology of both diseases. The pathogenesis of the association between AR and OSA (AROSA) is quite complex. The nose and upper airway regulate more than 50% of airway resistance, which plays a very important role in performing the physiological function of the respiratory system. Frequent unilateral or bilateral nasal congestion results in a significant increase in total airway resistance [5]. In addition, according to the Starling resistance model, the upper airway acts as a hollow tube which is capable of constricting proximal of the inlet (nostril) and in the posterior segment of the collapse of the pharynx; the presence of an obstruction upstream (nose) will create a negative pressure (suction) in the downstream (pharynx), leading to collapse of the pharynx (soft tissue structure) in “at-risk individuals” (Figure 1). Moreover, the increased resistance in the nose will increase the habit of breathing through the mouth, making the upper airway unstable (easy to collapse) and inducing OSA [6][7].

Figure 1. Mechanism of obstructive sleep apnea (OSA) related to pharynx collapse due to the increase of upstream and downstream pressure and resistance.
The mediators produced from AR, such as histamine, cysteinyl leukotrienes, and interleukins, also cause the manifestations of nasal obstruction and sleep disruption in patients with OSA. Patients with OSA also have elevated levels of mediators such as tumor necrosis factor TNF, interleukin 6, and interleukin 1, which are Th2-activating cytokines, which may aggravate symptoms of AR [8]. Thus, the personalized management of AROSA should be based on the use of topical corticosteroids and leukotriene receptor antagonists [2][8]. The treatment of AR with intranasal corticosteroids may reduce the symptoms of the disease, especially nasal congestion. This personalized treatment combined with improved sleep hygiene or weight loss may improve fatigue, excessive daytime sleepiness, and quality of life in patients with AROSA [2].
1.2. OSA and Obstructive Lung Diseases
Obstructive lung diseases (OLDs) such as COPD (chronic obstructive pulmonary disease) and asthma are usually comorbid with OSA and named OLDOSA (obstructive lung disease and OSA) (Figure 2). OLDs share common risk factors with OSA such as smoking, obesity, and GERD [9][10][11]. In a 10-year longitudinal follow-up study of 4980 patients with asthma, COPD, and OSA as comorbidities, the 10-year cumulative all-cause mortality was 52.8%; median time to death was 2.7 years. Rate of death in the comorbid group was: COPD–OSA 53.2%, asthma–COPD 62.1%, asthma–OSA 63.5%, and asthma–COPD–OSA 67.8% [12]. In the multicenter research published previously, the percentage of patients having moderate or severe OSA, defined as AHI > 15/h, was significantly higher in subjects with asthma–COPD overlap (ACO) than that in subjects with asthma and COPD (64.4% versus 35.5% and 36.4%; p < 0.01 and p < 0.01, respectively). In addition, the mean AHI in patients with ACO was significantly higher than that in those with asthma and COPD (p < 0.05 and p < 0.05, respectively) [11].
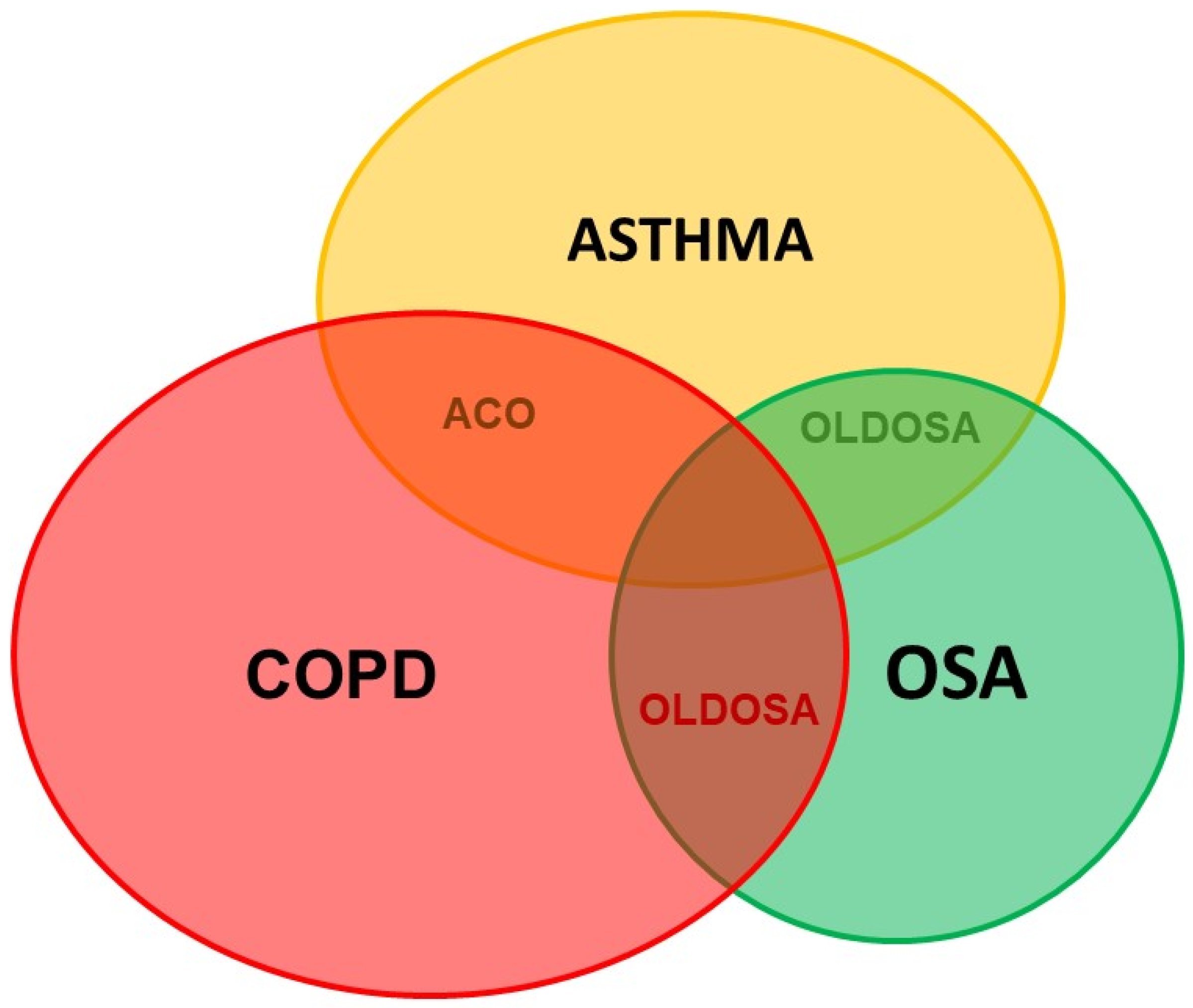
Figure 2. Comorbid obstructive lung disease and obstructive sleep apnea. ACO: Asthma–COPD overlap; COPD: chronic obstructive lung disease; OSA: obstructive sleep apnea; OLDOSA: obstructive lung disease and obstructive sleep apnea.
OSA and OLD have a bidirectional effect probably as a result of the common risk factors, nasopharyngeal pathology, increased airway resistance, hypoxemia, bronchospasm, local and systemic inflammation, and anti-inflammatory therapy [9]. All of these conditions have nocturnal hypoxemia, but OSA is characterized by intermittent hypoxemia during the night, while in COPD or asthma hypoxemia is continuous, both day and night, and is often aggravated at night. To diagnose OSA in patients with OLD, the attended PSG in sleep lab should be performed.
The management of patients with OLDOSA should be personalized. Treatment with CPAP in patients with OLDOSA may reduce mortality and improve quality of life compared with no CPAP [13]. CPAP improves day and night symptoms in asthmatic patients with OSA, reduces bronchodilator use and exacerbations, and improves lung function and quality of life. Treatment with CPAP in patients with OLDOSA needs to be personalized to improve patient adherence. CPAP treatment in adults and tonsillectomy in children with OLDOSA should be done only after optimizing the treatment of OLD. Due to the high prevalence of OSA in OLD, OSA should be screened in patients with OLD in order to confirm OLDOSA, personalize the therapy, and improve quality of life and mortality in these patients.
2. Personalized Approaches for Patients with OSA and Cardiovascular Diseases
2.1. General Considerations
The personalized management of OSA in patients with cardiovascular diseases is very important because the prevalence of comorbid cardiovascular diseases and OSA (CAVADOSA) is very high [14][15]. CAVADOSA has a negative impact on patient outcomes due to the increase in fatal and non-fatal cardiovascular morbidity and mortality. CAVADOSA may also reduce the patient’s quality of life and increase medical costs due to the severity of OSA [14]. Therefore, the personalization of CAVADOSA management is based on the early diagnosis and the appropriate treatment of cardiovascular diseases with comorbid OSA to effectively improve patient outcomes [16].
2.2. Personalization of OSA Diagnosis in Patients with Cardiovascular Diseases
Despite the high frequency of CAVADOSA, it is often underdiagnosed. This may be due to the lack of interest from cardiologists or the lack of diagnostic facilities such as PSG or RPG and available sleep labs. Furthermore, patients with CAVADOSA rarely complain about their sleep quality and snoring to cardiologists. In addition, a significant proportion of patients with CAVADOSA do not present with common characteristics of an OSA patient, such as male gender, daytime sleepiness, or snoring. In patients with CAVADOSA, the predominant symptoms are often related to the underlying cardiovascular disease [17][18]. Therefore, to diagnose and treat OSA early in cardiovascular patients, a personalized approach will help physicians to actively identify high-risk patients for CAVADOSA with resistant hypertension, recurrent atrial fibrillation, or nocturnal angina. Appropriate investigations such as sleep medicine consultation within attended PSG in available sleep labs should be used to further evaluate these patients.
2.3. Personalization of OSA Treatment in Patients with Cardiovascular Diseases
In patients with CAVADOSA, both conditions should be treated concurrently. Lifestyle modifications are important for CAVADOSA. The role of ideal body weight maintenance has been confirmed in these patients [19]. Interestingly, 10% of weight gain may increase the risk of OSA by six times [20]; inversely, 10% of weight loss might reduce the severity of AHI by 26% [21]. The personalized treatment of OSA in subjects with CAVADOSA should also focus on alcohol cessation and smoking cessation which would simultaneously reduce the risk of cardiovascular disease.
Among the specific treatments for OSA in patients with CAVADOSA, PAP (positive airway pressure) with CPAP, BiPAP (bilevel positive airway pressure), or auto SERVO should be personalized to achieve optimal outcomes. Although CPAP therapy is the most studied treatment in patients with CAVADOSA, the alternative methods should be individualized in these patients. Adequate treatment with CPAP might reduce major cardiovascular and cerebrovascular events, risk of stroke, transient ischemic accident, and fatal and nonfatal cerebrovascular events [14]. The personalized treatment of CPAP for patients with CAVADOSA should be prioritized as current recommendations suggest the use of CPAP at least four hours per night and five days per week [14]. Measures to improve CPAP adherence and effectiveness should be personalized for each patient with CAVADOSA.
3. Personalized Approaches for Patients with OSA and Diabetes
3.1. General Considerations and Personalized Approach
Type 2 diabetes mellitus (T2DM) and OSA are closely related and comorbid with significant public health implications. Both diseases have a high incidence and overlap in common risk factors, including obesity, older age, and higher prevalence in men [22]. OSA is an independent risk factor for the development of T2DM and the prevalence of T2DM in OSA patients is between 15–30%. In addition, severe OSA also leads to poorer glycemic control in T2DM patients [23]. The meta-analysis conducted by Huang et al. demonstrated that the risk of developing OSA was 2.14-fold (95% CI, 1.49, 3.07) in patients with T2DM after adjusting for age; the risk of T2DM after 10–18 years in OSA patients was 2.97-fold (95% CI, 2.40, 3.69) and decreased to 2.06 (1.86, 2.28) after adjusting for multiple confounding factors [22]. The prevalence of OSA in obese T2DM is up to 86.6% [23].
3.2. Personalized Treatment of OSA Patients with Comorbid Diabetes and Metabolic Syndrome
OSA has also been shown to be closely comorbid with metabolic syndrome. The term “Z syndrome” has been used to describe the association between obesity, insulin resistance, hypertension, and dyslipidemia with OSA. The OR (odds ratio) index of metabolic syndrome in OSA patients ranges from 5 to 9-fold when compared with subjects without OSA, and independent of age and BMI [24][25][26]. Due to the bidirectional association between T2DM or metabolic syndrome and OSA [2][23][27][28][29], the personalized management of these comorbid diseases should be performed to avoid the high risk of fatal cardiovascular events.
In severe or symptomatic OSA patients, CPAP therapy is the main approach because of its effectiveness in reducing AHI and symptoms. Although several trials have shown that CPAP therapy in OSA patients with T2DM did not significantly reduce HbA1C levels or BMI, nor improve blood glucose, CPAP therapy could improve insulin sensitivity and reduce insulin resistance assessed by the HOMA-IR (Homeostatic Model Assessment Index) [30][31][32]. However, the common limitation of most trials is that the duration of CPAP used during sleep is usually less than 4 h. In another study [33] patients who took CPAP for at least 4 h/night (6.6 h/night) could achieve a significant improvement in HbA1C. The personalization of OSA management in patients with comorbid T2DM should be based on weight loss (for overweight and obese people) for helping to better control blood sugar and reducing the AHI; in particular, 10% of weight loss can predict a 26% decrease in AHI [21]. More recently, randomized controlled trials with 4 years of follow-up have indicated that weight loss can alter OSA severity with a mean AHI reduction of 0.78 events/h for every kilogram of weight lost [34][35].
4. Personalized Approaches for Patients with OSA and Insomnia
4.1. Personalized Diagnosis
A typical symptom of OSA is hypersomnia which is defined by daytime sleepiness. However, some patients with OSA have the symptoms of insomnia, named COMISA (comorbid insomnia and OSA). Due the high prevalence of insomnia and COMISA in the general population and in patients with OSA [36][37], and due to the complexity in diagnosis and treatment of COMISA, the management of this disease should be personalized. Insomnia is defined as difficulty falling asleep, waking up in the middle of the night and having difficulty getting back to sleep, or waking up earlier than desired with an impact on daytime functioning [38]. Patients with COMISA often have more severe daytime symptoms, and the frequency of neuropsychiatric and cardiovascular disorders is also higher than in the general population. A previous study showed that, among patients with COMISA, 35% had AHI ≥ 5 times/hour and 29% had AHI ≥ 15 times/hour [39].
4.2. Personalized Treatment
For patients with COMISA, personalized treatment aims to resolve different challenges including sedative-induced severe OSA, CPAP treatment-induced insomnia, poor CPAP adherence, and the efficiency of balanced OSA—insomnia treatment. Luyster et al. reported on symptoms of OSA and insomnia and showed that only a few clinical features were distinct, with most daytime and nocturnal complaints shared by both patients; and there was a significant overlap in symptoms between insomnia and OSA (COMISA). Hence, it is difficult to determine a causal relationship between OSA and insomnia in individual patients. Different treatment responses may indicate whether OSA is primary or secondary to the insomnia component and vice versa.
The personalized diagnosis and treatment of COMISA always requires multidisciplinary collaboration. Combined therapy, including cognitive behavior therapy (CBT) for the treatment of insomnia and for OSA, may result in a greater improvement than treatment with CPAP alone [37]. The assessment of symptom improvement in patients with COMISA should be measured by the degree of insomnia and daytime function. In these patients, pre-administered CBT for insomnia might increase CPAP tolerance and improve insomnia in comparison with CPAP alone. The effectiveness of CBT remains unaffected by OSA.
5. Personalized Approaches for Subjects with OSA and Genetic Defects and Other Disorders
5.1. Personalized Approach and Diagnosis
The personalized approach in patients with genetic defects and OSA is necessary for optimizing the patients’ outcomes. Particularly in individuals with Down syndrome (DS), OSA is observed in up to 85–93% compared with 7 to 13% of the general population [40][41]. The prevalence of OSA in these subjects is associated with a variety of genetic, anatomical, endocrine, and metabolic abnormalities. Its main cause is an extra chromosome at position 21 causing asymmetrical skull structure, upper airway narrowing, hypotonia, hypothyroidism, or obesity [42][43][44]. Both children and adults with DS are particularly affected by OSA.
The personalized diagnosis of OSA in patients with DS (DOSOSA: Down syndrome and OSA) is based on the clinical characteristics and PSG. Neurophysiological analysis should be performed to identify the specific features of PSG. In the DS population, PSGs usually report a high prevalence of OSA and an association between degree of mental retardation and duration of REM sleep, which might play a potential role in deficit perception of people with DS [45][46]. Sleep fragmentation caused by apnea/hypopnea episodes might be considered as the cause of neurocognitive dysfunction, impaired quality of life, and increased risk of occupational accidents in patients with DOSOSA. In the DS population, obesity is very common and develops from childhood. The relationship between obesity, fat deposition, and OSA severity has been well established in the DOSOSA population [32][47][48][49]. Obesity is a pathology more frequent in people with DS because they have the genetic predisposition to develop obesity [50].
Moreover, there seems to be a link between genetic factors and the development of OSA-related cognitive decline. The ε4 isoform of apolipoprotein ε (Apoε4) has been found to be an early marker of Alzheimer’s disease [51]. It is overexpressed in subjects with DS, confirming the risk of developing this disease. Thus, OSA-related cognitive disorders and the development of Alzheimer’s disease are also closely linked [52]. Other genetic factors may also influence craniofacial morphology and obesity [53][54], and thus may increase the risk of OSA [55][56].
5.2. Personalized Treatment
Currently, there is no personalized and potential treatments for OSA in subjects with genetic defects. CPAP therapy may be an ineffective treatment option because of poor tolerability and adherence [57]. Therefore, the personized approach should focus on the necessity of early diagnosis of OSA in this population to minimize its harmful complications. The current personalized and alternative therapy without CPAP in people with DOSOSA with non-severe AHI is based on a program of physical activity combined with the implementation of rigorous dietary and lifestyle measures [58].
Finally, the diagnosis and treatment of OSA comorbid with other disorders should be personalized for having an accurate diagnosis and optimal treatment for each given patient in clinical practice. For each given comorbid disease, the personalized patient with OSA should benefit from a multidimensional approach and treatment (Figure 3).
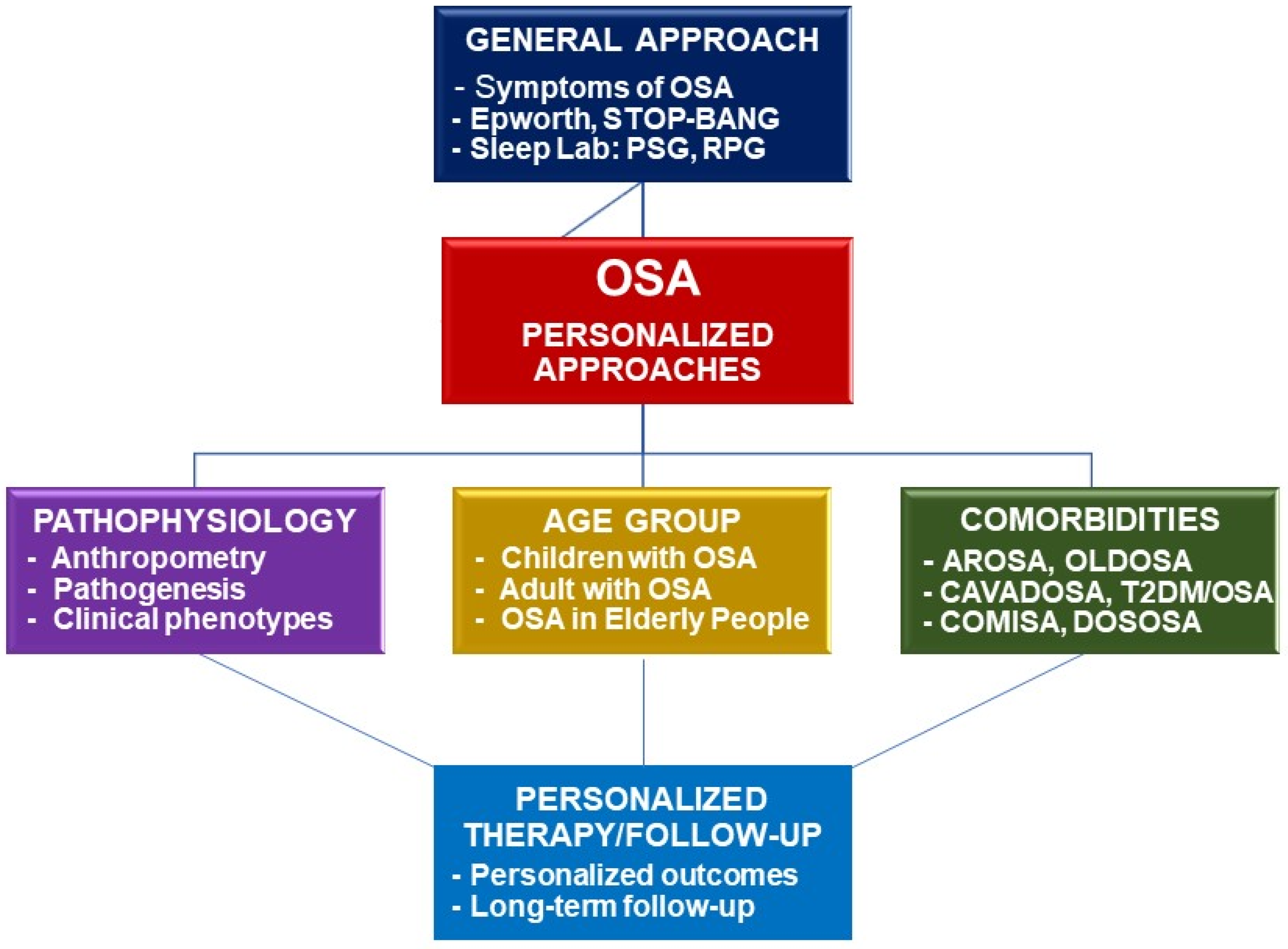
Figure 3. Framework of personalized approaches for diagnosis and treatment of OSA. OSA: obstructive sleep apnea; PSG: polysomnography; RPG: respiratory polygraphy; AROSA: allergic rhinitis and OSA; OLDOSA: obstructive lung disease and OSA; CAVADOSA: cardiovascular diseases and OSA; T2DM: type 2 diabetes mellitus; COMISA: comorbid insomnia and OSA; DOSOSA: Down syndrome and OSA.
References
- Lunn, M. Timothy Craig. Rhinitis and sleep. Sleep Med. Rev. 2011, 15, 293–299.
- Pawanker, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F. World Health Organization White Book on Allergy 2011–2012 Executive Summary; World Health Organization: Geneva, Switzerland, 2011.
- Cao, Y.; Wu, S.; Zhang, L.; Yang, Y.; Cao, S.; Li, Q. Association of allergic rhinitis with obstructive sleep apnea. Medicine 2018, 97, e13783.
- Vo-Thi-Kim, A.; Van-Quang, T.; Nguyen-Thanh, B.; Dao-Van, D.; Duong-Quy, S. The effect of medical treatment on nasal exhaled nitric oxide (NO) in patients with persistent allergic rhinitis: A randomized control study. Adv. Med. Sci. 2020, 65, 182–188.
- Sianturi, M.; Marliyawati, D.; Yusmawan, W.; Yunika, K. The Correlation of Allergic Rhinitis with Obstructive Sleep Apnea Syndrome (OSAS) in Young Adults. Diponegoro. Int. Med. J. 2020, 1, 21–25.
- Olsen, K.D.; Kern, E.B. Nasal Influences on Snoring and Obstructive Sleep Apnea. Mayo Clin. Proc. 1990, 65, 1095–1105.
- Smith, P.L.; Wise, R.A.; Gold, A.R.; Schwartz, A.R.; Permutt, S. Upper airway pressure-flow relationships in obstructive sleep apnea. J. Appl. Physiol. 1988, 64, 789–795.
- Tan, S.N.; Abdullah, B. The Association Between Obstructive Sleep Apnea and Allergic Rhinitis: Current Literature Review. Curr. Respirat. Med. Rev. 2021, 17, 13–19.
- Ioachimescu, O.C.; Teodorescu, M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology 2013, 18, 421–431.
- Duong-Quy, S.; Dang Thi Mai, K.; Tran Van, N.; Nguyen Xuan Bich, H.; Hua-Huy, T.; Chalumeau, F.; Dinh-Xuan, A.T.; Soyez, F.; Martin, F. Study about the prevalence of the obstructive sleep apnoea syndrome in Vietnam. Rev. Mal. Respir. 2018, 35, 14–24.
- Duong-Quy, S.; Van, H.T.; Kim, A.V.T.; Huy, Q.P.; Craig, T.J. Clinical and Functional Characteristics of Subjects with Asthma, COPD, and Asthma-COPD Overlap: A Multicentre Study in Vietnam. Can. Respir. J. 2018, 2018, 1–11.
- Ioachimescu, O.C.; Janocko, N.J.; Ciavatta, M.-M.; Howard, M.; Warnock, M.V. Obstructive Lung Disease and Obstructive Sleep Apnea (OLDOSA) cohort study: 10-year assessment. J. Clin. Sleep Med. 2020, 16, 267–277.
- Stanchina, M.L.; Welicky, L.M.; Donat, W.; Lee, D.; Corrao, W.; Malhotra, A. Impact of CPAP Use and Age on Mortality in Patients with Combined COPD and Obstructive Sleep Apnea: The Overlap Syndrome. J. Clin. Sleep Med. 2013, 9, 767–772.
- BaHammam, A.S.; Han, F.; Gupta, R.; Duong-Quy, S.; Al-Abri, M.A.; Jahrami, H.A.; Song, P.; Desudchit, T.; Xu, L.; Hong, S.B. Asian accreditation of sleep medicine physicians and technologists: Practice guidelines by the Asian Society of Sleep Medicine. Sleep Med. 2021, 81, 246–252.
- Dinh-Thi-Dieu, H.; Vo-Thi-Kim, A.; Tran-Van, H.; Duong-Quy, S. Efficacy and adherence of auto-CPAP therapy in patients with obstructive sleep apnea: A prospective study. Multidiscip. Respir. Med. 2020, 15, 468.
- Bock, J.; Needham, K.; Gregory, D.A.; Ekono, S.M.M.; Wickwire, E.M.; Somers, V.K.; Lerman, A. CPAP adherence reduces tratment cost in patients with Obstructive Sleep Apnea and Cardiovascular disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 166–175.
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep Apnea and Cardiovascular Disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement From the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing In Collaboration With the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). J. Am. Coll. Cardiol. 2008, 52, 686–717.
- Bitter, T.; Westerheide, N.; Hossain, S.M.; Prinz, C.; Horstkotte, D.; Oldenburg, O. Symptoms of sleep apnoea in chronic heart fail-ure-results from a prospective cohort study in 1500 patients. Sleep Breath 2012, 16, 781–791.
- Bauters, F.; Rietzschel, E.R.; Hertegonne, K.B.C.; Chirinos, J.A. The Link Between Obstructive Sleep Apnea and Cardiovascular Disease. Curr. Atheroscler. Rep. 2016, 18, 1–11.
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions Between Obesity and Obstructive Sleep Apnea: Implications for Treatment. Chest 2010, 137, 711–719.
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal Study of Moderate Weight Change and Sleep-Disordered Breathing. JAMA 2000, 284, 3015–3021.
- Viswanathan, V.; Ramakrishnan, N.; Saboo, B.; Agarwal, S. RSSDI clinical practice recommendations for screening, diagnosis, and treatment in type 2 diabetes mellitus with obstructive sleep apnea. Int. J. Diabetes Dev. Ctries. 2021, 41, 4–21.
- Rajan, P.; Greenberg, H. Obstructive sleep apnea as a risk factor for type 2 diabetes mellitus. Nat. Sci. Sleep 2015, 7, 113–125.
- Jehan, S.; Myers, A.; Zizi, F. Obesity, obstructive sleep apnea, and type 2 diabetes mellitus: Epidemiology and pathophysiologic insights. Sleep Med. Dis. Int. J. 2018, 2, 54–60.
- Coughlin, S.R.; Mawdsley, L.; Mugarza, J.A.; Calverley, P.M.; Wilding, J.P. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur. Heart J. 2004, 25, 735–741.
- Gruber, A.; Horwood, F.; Sithole, J.; Ali, N.; Idris, I. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc. Diabetol. 2006, 5, 22.
- Shaw, J.; Sicree, R.; Zimmet, P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pr. 2010, 87, 4–14.
- Altaf, Q.-A.A.; Ali, A.; Piya, M.K.; Raymond, N.T.; Tahrani, A.A. The relationship between obstructive sleep apnea and intra-epidermal nerve fiber density, PARP activation and foot ulceration in patients with type 2 diabetes. J. Diabetes Its Complicat. 2016, 30, 1315–1320.
- Mok, Y.; Tan, C.W.; Wong, H.S.; How, C.H.; Tan, K.L.A.; Hsu, P.P. Obstructive sleep apnoea and type 2 diabetes mellitus: Are they connected? Singap. Med. J. 2017, 58, 179.
- Khalyfa, A.; Wang, Y.; Zhang, S.X.; Qiao, Z.; Abdelkarim, A.; Gozal, D. Sleep Fragmentation in Mice Induces Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2-Dependent Mobilization, Proliferation, and Differentiation of Adipocyte Progenitors in Visceral White Adipose Tissue. Sleep 2014, 37, 999–1009.
- Feng, Y.; Zhang, Z.; Dong, Z.Z. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: A systematic review and meta-analysis. NPJ Prim. Care Respir. Med. 2015, 25, 15005.
- Yang, D.; Liu, Z.; Yang, H.; Luo, Q. Effects of continuous positive airway pressure on glycemic control and insulin resistance in patients with obstructive sleep apnea: A meta-analysis. Sleep Breath 2013, 17, 33–38.
- Babu, A.R.; Herdegen, J.; Fogelfeld, L.; Shott, S.; Mazzone, T. Type 2 Diabetes, Glycemic Control, and Continuous Positive Airway Pressure in Obstructive Sleep Apnea. Arch. Internt. Med. 2005, 165, 447–452.
- Mitchell, L.; Davidson, Z.E.; Bonham, M.; O'Driscoll, D.M.; Hamilton, G.; Truby, H. Weight loss from lifestyle interventions and severity of sleep apnoea: A systematic review and meta-analysis. Sleep Med. 2014, 15, 1173–1183.
- Joosten, S.A.; Hamilton, G.; Naughton, M.T. Impact of Weight Loss Management in OSA. Chest 2017, 152, 194–203.
- Ford, E.S.; Cunningham, T.J.; Giles, W.H.; Croft, J.B. Trends in insomnia and excessive daytime sleepiness among U.S. Adults from 2002 to 2012. Sleep Med. 2015, 16, 372–378.
- Ragnoli, B.; Pochetti, P.; Raie, A.; Malerba, M. Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management. Int. J. Environ. Res. Public Health 2021, 18, 9248.
- Sateia, M.J. International classification of sleep disorders-third edition highlights and modifications. Chest 2014, 146, 1387–1394.
- Sweetman, A.M.; Lack, L.C.; Catcheside, P.; Antic, N.A.; Chai-Coetzer, C.L.; Smith, S.; Douglas, J.A.; McEvoy, D. Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Med. Rev. 2016, 33, 28–38.
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014.
- Trois, M.S.; Capone, G.T.; Lutz, J.A.; Melendres, M.C.; Schwartz, A.R.; A Collop, N.; Marcus, C.L. Obstructive Sleep Apnea in Adults with Down Syndrome. J. Clin. Sleep Med. 2009, 5, 317–323.
- Murdoch, J.; Ratcliffe, W.; McLarty, D.; Rodger, J.; Ratcliffe, J. Thyroid function in adults with Down's syndrome. J. Clin. Endocrinol. Metab. 1977, 44, 453–458.
- Baynard, T.; Pitetti, K.H.; Guerra, M.; Unnithan, V.B.; Fernhall, B. Age-related changes in aerobic capacity in individuals with mental retardation: A 20-yr review. Med. Sci. Sport. Exerc. 2008, 40, 1984–1989.
- Fernhall, B.; Mendonca, G.V.; Baynard, T. Reduced work capacity in individuals with Down syndrome: A consequence of autonomic dysfunction? Exerc. Sport Sci. Rev. 2013, 41, 138–147.
- Ferri, R.; Curzi-Dascalova, L.; Del Gracco, S.; Elia, M.; Pettinato, S.; Musumeci, S. Sleep Neurophysiopathology in Down syndrome. Down Syndr. Res. Pr. 1998, 5, 105–110.
- Marcus, C.L.; Keens, T.G.; Bautista, D.B.; von Pechmann, W.S.; Ward, S.L. Obstructive sleep apnea in children with Down syn-drome. Pediatrics 1991, 88, 132–139.
- Basil, J.S.; Santoro, S.L.; Martin, L.J.; Healy, K.W.; Chini, B.A.; Saal, H.M. Retrospective Study of Obesity in Children with Down Syndrome. J. Pediatr. 2016, 173, 143–148.
- Chen, C.C.; Ringenbach, S.D.R.; Albert, A.R. Assisted cycling exercise improves fine manual dexterity in persons with Down's syndrome. J. Appl. Res. Intellect. Disabil. 2014, 27, 264–272.
- Skotko, B.G.; Macklin, E.A.; Muselli, M.; Voelz, L.; McDonough, M.E.; Davidson, E.; Allareddy, V.; Jayaratne, Y.S.N.; Bruun, R.; Ching, N.; et al. A predictive model for obstructive sleep apnea and Down syndrome. Am. J. Med. Genet. Part A 2017, 173, 889–896.
- Seo, D.S.; Chau, G.C.; Baek, K.-H.; Um, S.H. A single extra copy of Down syndrome critical region 1–4 results in impaired hepatic glucose homeostasis. Mol. Metab. 2018, 21, 82–89.
- Wiseman, F.K.; Al-Janabi, T.; Hardy, J.; Karmiloff-Smith, A.; Nizetic, D.; Tybulewicz, V.L.J.; Fisher, E.M.C.; Strydom, A. A genetic cause of Alzheimer disease: Mechanistic insights from Down syndrome. Nat. Rev. Neurosci. 2015, 16, 564–574.
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive Impairment in Obstructive Sleep Apnea. Chest 2012, 141, 1601–1610.
- Patel, S.R.; Larkin, E.K.; Redline, S. Shared genetic basis for obstructive sleep apnea and adiposity measures. Int. J. Obes. 2008, 32, 795.
- Schwartz, A.R.; Patil, S.P.; Laffan, A.M.; Polotsky, V.; Schneider, H.; Smith, P.L. Obesity and Obstructive Sleep Apnea: Pathogenic Mechanisms and Therapeutic Approaches. Proc. Am. Thorac. Soc. 2008, 5, 185–192.
- Redline, S.; Tishler, P.V. The genetics of sleep apnea. Sleep Med. Rev. 2000, 4, 583–602.
- Redline, S.; Tishler, P.V.; Tosteson, T.D.; Williamson, J.; Kump, K.; Browner, I.; Ferrette, V.; Krejci, P. The familial aggregation of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995, 151, 682–687.
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931.
- Nguyen, T.D.; Baillieul, S.; Guinot, M.; Doutreleau, S.; Bricout, V.-A. Classification of Factors Effect on Sleep in Individuals with Down Syndrome. Brain Sci. 2021, 11, 1500.
More
Information
Subjects:
Respiratory System
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revision:
1 time
(View History)
Update Date:
21 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





