Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vishnu D. Rajput | -- | 2485 | 2022-12-21 08:26:46 | | | |
| 2 | Camila Xu | -1 word(s) | 2484 | 2022-12-21 11:26:31 | | | | |
| 3 | Camila Xu | Meta information modification | 2484 | 2022-12-21 11:28:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mathur, J.; Goswami, P.; Gupta, A.; Srivastava, S.; Minkina, T.; Shan, S.; Rajput, V.D. Nanoparticle Mechanisms for the Removal of Metals. Encyclopedia. Available online: https://encyclopedia.pub/entry/39020 (accessed on 07 February 2026).
Mathur J, Goswami P, Gupta A, Srivastava S, Minkina T, Shan S, et al. Nanoparticle Mechanisms for the Removal of Metals. Encyclopedia. Available at: https://encyclopedia.pub/entry/39020. Accessed February 07, 2026.
Mathur, Jyoti, Pooja Goswami, Ankita Gupta, Sudhakar Srivastava, Tatiana Minkina, Shengdao Shan, Vishnu D. Rajput. "Nanoparticle Mechanisms for the Removal of Metals" Encyclopedia, https://encyclopedia.pub/entry/39020 (accessed February 07, 2026).
Mathur, J., Goswami, P., Gupta, A., Srivastava, S., Minkina, T., Shan, S., & Rajput, V.D. (2022, December 21). Nanoparticle Mechanisms for the Removal of Metals. In Encyclopedia. https://encyclopedia.pub/entry/39020
Mathur, Jyoti, et al. "Nanoparticle Mechanisms for the Removal of Metals." Encyclopedia. Web. 21 December, 2022.
Copy Citation
Different natural and anthropogenic global events and activities such as urban settlements and industrial development have led to a build-up of numerous pollutants in the environment, creating problems for nature and human health. Metal(loid)s are defined as elements with a density of more than 4–5 g/cm3 that are toxic to humans even at low levels.
metal(loid)s
nanoremediation
nanomaterials
pollution
water
1. Introduction
Water is an important resource for the survival of life on Earth, and all living organisms, including humans, need water. In recent decades, excessive metal and metalloid contamination in water has become a serious concern all around the world. Metal(loid)s are defined as elements with a density of more than 4–5 g/cm3 that are toxic to humans even at low levels [1]. Toxic metal(loid)s may harm the environment, plants, animals, and human health. The uncontrolled extraction and processing of metal(loid)s from natural geological sources owing to their demand by the rising population and numerous applications to support the modern living standards have resulted in increased metal(loid) contamination intensity and regarding area coverage. Simultaneously, agricultural operations have increased and resulted in the greater usage of soil and plant additives, fertilizers, and pesticides, which include metal(loid)s as impurities. Natural sources of metal(loid)s include the biogeochemical weathering of rocks and volcanic eruptions [1].
For biological systems, several metal(loid)s are essentially required from trace to large quantities. However, when present at higher concentrations than those optimally required, even the essential metal(loid)s induce toxicity. Metal(loid)s such as arsenic (As), copper (Cu), nickel (Ni), cadmium (Cd), mercury (Hg), chromium (Cr), and lead (Pb) are extremely detrimental to human health, and can enter the human body and cause various ailments. Arsenic can cause diseases such as skin lesions, and skin, bladder, and lung cancers [2]. Lead can cause various cardiovascular and neurological diseases due to accumulation in the human body if it is exposed to them for a long period of time [3]. The kidneys are an easy target for Hg toxicity. Mercury is easily spread through aquatic systems, and can cause renal dysfunction and proteinuria [4]. Chromium is a known human carcinogen that directly affects the nervous system, and can cause brain cancer and other motor neuron diseases [5]. Cadmium toxicity can cause various lung, kidney, and bone diseases, and affect the reproductive system [6]. The ill effects of Ni toxicity observed among workers in mining industries are lung cancer, skin allergies, and various cardiovascular diseases [7]. Considering the above-stated toxic side effects caused due to metal(loid) toxicity, it is important to alleviate this problem to prevent various harmful diseases in humans.
Contaminated drinking water acts as the major source of metal(loid)s for humans. Water is also used for irrigation purposes, and due to this, contaminated irrigation water can be a source of metal(loid)s for plants and grains, and subsequently for humans via food. The arsenic contamination of groundwater is a serious problem that has arisen due to natural and anthropogenic factors such as mining, overirrigation, and the natural presence of As in groundwater [2]. Mining and industrial activities discharge their wastewater in nearby streams, and this leads to the accumulation of metal(loid)s such as Cr, Cd, and Pb in water sources [3][4][5][6]. Mercury can volatilize in the air; thus, it can spread to longer distances from the original source via atmospheric deposition [4]. Contaminated water, wastewater, and drinking or irrigation water need to be remediated to render it suitable for discharge or other useful purposes. Metal(loid) resources are exhaustible. Hence, as much as possible, metal(loid)s must be extracted back from contaminated media for reuse.
A large number of physicochemical and biological methods are available for water clean-up. These include bioremediation, phytoremediation, chemical precipitation, membrane separation, adsorption, and ion exchange [8]. Adsorption-based methods are appropriate considering the possibility of removing adsorptive material and subsequent metal(loid) extraction. A number of materials such as microbial cells, crop residues, fly-ash, and red mud can be used for adsorption. Nanoremediation is the process of removing environmental toxins from polluted places by utilizing nanoparticles (NPs)/nanomaterials (NMs). These NPs/NMs can be generated either by chemical methods or biologically by plants, fungi, and bacteria [9]. Nanomaterials such as titanium oxide (TiO2), silver (Ag), nano zero valent iron (nZVI), cerium oxide (CeO2), zinc oxide (ZnO2), nanohydroxyapatite (NHAP), and nano carbon black (NCB) were successful in eliminating metal(loid)s and other contaminants in several studies (Figure 1) [10][11]. Because of its enhanced properties, such as a high surface-area-to-volume ratio and high reactivity, nanotechnology has emerged as the most effective approach for remediation. The ultraminute size of NPs/NMs facilitates handling them for example via packing small filter cartridges. Further, even a small volume of NPs/NMs, can offer many-fold large surface area for interaction with metal(loid)s. Further, the surface of NPs/NMs can be modified by addition of other chemical ligands as per the requirement, and this can further enhance the reactivity, usability, and efficiency of NPs/NMs [12]. Nanotechnology can find application in remediation of metal(loid)s either alone or in conjunction with plants and other methods. The present research discusses the application of various NPs in metal(loid) remediation.
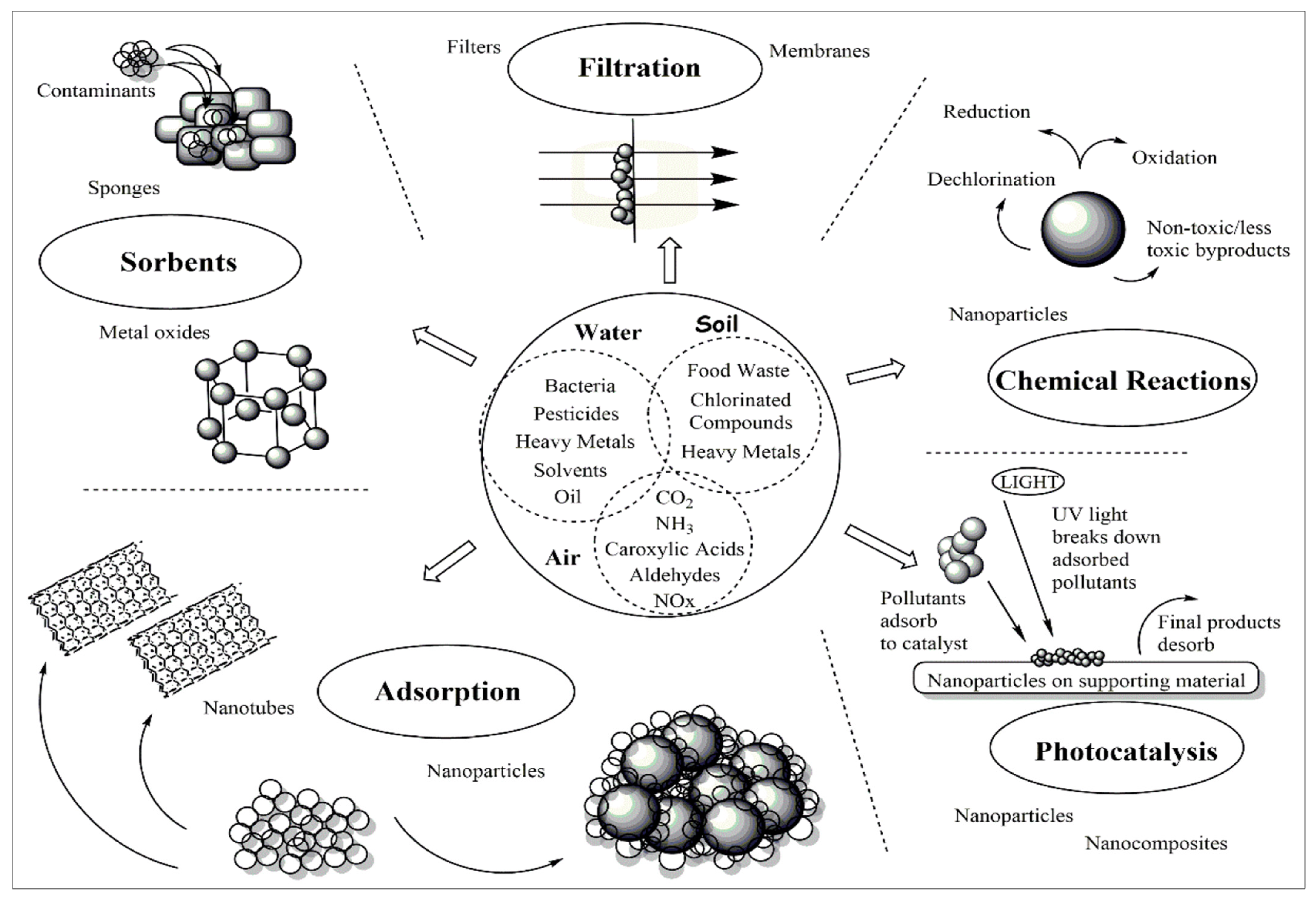
Figure 1. General overview of environmental remediation approaches with the use of nanotechnology.
Nanoremediation uses nanotubes, carbon and carbon-based NMs, NPs, nanofibers, nanoclusters, and nanocomposites to remove water pollutants. Due to their magnetic features, low toxicity, high chemical stability, ease of manufacture, and high recycling capacity, magnetic NPs are frequently utilized in remediation operations, particularly for the removal of pollutants from aqueous solutions. Carbon-based nanomaterials (CNMs) are effective adsorbents due to their unique morphological and structural features [13]. Despite the fact that their hydrophobic qualities and low solvent solubility set them apart from the competition in terms of cost, they have limited application in wastewater treatment. After functionalization, the problem of the hydrophobicity-related low solubility of CNMs can be tackled, and their selectivity for contaminants can be increased. Graphene is a single atomic sheet of graphite with remarkable mechanical, chemical, electrical, and physical characteristics, and low manufacturing cost. The graphene family has been applied as nanoadsorbents for remediation purposes [12]. Organic NMs prepared from synthetic polymers or natural compounds such as cellulose and chitosan are also often used, and they offer great metal(loid) removal due to presence of functional groups such as –NH2, –COOH, and –OH [12]. Metal–organic frameworks (MOFs) are hybrid materials of organic and inorganic mixtures. These contain metals as an inorganic component, and zirconium (Zr)-based MOFs have gained interest for metal(loid) removal [14]. There are several other NPs of different metals and metal oxides whose application is continually increasing for the remediation of metal(loid)-contaminated media (Figure 2).
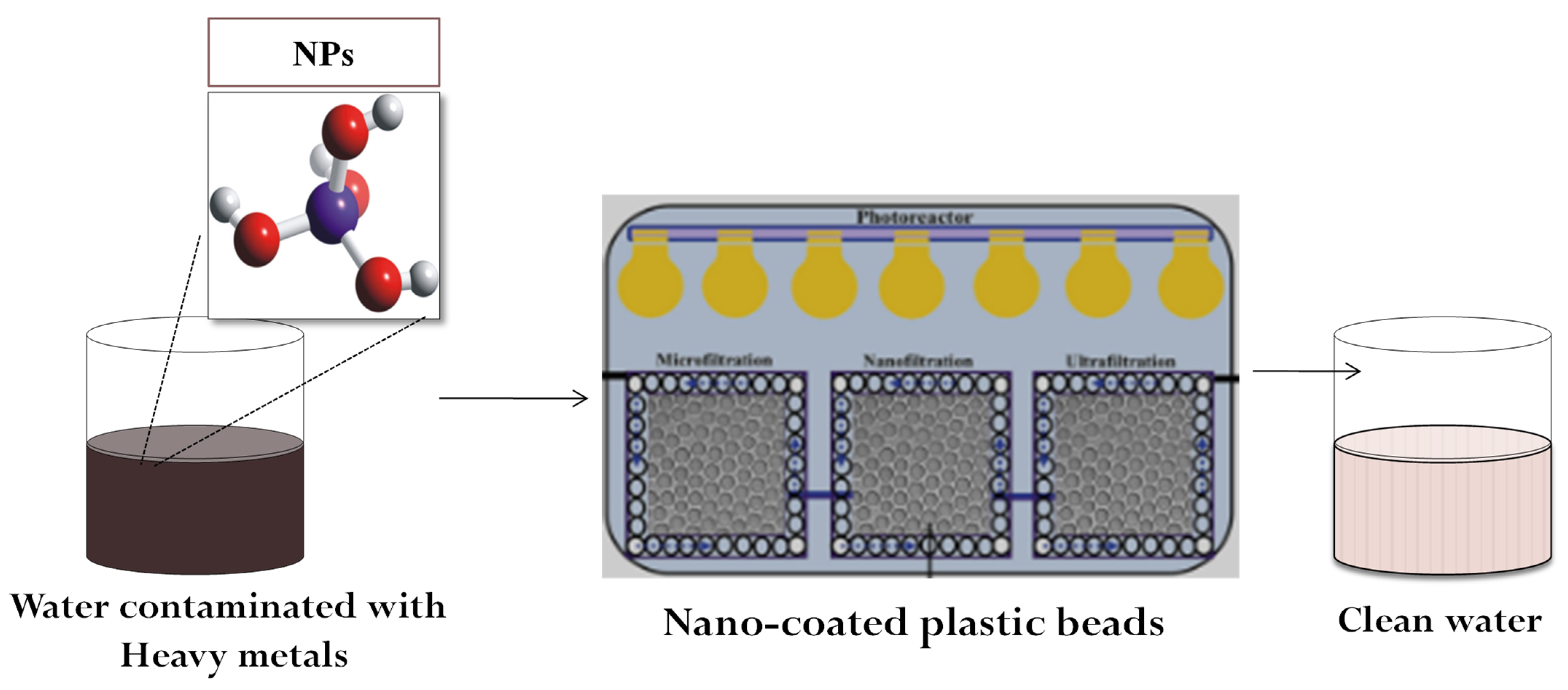
Figure 2. Nanoparticle-mediated water remediation.
The adsorption capabilities of adsorbents are analyzed using adsorption isotherms. There are various models that can be used to determine the adsorption isotherms. In the Langmuir model, adsorption occurs uniformly on the active sites of the adsorbents, and there is no further adsorption behavior on these sites once the adsorbates have occupied them. The Freundlich model is used in the case of nonideal sorptions and is based on multilayer adsorption, which renders it different from Langmuir model where only a single adsorbate is bound to a site. The Sips model is a hybrid of the two above models that converts into a Freundlich isotherm at a low concentration, and a Langmuir isotherm at a high concentration of adsorbates [15]. So, on the basis of these isotherms, the adsorption capacities of various NPs are analyzed, and then these NPs are used for the removal of metal(loid)s from water. Table 1 summarizes the key findings of recent studies with respect to the NP-mediated remediation of metal(loid)s from water.
Table 1. Applications of nanomaterials in metal(loid) remediation from the environment.
| Nanomaterial Types | Metal(loid) | Key Results | References |
|---|---|---|---|
| Fe3O4 magnetic nanoparticles (MNPs) coated with hyperbranched polyamidoamine (PAMAM) dendrimer, MNP-PAMAM; 0.1–0.2 g/L | Pb(II), Cd(II), Ni(II); 10–100 mg/L | The maximal adsorption capacities were 92.82, 80.10, and 57.72 mg/g in a single system, and 37.00, 31.91, and 24.94 mg/g in a ternary system for Pb(II), Ni(II) and Cd(II), respectively | [16] |
| FeONPs synthesized with Rosa indica flower petal extract | Cr(VI) | Cr(VI) (10–50 ppm) adsorption was good with 0.1 to 0.5 g/L NPs | [17] |
| Nanocomposite hydrogels of polyaniline–polypyrrole-modified graphene oxide in an alginate matrix (GO@PAN-PPy/SA) | Cr(VI) and Cu(II) (5–25 mg/L) | The maximal adsorption for Cr(VI) and Cu(II) was 133.7 and 87.2 mg/g at pH 3.0 | [18] |
| Bilayer–oleic coated FeO NPs (bilayer–OA@FeO NPs) (0.1–3 g/L) | As(V) (0.01–0.15 mg/L) | High As(V) sorption (32.8 μg/g) occurred at pH 7.0 at 1 g/L dose | [19] |
| Calcium alginate entrapped in magnetic NPs and functionalized with methionine | As(III) (10–35 mg/L) | About 99.56% As(III) was removed from 10 mg/L solution at pH 7.0 with 1.6 g of adsorbent in less than 2 h | [20] |
| Aminopropyltrimethoxysilane (APTMs)-modified bamboo-derived TEMPO-oxidized nanofibrillated cellulose (TO-NFC) aerogels ((APTM-modified TO-NFC)) | Cu(II), Cd(II), Hg(II) (0–200 mg/L) | Aerogel showed adsorption capacity of 99.0, 124.5, and 242.1 mg/g for Cu(II), Cd(II), and Hg(II), respectively; optimal adsorption efficiency at pH 3–7 | [21] |
| Carboxymethyl cellulose (CMC) bridged chlorapatite (CMC-CAP) NPs |
Cd(II) (5 mg/L) Zn(II) (7 mg/L) |
Maximal sorption capacity of CMC-CAP was 141.1 and 150.2 mg/g, respectively, for Zn and Cd | [22] |
| Fe3O4 NP-modified activated carbon prepared from biochar (FAC) | As(V) (15–600 mg/L) | The maximal adsorption of As(V) on FAC was 32.57 mg/g | [23] |
| Simarouba glauca leaf-extract-synthesized CuFe2O4 NPs; 0.025 to 0.1 g | Pb(II); 10–40 mg/L | Good Pb removal was achieved with NPs at pH 6 with 0.05 g adsorbent from Pb solution of 20 mg/L | [24] |
| Moringa oleifera activated carbon (AC) + chitosan (CS) and Fe3O4 NPs; 1 g/L | Cr(VI); 20 mg/L | Adsorption capacity of AC, CS/AC, AC/Fe3O4, and CS/AC/Fe3O4 adsorbers for Cr(VI) was 56.78, 114.80, 121.70, and 130.80 mg/g | [25] |
| Humic acid (HA)-coated hydrated ferric oxide (HFO)-porous resin D-201 nanocomposites (HA-HFO-D-201) | Cu(II), Cd(II) and Pb(II) | Excellent metal removal in pH range of 3–9, >90% metal removal achieved with nanocomposite | [26] |
| Biochar-loaded Ce3+-enriched ceria NPs (Ce-BC) (20–50 mg/L) | As(V) (10 mg/L) | Up to 99.7–100% As was removed from 0.05 and 0.1 mg/L solution of As(V) by Ce-BC | [27] |
| SnO2 nanoparticles (NPs) synthesized using Vitex agnus-castus fruit extract; 0.03–0.24 g/L | Co(II); 100 mg/L | The removal efficiency was higher than 94% at 298 K after 60 min at an adsorbent dosage of 0.12 g/L | [28] |
| Biochar fabricated with MgAl layered double hydroxide (MgAl-LDH) nanosheets; 0.2–1.0 g/L | Pb(II) and CrO42−; 10–500 mg/L and 10–300 mg/L | The adsorption capacity for lead was 591.2 and 330.8 mg/g for CrO42, which is 263% and 416% higher than the adsorption capacity of only the biochar | [29] |
| Superparamagnetic amino/thiol nanoparticle (Fe3O4@SiO2@GLYMO(S)-en) (Glymo(S)-en; thio-(3-Glycidyloxopropyl)trimethoxysilane); 16 mg | Pb(II) and Cd(II); 50 mg/L | Adsorption capacity of 93.5 mg/g for lead and 89.64 mg/g for cadmium at pH 7 and contact time of 55 min | [30] |
| Ccarboxymethyl cellulose stabilized FeS NPs (CMC-FeS) (0.15 mg/L) | Hg (0.6 mg/L) | The maximal sorption of 3358.28 mg/g Hg by CMC-FeS | [31] |
| NiO-MgO-SBNs; 25 mg | Cu(II), Cr(III), and Zn(II); 50–400 mg/L | The adsorption capacity for Zn(II), Cu(II), and Cr(III) was 37.69, 69.68, and 209.5 mg/g, respectively, at pH 5.5 | [32] |
| Fe and Cu oxide NPs stabilized by rice-husk biochar; 10 g | As(III) and As(V); 0.5–128 mg/L | The removal efficiency of As(III) + As(V) (70 mg/L) was 95.3% at pH 7 in 60 min of contact time | [33] |
| HTO NPs supported by rice straw (RS-HTO) via sol-gel method; 2 g/L | Cu(II); 10 mg/L | The adsorptive removal efficiency was more than 99% by RS-HTO at pH 7.5 | [34] |
| Humic acid coated magnetic nanoadsorbent (HA/Fe3O4); 2–20 mg/ml | V(IV); 50 mg/L | With the Langmuir isotherm model, the maximal adsorption capacity for vanadium was 8.97 mg/g at pH 5 | [35] |
| Polypyrrole functionalized magnetic Fe3O4 nanoparticle (Ppy@Fe3O4); 0.05 g/L | Ni(II) and Cr(VI); 10–40 mg/L | The maximal adsorption caapacity was 19.92 mg/g for Ni(II) at pH 6 in 150 min and 344.82 mg/g for Cr(VI) at pH 2 in 60 min of contact time | [36] |
| Fe3O4 sulfonated magnetic NP (Fe3O4-SO3H MNP); 10 mg | Cd(II) and Pb(II) 10–200 mg/L | Fe3O4-SO3H MNP showed maximal adsorption of 108. 93 and 80.9 mg/g for Pb and Cd, respectively | [37] |
| Lignin hydrogels loaded with nano-FeS with variable level of polymerization (LH1–LH6) and NPs concentration | Cd(II) (100 mg/L) and tetracycline | The removal capacity of hydrogels for both contaminants was increased initially (up to LH3/4) due to the increasing content and decreasing size of FeS NPs | [38] |
2. Nanoparticle Mechanisms for the Removal of Metals
There are a number of methods that can be applied to treat wastewater and remove metals, such as chemical precipitation, oxidation reduction, ion exchange, and adsorption. An important and beneficial condition for the removal of metals is the availability of a large surface area for achieving greater metal removal in one cycle of operation, and this condition is well-satisfied by nanomaterials. Nanomaterial adsorbents have a nanoscale size providing larger surface area in a specific volume as compared to the bulk material. The larger surface area also provides more reactive surfaces in the case of chemically modified NPs. The interaction mechanisms that enable the removal of metal ions from aqueous solutions are still unknown [39]. The majority of remediation techniques used to remove these contaminants involve sorption, sorption reduction, photocatalysis, and precipitation (Figure 3) [40]. Due to the chemical interactions between nanomaterials and metal ions, sorption is one of the most basic methods for removing metal ions from contaminated water [41]. In the adsorption mechanism, nanomaterials such as mesoporous silica entrap metal ions with large adsorptive surfaces and via functional groups involving both physical and chemical interactions. Nanoadsorbents also offer the unique opportunity of regenerating them through chemical processes such as desorption and reusing for several cycles [42]. Similar to immobilization, sorption reduction is a technique for changing high-valent metal ions into low-valent ones. First, high-valent metal ions are reduced, resulting in denser particles or clusters that precipitate more easily. Similarly, in precipitation, various nanoparticles are used to modify metal ions to insoluble precipitates by converting them into hydroxides or carbonates etc. The solid precipitate can be separated later with filtration [43]. The conversions of Se4+ into Se2+ and Cr6+ into Cr3+ are two frequent instances of these sorption-reduction processes. The photocatalytic degradation process has been extensively used to remove low concentrations of metals [44][45] despite the fact that it is frequently used to remove various organic contaminants. This method is based on photocatalytic reactions that are greatly influenced by the catalyst’s shape, the absorption of visible light, and active sites. Depending on the type of metal ions and light sources, the mechanisms for metal ions may change [46]. Moreover, the ion exchange mechanisms operate on the basis of the mutual exchange of cationic or anionic metal species with the ionic ligands attached to the nanomaterials [47].
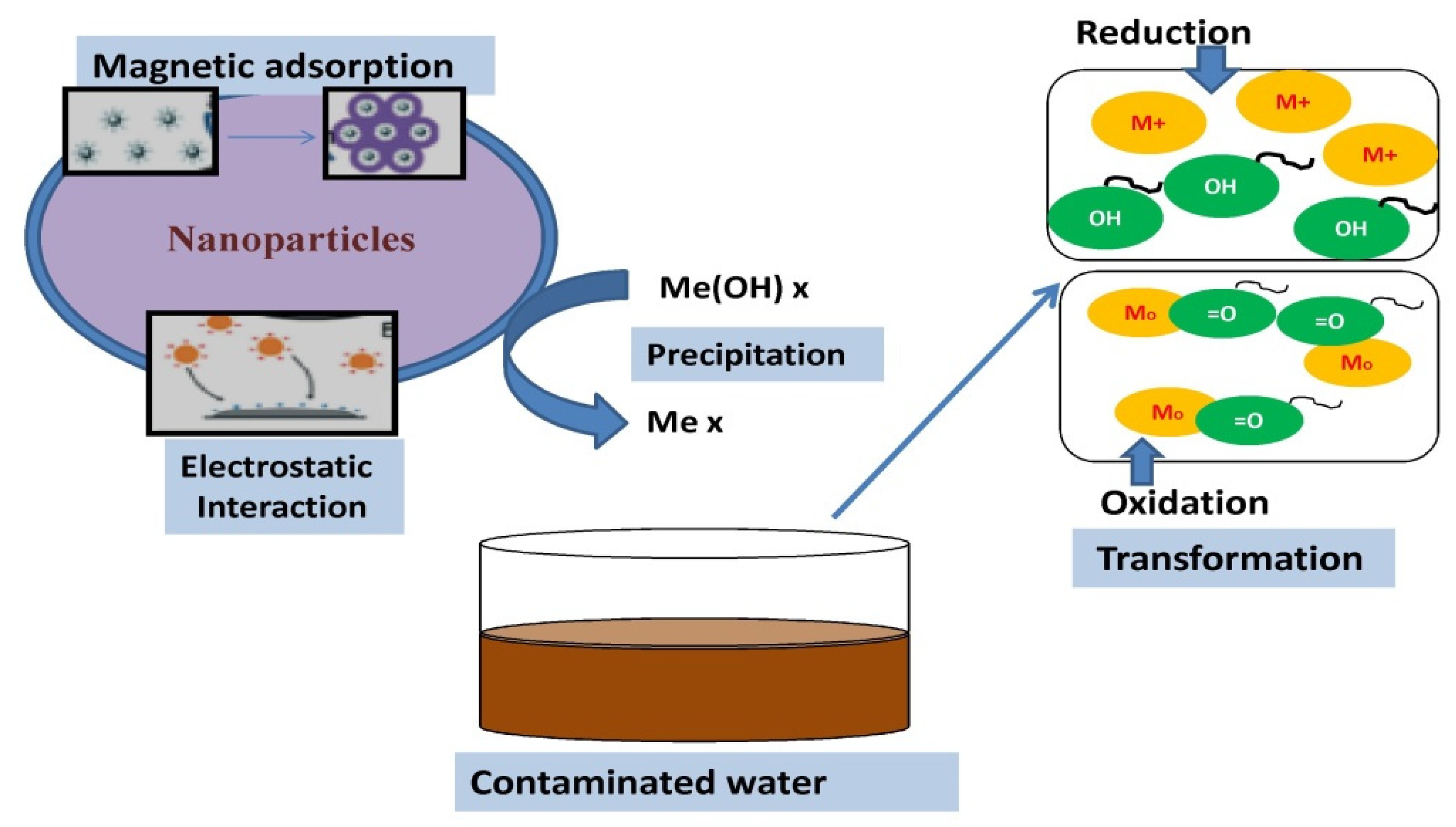
Figure 3. Key processes of the remediation of pollutants in contaminated water by nanoparticles.
References
- Upadhyay, M.K.; Majumdar, A.; Srivastava, A.K.; Bose, S.; Suprasanna, P.; Srivastava, S. Antioxidant enzymes and transporter genes mediate arsenic stress reduction in rice (Oryza sativa L.) upon thiourea supplementation. Chemosphere 2022, 292, 133482.
- Shahid, M.; Khalid, S.; Saleem, M. Unrevealing arsenic and lead toxicity and antioxidant response in spinach: A human health perspective. Environ. Geochem. Health 2022, 44, 487–496.
- Abdeldayem, R. Domestic water and accumulating mercury toxicity in the kidney. Appl. Water Sci. 2022, 12, 114.
- Wise, J.P., Jr.; Young, J.L.; Cai, J.; Cai, L. Current understanding of hexavalent chromium neurotoxicity and new perspectives. Environ. Int. 2022, 158, 106877.
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology—Historical Review and Commentary. Biomolecules 2022, 12, 360.
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Mondal, M.H.; Bhattarai, A.; Saha, B. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Adv. 2022, 12, 9139–9153.
- Rajput, V.D.; Minkina, T.; Upadhyay, S.K.; Kumari, A.; Ranjan, A.; Mandzhieva, S.; Sushkova, S.; Singh, R.K.; Verma, K.K. Nanotechnology in the Restoration of Polluted Soil. Nanomaterials 2022, 12, 769.
- Nikam, P.B.; Salunkhe, J.D.; Minkina, T.; Rajput, V.D.; Kim, B.S.; Patil, S.V. A review on green synthesis and recent applications of red nano Selenium. Results Chem. 2022, 4, 100581.
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. In Heavy Metals; Saleh, H.E.M., Aglan, R.F., Eds.; IntechOpen: London, UK, 2018; Volume 10, pp. 115–132.
- Zhang, Y.; Tang, Z.R.; Fu, X.; Xu, Y.J. TiO2−graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2−graphene truly different from other TiO2−carbon composite materials? ACS Nano 2010, 4, 7303–7314.
- Chang, Z.; Zeng, L.; Sun, C.; Zhao, P.; Wang, J.; Zhang, L.; Zhu, Y.; Qi, X. Adsorptive recovery of precious metals from aqueous solution using nanomaterials—A critical review. Coord. Chem. Rev. 2021, 445, 214072.
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Siddiqui, M.N.; Agarwal, S. Chromium removal from water by activated carbon developed from waste rubber tires. Environ. Sci. Pollut. Res. 2013, 20, 1261–1268.
- Zha, M.; Liu, J.; Wong, Y.L.; Xu, Z. Extraction of palladium from nuclear waste-like acidic solutions by a metal–organic framework with sulfur and alkene functions. J. Mater. Chem. A 2015, 3, 3928–3934.
- Srivastava, A.; Singh, R.; Rajput, V.D.; Minkina, T.; Agarwal, S.; Garg, M.C. A systematic approach towards optimization of brackish groundwater treatment using nanofiltration (NF) and reverse osmosis (RO) hybrid membrane filtration system. Chemosphere 2022, 303, 135230.
- Kothavale, V.P.; Sharma, A.; Dhavale, R.P.; Chavan, V.D.; Shingte, S.R.; Selyshchev, O.; Dongale, T.D.; Park, H.H.; Zahn, D.R.T.; Salvan, G.; et al. Hyperbranched amino modified magnetic nanoparticles for simultaneous removal of heavy metal ions from aqueous solutions. Mater. Chem. Phys. 2022, 292, 126792.
- Prema, P.; Nguyen, V.H.; Venkatachalam, K.; Murugan, J.M.; Ali, H.M.; Salem, M.Z.M.; Ranvindran, B.; Balaji, P. Hexavalent chromium removal from aqueous solutions using biogenic iron nanoparticles: Kinetics and equilibrium study. Environ. Res. 2022, 205, 112477.
- Zhang, W.; Ou, J.; Wang, B.; Wang, H.; He, Q.; Song, J.; Zhang, H.; Tang, M.; Zhou, L.; Gao, Y.; et al. Efficient heavy metal removal from water by alginate-based porous nanocomposite hydrogels: The enhanced removal mechanism and influencing factor insight. J. Hazard. Mater. 2021, 418, 126358.
- Raval, N.P.; Kumar, M. Geogenic arsenic removal through core–shell based functionalized nanoparticles: Groundwater in-situ treatment perspective in the post–COVID anthropocene. J. Hazard. Mater. 2021, 402, 123466.
- Lilhare, S.; Mathew, S.B.; Singh, A.K.; Carabineiro, S.A.C. Calcium alginate beads with entrapped iron oxide magnetic nanoparticles functionalized with methionine—A versatile adsorbent for arsenic removal. Nanomaterials 2021, 11, 1345.
- Geng, B.; Xu, Z.; Liang, P.; Zhang, J.; Christie, P.; Liu, H.; Wu, S.; Liu, X. Three-dimensional macroscopic aminosilylated nanocellulose aerogels as sustainable bio-adsorbents for the effective removal of heavy metal ions. Int. J. Biol. Macromol. 2021, 190, 170–177.
- Li, Z.; Gong, Y.; Zhao, D.; Dang, Z.; Lin, Z. Enhanced removal of zinc and cadmium from water using carboxymethyl cellulose-bridged chlorapatite nanoparticles. Chemosphere 2021, 263, 128038.
- Ha, H.T.; Phong, P.T.; Minh, T.D. Synthesis of iron oxide nanoparticle functionalized activated carbon and its applications in arsenic adsorption. J. Anal. Meth. Chem. 2021, 2021, 6668490.
- Sreekala, G.; Beevi, A.F.; Resmi, R.; Beena, B. Removal of lead (II) ions from water using copper ferrite nanoparticles synthesized by green method. Mater. Today Proc. 2021, 45, 3986–3990.
- Bahador, F.; Foroutan, R.; Esmaeili, H.; Ramavandi, B. Enhancement of the chromium removal behavior of Moringa oleifera activated carbon by chitosan and iron oxide nanoparticles from water. Carbohydr. Polym. 2021, 251, 117085.
- Hao, L.; Li, L.; Yu, S.; Liu, J. Humic acid-coated hydrated ferric oxides-polymer nanocomposites for heavy metal removal in water. Sci. Total Environ. 2022, 834, 155427.
- Wang, Y.; Chen, X.; Yan, J.; Wang, T.; Xie, X.; Yang, S. Efficient removal arsenate from water by biochar-loaded Ce3+-enriched ultra-fine ceria nanoparticles through adsorption-precipitation. Sci. Total Environ. 2021, 794, 148691.
- Ebrahimian, J.; Mohsennia, M.; Khayatkashani, M. Photocatalytic-degradation of organic dye and removal of heavy metal ions using synthesized SnO2 nanoparticles by Vitex agnus-castus fruit via a green route. Mater. Lett. 2020, 263, 127255.
- Wang, H.; Wang, S.; Chen, Z.; Zhou, X.; Wang, J.; Chen, Z. Engineered biochar with anisotropic layered double hydroxide nanosheets to simultaneously and efficiently capture Pb2+ and CrO42− from electroplating wastewater. Bioresour. Technol. 2020, 306, 123118.
- Masjedi, A.; Askarizadeh, E.; Baniyaghoob, S. Magnetic nanoparticles surface-modified with tridentate ligands for removal of heavy metal ions from water. Mater. Chem. Phys. 2020, 249, 122917.
- Wang, M.; Li, Y.; Zhao, D.; Zhuang, L.; Yang, G.; Gong, Y. Immobilization of mercury by iron sulfide nanoparticles alters mercury speciation and microbial methylation in contaminated groundwater. Chem. Eng. J. 2020, 381, 122664.
- Abuhatab, S.; El-Qanni, A.; Al-Qalaq, H.; Hmoudah, M.; Al-Zerei, W. Effective adsorptive removal of Zn2+, Cu2+, and Cr3+ heavy metals from aqueous solutions using silica-based embedded with NiO and MgO nanoparticles. J. Environ. Manag. 2020, 268, 110713.
- Priyadarshni, N.; Nath, P.; Chanda, N. Sustainable removal of arsenate, arsenite and bacterial contamination from water using biochar stabilized iron and copper oxide nanoparticles and associated mechanism of the remediation process. J. Water Process Eng. 2020, 37, 101495.
- Chen, Y.; Shi, H.; Guo, H.; Ling, C.; Yuan, X.; Li, P. Hydrated titanium oxide nanoparticles supported on natural rice straw for Cu(II) removal from water. Environ. Technol. Innov. 2020, 20, 101143.
- Zeinali, S.; Tatian, S. Vanadium Removal from Fuel Oil and Waste Water in Power Plant Using Humic Acid Coated Magnetic Nanoparticles. Int. J. Nanosci. Nanotechnol. 2019, 15, 249–263.
- Chithra, K.; Akshayaraj, R.T.; Pandian, K. Polypyrrole-protected magnetic nanoparticles as an excellent sorbent for effective removal of Cr(VI) and Ni(II) from effluent water: Kinetic studies and error analysis. Arab. J. Sci. Eng. 2018, 43, 6219–6228.
- Chen, K.; He, J.; Li, Y.; Cai, X.; Zhang, K.; Liu, T.; Hu, Y.; Lin, D.; Kong, L.; Liu, J. Removal of cadmium and lead ions from water by sulfonated magnetic nanoparticle adsorbents. J. Colloid Interface Sci. 2017, 494, 307–316.
- Liu, Y.; Chen, H.; Mo, Q.; Yang, X.; Wang, J.; Lin, X.; Shang, D.; Li, Y.; Zhang, Y. Removal of cadmium and tetracycline by lignin hydrogels loaded with nano-FeS: Nanoparticle size control and content calculation. J. Hazard Mater. 2021, 416, 126262.
- Hubicki, Z.; Kołodynska, D. Selective removal of heavy metal ions from waters and waste waters using ion exchange methods. Ion Exch. Technol. 2012, 7, 193–240.
- Lee, J.; Lee, J.K.; Uhm, S.; Lee, H.J. Electrochemical technologies: Water treatment. Appl. Chem. Eng. 2011, 22, 235–242.
- Xiong, C.; Wang, W.; Tan, F.; Luo, F.; Chen, J.; Qiao, X. Investigation on the efficiency and mechanism of Cd(II) and Pb(II) removal from aqueous solutions using MgO nanoparticles. J. Hazard. Mater. 2015, 299, 664–674.
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora, L.E.; Ni, M.; Alexis, F.; Guerrero, V. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504.
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621.
- Ettre, L.S. Nomenclature for chromatography (IUPAC Recommendations 1993). Pure Appl. Chem. 1993, 65, 819–872.
- Shukor, S.A.A.; Hamzah, R.; Bakar, M.A.; Noriman, N.Z.; Al-Rashdi, A.A.; Razlan, Z.M.; Shahriman, A.B.; Zunaidi, I.; Khairunizam, W. Metal oxide and activated carbon as photocatalyst for waste water treatment. IOP Conf. Ser. Mater. Sci. Eng. 1993, 557, 012066.
- Le, A.T.; Pung, S.Y.; Sreekantan, S.; Matsuda, A.; Huynh, D.P. Mechanisms of removal of heavy metal ions by ZnO particles. Heliyon 2019, 5, e01440.
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946.
More
Information
Subjects:
Water Resources
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
21 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




