Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Naser Alsharairi | -- | 3334 | 2022-12-20 07:23:32 | | | |
| 2 | Amina Yu | + 11 word(s) | 3345 | 2022-12-21 02:15:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Alsharairi, N.A. Dietary Antioxidants and Lung Cancer in Smokers, Non-Smokers. Encyclopedia. Available online: https://encyclopedia.pub/entry/38984 (accessed on 07 February 2026).
Alsharairi NA. Dietary Antioxidants and Lung Cancer in Smokers, Non-Smokers. Encyclopedia. Available at: https://encyclopedia.pub/entry/38984. Accessed February 07, 2026.
Alsharairi, Naser A.. "Dietary Antioxidants and Lung Cancer in Smokers, Non-Smokers" Encyclopedia, https://encyclopedia.pub/entry/38984 (accessed February 07, 2026).
Alsharairi, N.A. (2022, December 20). Dietary Antioxidants and Lung Cancer in Smokers, Non-Smokers. In Encyclopedia. https://encyclopedia.pub/entry/38984
Alsharairi, Naser A.. "Dietary Antioxidants and Lung Cancer in Smokers, Non-Smokers." Encyclopedia. Web. 20 December, 2022.
Copy Citation
Smoking is the major cause of cancer mortality, responsible for 64.2% of global lung cancer (LC)-related deaths in 2019. There is clear evidence from longitudinal and/or case–control studies that support the link between current smoking and LC risk. Secondhand smoke (SHS) as well as other environmental and genetic factors are potential risk factors for the development of LC in non-smokers. Approximately 6% of global non-smoker deaths from LC in 2019 are caused by SHS exposure.
dietary antioxidants
vitamins
minerals
oxidative stress
smokers
1. Cigarette Smoke-Induced Oxidative Stress in Lung Cancer
Oxidative stress (OS) is considered the key mechanism responsible for the development of smoking-related lung cancer (LC). Levels of OS and lipid peroxidation (LP) were found to be higher, and antioxidant enzymes and vitamins/minerals were lower in current and former smokers than non-smokers. A recent review suggests that there are lower plasma levels of β-carotene, BCX, lycopene, vitamin E, vitamin C and selenium in current smokers than non-smokers [1]. In one cross-sectional study, female current, but not former smokers have higher levels of urinary 8-hydroxy-20-deoxyguanosine (8OHdG), a biomarker for cigarette smoke (CS)-induced oxidative damage, than their male counterparts; although they had elevated serum levels of β-carotene and BCX. This is possibly due to low levels of erythrocyte superoxide dismutase (SOD), an antioxidant enzyme that degrades superoxide free radicals, thereby increasing oxidative DNA damage and driving gene mutation in LC cells [2]. SOD activity was found to be lower in male heavy smokers compared to male non-smokers [3]. In a case-control study that examined the effects of CS on salivary antioxidant activity found that SOD activity was lower in male current smokers than non-smokers [4]. Results from a case-control study showed that current smokers have higher nitrite/nitrate, carbonyl and iron levels, erythrocytes nitric oxide synthase (NOS) protein expression, and lower plasma vitamin C and glutathione (GSH) enzyme, compared to non-smokers [5]. In a case-control study of antioxidant activity in erythrocytes of NSCLC cases compared with healthy controls, SOD activity was found to be lower, while glutathione peroxidase (GPx) was found to be higher in current and non-smokers [6]. Evidence from an randomised controlled trial (RCT) revealed an increase in the urinary 8OHdG levels of men and women current smokers compared to their non-smoker counterparts [7]. Experimental studies showed that high levels of 8-OHdG and low levels of antioxidant capacity (AOC) were associated with non-smoking patients with NSCLC compared to smokers [8][9]. High urinary 8-OHdG levels have been reported in non-smokers [10]. An experimental study suggested that an oxidant and antioxidant imbalance exists in smokers as compared with non-smokers. Results showed that the serum malondialdehyde (MDA) level, a lipid peroxidation product assessed as a biomarker of OS, is increased, whereas the serum paraoxonase 1 (PON1) level, an antiatherogenic enzyme that hydrolyzes oxidized lipids and paraoxon in Clara cells of the lung is decreased in current smokers compared with those of non-smokers [11].
2. Potential Role of Dietary Antioxidants against Cigarette Smoke-Induced Oxidative Stress in Lung Cancer
2.1. Antioxidant Vitamins
2.1.1. Retinol
Retinol, retinal or retinoid acid (RA), which is derived from protein/animal sources (e.g., chicken, egg), may explain the association with LC risk in smokers [12]. RA was reported to promote inflammation-induced oxidative DNA damage in NSCLC cell line A549 in smokers by suppressing hydrogen peroxide (H2O2)-utilizing myeloperoxidase (MPO) enzyme produced by activated neutrophils, thereby increasing reactive oxygen species (ROS) and hydroxyl radical production [13]. Nicotine has been shown to suppress the growth inhibitory effects of trans-RA in NSCLC cell line H460 by inhibiting its ability to enhance RA receptor beta (RARβ) expression due to the increase an orphan RA receptor TR3 expression, suggesting that RA may be ineffective in reducing LC risk in smokers [14]. Bexarotene (Targretin, LGD1069), a synthetic retinoid, has demonstrated significant inhibition of the metastasis and angiogenesis of NSCLC cells by suppressing vascular endothelial growth factor (VEGF) and metalloproteinase (MMP) expression through downregulation of the α7nAChR-mediated c-JUN NH2-terminal kinase/extracellular signal-regulated kinase (JNK/ERK) signaling pathway [15]. Treatment with RA in combination with ERK inhibitors may suppress the migration of NSCLC cells in vitro [15]. Herein, it is showed that dietary retinol intake was associated with increased LC risk in male current smokers. Such an association may be due to the interaction of retinol and the CS in LC cells, resulting in a reduction of RAR-β expression and activation of proliferative markers. However, the results demonstrated a reduction in LC risk among female current smokers with dietary retinol intake. The reasons for the contrast of such an association require further clarification as to possible mechanisms.
2.1.2. β-Cryptoxanthin and Lycopene
BCX and lycopene, which are commonly derived from fruits, may play a vital role as LC preventive agents in smokers. BCX exerts anti-proliferative and apoptotic/autophagic effects on nicotine-induced A549 cells in vitro by induction of G1/G0 phase arrest via inhibiting cyclinD1/E and upregulating P21 expression [16]. BCX also exerts a protective effect against NNK-induced lung tumours in vivo by inhibiting the protein and mRNA levels of the α7nAChR via downregulating the α7nAChR/phosphatidylinositol-3 kinase (PI3K) signaling pathway [17]. In vivo, BCX supplementation has been shown to inhibit CS exposure-induced lung inflammation, as evident by reducing the levels of 8OHdG, tumour necrosis factor-α (TNFα), nuclear transcription factor-kappaB (NF-κB) and activator protein-1 (AP)-1 in lung macrophages [18]. Another in vivo study showed that BCX exerts protective effects against LC and emphysema by reducing nicotine/NNK-induced Sirtuin 1 (SIRT1) mRNA, reducing RARβ and p53 mRNA levels and increasing serine-threonine kinase (AKT) phosphorylation levels [19]. Treatment of A549 cells with lycopene resulted in inhibition of CS-induced OS in vitro by enhancing connexin-43 (Cx43) protein, 8-oxoguanine DNA glycosylase (OGG1) and Nei-like DNA glycosylases (NEIL1, NEIL2, NEIL3) levels, suggesting that the carotenoid lycopene may have therapeutic potential in smoke-induced LC by modulating the expression of multiple DNA repair genes [20]. Treatment with lycopene also triggers a protective effect against LC in vivo by inhibiting NNK-induced α7nAChR expression [21]. Lycopene exerts antioxidant effects in vivo by improving pulmonary emphysema by reducing CS-derived MPO/NO-induced LP/DNA damage and increasing erythrocyte SOD and GPx activity. It also exerts anti-inflammatory effects by reducing TNF-α, interferons (IFN-γ) and IL-10 cytokine production [22]. High dietary lycopene intake was shown to suppress CS/NNK-induced emphysema in vivo by reducing mRNA expression of ATP-binding cassette transporter G1 (ABCG1), liver X receptor-α (LXRα) and peroxisome proliferator-activated receptor α (PPARα) involved in reverse cholesterol transport, which is the main driver of persistent and excessive inflammation after CS exposure [23]. It is suggested that lycopene and BCX may play a significant role in reducing LC risk in smokers.
2.1.3. β-Carotene, Vitamins C and E
Several studies of vitamin supplements (e.g., vitamins C and E, β-carotene) provide contradictory results for protection against LC in smokers [24]. Limited evidence from RCTs showed that supplementation with β-carotene increases LC risk and mortality among current smokers [24]. β-carotene is considered an antioxidant-beneficial agent in human and animal lung tissues due to its ability to reduce CS-induced LP. However, their benefits may shift from being an antioxidant to a prooxidant causing oxidative DNA damage in lung tissue when exposed to tobacco smoke condensate via a range of mechanisms including downregulating RA signaling, elevating markers of OS, increasing molecular expressions involved in cellular proliferation/apoptosis, and the production of carotenoid oxidation products [25]. An in vitro experimental study showed that β-carotene does not promote LP levels in liposomal membrane tissue caused by smoking or reduce other antioxidant vitamins such as α-tocopherol and ascorbate. β-carotene, the so-called non-polar carotenoid, was found to be sensitive to CS-induced oxidation and its levels in methanol were depleted in response to CS exposure [26]. β-carotene is sensitive to CS-dependent oxidation in such a way that it can react with a mixture of nitrogen oxides (NO2), peroxynitrite and volatile peroxyl radicals to generate a β-carotene radical via the synthesis of the cation radical [27][28]. It can be suggested that, although β-carotene is oxidized by smoke, it is unlikely to increase LC risk in smokers. The treatment of pneumocyte cells with β-carotene and its short-chained volatile aldehydes in the presence of oxidative stress resulted in a significant decrease in DNA damage even at a concentration of 50μM [29], suggesting that β-carotene acts as an antioxidant and may have a protective effect against LC in smokers.
β-carotene, α-tocopherol and ascorbic acid have been shown to attenuate CS-induced NO2 and aldehydes, which cause biomolecular damage enhancing LP through free radical mechanisms [30]. β-carotene and vitamin E levels in heavy smokers were associated with lung function decline in longitudinal 8 years of follow-up, suggesting that such antioxidants may protect lung tissue by reducing OS induced by CS [31]. Low plasma α-tocopherol and ascorbic acid levels have been observed in smokers more than non-smokers [32]. In an in vitro experimental study, vitamin C was been shown to reduce aqueous extract of CS-induced A549 cell proliferation by deactivating p-benzoquinone, thereby downregulating EGFR and its downstream molecules, suggesting that vitamin C may have potential as an antioxidant for reducing LC risk in smokers [33].
Antioxidant supplementation with vitamins C, E and β-carotene resulted in significantly decreased urinary 8OHdG and carbonyl levels in current smokers [34]. Vitamin C and E supplementation were found to be effective in reducing 8-epiPGF2α in the plasma of current smokers [35]. A randomized placebo-controlled trial resulted in decreased urine 8-iso-PGF2α by 21% in male current smokers after receiving 400IU/d of vitamin E (rac-α-tocopheryl acetate) over 36 months compared to those in the placebo group (no vitamin E supplementation) [36]. An RCT which examined the effect of antioxidant vitamins consumption on LP in male smokers showed a significant reduction in plasma MDA levels over one month of vitamin supplementation (500 mg vitamin C, 9 µg β-carotene, 200 IU vitamin E) compared to non-smokers [37]. Vitamin E supplementation has been shown to improve lung function in current smokers by reducing plasma MDA levels and increasing SOD and GPx activity [38]. A case-control study showed that supplementation with antioxidant vitamins (60 mg vitamin C, 3000 µg β-carotene, 30 IU vitamin E) over 60 days decreased 8-oxodGuo by 62% in groups exposed to tobacco smoke compared with controls [39].
A few cohort studies have demonstrated a reduction in LC risk among male smokers receiving α-tocopherol supplementation [40][41]. CS oxidizes α- and γ-tocopherol, leading to reduced plasma levels in smokers compared with non-smokers [42][43], and supplementation with ascorbic acid could have a significant effect in decreasing α-and γ-tocopheroxyl free radicals to nonoxidized forms, thereby increasing tocopherol plasma levels [43]. A placebo-controlled parallel intervention study showed a reduction in oxidative DNA damage in mononuclear blood cells in male smokers exposed to the vitamin C (500 mg/d) and vitamin E (182 mg/d) intervention over a four week period compared to those in the placebo group (no vitamin C or E supplementation) [44]. In another double-blind, placebo-controlled investigation, male smokers received either the daily dietary supplement (31 mg all-rac-α-tocopheryl acetate 272 mg vitamin C) or placebo (non-digestible carbohydrate and <2% stearic acid) for three months. Results showed that plasma α-tocopherol levels were increased in both groups, while plasma vitamin C levels were only significantly repleted in the intervention group [45].
One RCT study suggests that high-dose multiple antioxidant vitamins (1050 mg/day vitamin E, 6100 mg/day ascorbic acid and 60 mg/day β-carotene) used in combination with chemotherapy (carboplatin and paclitaxel) failed to protect NSCLC cells from free radical damage [46]. Treatment of H460 cells with the chemotherapeutic drug paclitaxel in the presence of α-tocopheryl succinate, a powder form of α-tocopherol, showed apoptotic effects through increased poly ADP ribose polymerase (PARP) and caspase 8 expression [47].
Taken together, β-carotene and vitamins C and E appear to have protective effects against CS-induced OS in LC smokers. Supplementation with these antioxidants resulted in reduced OS biomarkers. Vitamin E in combination with chemotherapy may have useful apoptotic effects against nicotine-induced NSCLC.
Herein, a reduction in LC risk among male current smokers with a high intake of dietary carotenoids (β-carotene, BCX and lycopene) was domenstrated, and female current smokers with a high intake of vitamin C. High dietary intake of β-carotene and vitamin E was associated with reduced LC risk in non-smokers, while high dietary intake of vitamin E only was associated with reduced risk of LC in both current and former smokers. The underlying reasons for such effects are unclear. Given that smokers and non-smokers are susceptible to CS-induced OS, but current and former smokers to higher levels than non-smokers, dietary carotenoids, vitamins C and E may play a significant role in protecting lung cells against CS-derived free radicals/oxidant-induced oxidative damage in LC. Further studies are needed to explore gender differences in dietary antioxidant vitamins intake and LC risk, and how these relationships may relate to smoking status.
2.1.4. Vitamin D
Vitamin D as a potent antioxidant has been implicated in reducing NSCLC risk via α7nAChR-mediated cellular signaling pathway inhibition, but there is no evidence to date on its association with reducing CS-induced OS/LP in LC smokers and non-smokers. A meta-analysis of 24 case-control and cohort studies showed that smoking was associated with lower blood 25(OH)D levels in smokers and non-smokers [48]. In a longitudinal cohort study with follow-up over 20 years, vitamin D deficiency was found to be associated with lung function decline in current smokers [49]. In vivo, vitamin D has been shown to protect lung cells from CS-induced oxidative damage [50]. A randomized, double-blind trial demonstrated significantly improved survival of lung ADC patients with lower 25(OH)D levels receiving vitamin D supplements (1200 IU/day) over a one-year period compared with those in placebo groups (not taking a supplement) [51]. In vitro, vitamin D suppresses proliferation/invasion and induces apoptosis of A549/NCI-H1975 cells, and enhances the sensitivity of these cells to the chemotherapeutic drug cisplatin. Such an effect is mediated by downregulating the PI3K/AKT/mammalian target of the rapamycin (mTOR) signaling pathway [52]. Treatment with platinum-based doublet first-line chemotherapy resulted in a significant reduction of plasma vitamin D (25(OH)D) levels in advanced NSCLC patients [53]. This suggests that vitamin D could be beneficial alone or in combination with chemotherapy to reduce LC risk in smokers. Herein, the preventive effect of vitamin D was observed in LC non-smokers exposed to passive smoke. The reason underlying this is that non-smokers might benefit from eating a diet rich in vitamin D, which could mitigate CS-induced OS, given that non-smokers are susceptible to CS-induced OS but to a lower extent than smokers.
2.2. Antioxidant Minerals
2.2.1. Iron
The high intake of iron is not advocated as it results in significant increases in CS-induced OS in LC smokers. A recent meta-analysis of 20 prospective and case-control studies which examined the associations between different iron biomarkers/iron intake and LC risk, showed that transferrin saturation and serum ferritin high levels were associated with LC risk, whereas iron intake (heme iron, non-heme iron or total iron) had no effect [54]. High dietary iron intake has been shown to increase urinary MDA and cotinine levels in men and women exposed to CS [55]. It has been found that the 8-isoprostane levels were increased among smokers with a high iron intake [56]. High levels of iron in LC cells not only induce oxidative DNA damage but also ferroptosis, a novel iron-dependent type of regulated necrosis, caused by the accumulation of ROS through LP [57]. Cigarette smoke extract exposure upregulates heme oxygenase-1 (HO-1) expression in human lung cells [58][59], an enzyme responsible for catalyzing the degradation of exogenous heme to produce biliverdin, carbon monoxide and ferrous ions, in parallel with a reduction in GSH, resulting in induced ferroptosis and increased deca-pentaplegic homolog (Smad)-dependent pathways (e.g., c-Fos and c-Jun) expression [59]. In vivo, NNK has been shown to induce HO-1 expression in LC cells via the upregulation of NF-κB and ERK1/2 signaling pathways [60]. Results obtained here demonstrated that a reduction in LC risk was associated with total iron, but not with haem and non-haem intake in former smokers. Such an association is inconsistent with the fact that iron intake increases LC risk in smokers. The mechanism behind such an effect is largely unknown. It is hypothesized that lymphoid-specific helicase (LSH), a DNA methylation modifier stabilizing the transcripts that enhances tumourigenesis in NSCLC [61], reduces CS-induced ferroptosis by interacting with stearoyl-coa desaturase-1 (SCD-1), which is an iron-containing microsomal enzyme which exists in the endoplasmic reticulum responsible for the degradation of saturated fatty acid into monounsaturated fatty acid [62]. The reduction of SCD-1 expression inhibits proliferation and induces apoptosis in NSCLC cells through reducing α7nAChR-mediated Akt phosphorylation [63]. Thus, LSH and its ferroptosis-related gene SCD-1 exert anti-tumour effects, which may reduce CS-induced ferroptosis by reducing intracellular haem and non-haem levels in NSCLC cells.
2.2.2. Copper, Zinc and Selenium
There is controversy over the effects of copper on LC risk in smokers, whereas zinc and selenium could be beneficial in reducing the risk. Here, it is demonstrated that a high intake of dietary zinc and copper was associated with a reduction in LC risk in current smokers. On the other hand, selenium intake had no effect on LC risk in smokers. A few meta-analyses of case-control studies have shown higher serum copper levels but lower serum zinc levels in LC patients than among healthy controls [64][65]. In vitro, treatment of human erythrocytes with hydroquinone, an ingredient of cigarette smoking, alone or in the presence of copper, increases H2O2 production and reduces GSH levels. This may be due to the binding of copper to the thiol group of reduced GSH or to the 1, 4 benzoquinone-GSH adduct production, suggesting that copper may increase erythrocyte membrane OS in smokers [66]. In a case-control study, an association between the plasma levels of copper and LP, assessed as fluorescent damage products of LP was observed in greater proportions among smokers as compared to non-smokers [67]. An in vitro study showed that treatment of A549 cells with the aqueous almond skin extract-capped copper nanorods reduced antioxidant enzyme GPx activity and mitochondrial membrane functions causing induction of cellular OS by increasing ROS production in high quantities [68].
In a case-control study that examined associations between serum trace elements status and redox status parameters in LC patients compared with healthy controls, zinc supplementation was positively associated with total antioxidant status and negatively associated with total oxidant status. Results of that study also demonstrated that copper and Cu:Zn ratios were positively associated with SOD activity and negatively associated with catalase activity [69]. An experimental study demonstrated apoptosis induction on A549 cells in vitro when treated with zinc in different concentrations in combination with docetaxel (Taxotere), a semisynthetic antineoplastic agent used for NSCLC treatment, through upregulating expression of p53. Such an effect is accompanied by an increase in GSH and GPx activity [70]. An experimental study that investigated associations between plasma mineral levels and erythrocyte antioxidative enzyme activity in smokers, demonstrated a positive association between consumption of selenium and GPx, copper and copper-zinc superoxide dismutase (CuZnSOD), and a negative association between selenium and plasma thiocyanate levels used as an indicator of smoking status [71]. In an in vivo experimental study, CuZnSOD has been shown to reduce oxidative damage and LP in the lungs of mice in response to CS exposure. Results also showed that treatment with SOD causes a significant reduction in the number of A549 cells/macrophages and inhibition of neutrophil chemotaxis following CS exposure [72]. Evidence showed that smokers have higher serum thiobarbituric acid reactive substance levels, which are used as an indicator of CS-induced OS, but lower serum selenium GSH and GPx levels than non-smokers [73]. In vivo, selenium pretreatment of rats exposed to CS showed a significant reduction in the plasma LP, total cholesterol levels and triacylglycerol [74]. A case-control study showed that supplementation with 40 µg selenium, 40 mg zinc and 2 mg copper over 60 days reduced 8-oxodGuo levels in smokers compared with non-smokers [39]. In one experimental in vitro study, treatment with copper alone or in combination with the chemotherapeutic drug disulfiram induced G2/M cell cycle arrest in A549 and NCI-H2009-cells and reduced the mRNA expression of NSCLC-related genes [75]. These findings suggest that zinc and selenium could be helpful in protecting against CS-induced OS in LC smokers. Copper in combination with chemotherapeutics or supplemented with zinc and selenium could be beneficial for reducing CS-induced OS in LC current smokers. Increased CuSOD activity may have a potential antioxidant effect against LC risk in smokers by reducing CS-induced OS/LP. Figure 1 shows the role of dietary antioxidant vitamins and minerals against CS-induced OS in LC.
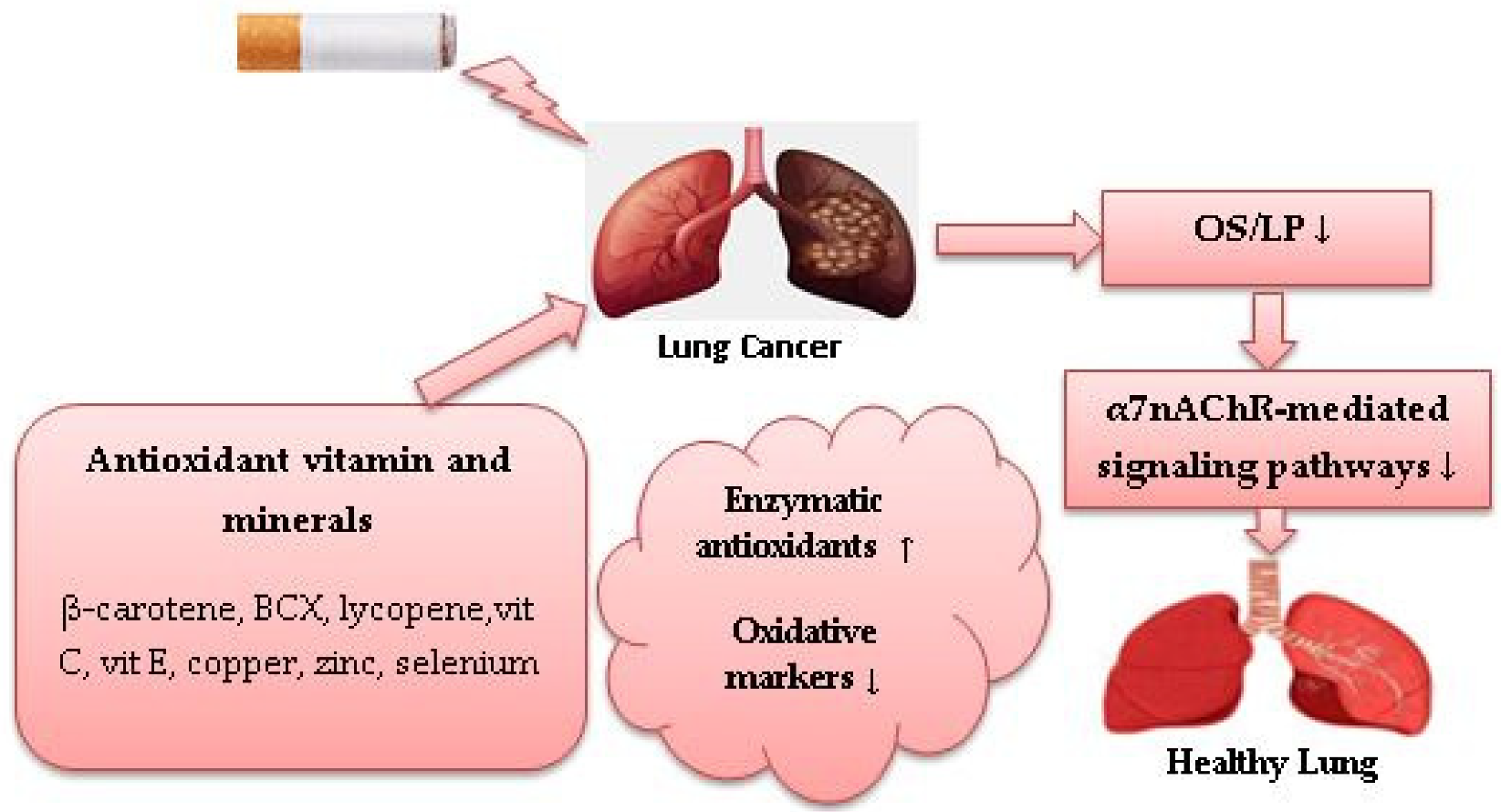
Figure 1. Role of dietary antioxidants against cigarette smoke-induced oxidative stress in lung cancer. (↓) decrease, (↑) increase.
References
- Astori, E.; Garavaglia, M.L.; Colombo, G.; Landoni, L.; Portinaro, N.M.; Milzani, A.; Dalle-Donne, I. Antioxidants in smokers. Nutr. Res. Rev. 2022, 35, 70–97.
- Hakim, I.A.; Harris, R.; Garland, L.; Cordova, C.A.; Mikhael, D.M.; Chow, H.-H.S. Gender difference in systemic oxidative stress and antioxidant capacity in current and former heavy smokers. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2193–2200.
- Orhan, H.; Evelo, C.T.A.; Sahin, G. Erythrocyte antioxidant defense response against cigarette smoking in humans--the glutathione S-transferase vulnerability. J. Biochem. Mol. Toxicol. 2005, 19, 226–233.
- Giuca, M.R.; Giuggioli, E.; Metelli, M.R.; Pasini, M.; Iezzi, G.; D’Ercole, S.; Tripodi, D. Effects of cigarette smoke on salivary superoxide dismutase and glutathione peroxidase activity. J. Biol. Regul. Homeost. Agents 2010, 24, 359–366.
- Padmavathi, P.; Raghu, P.S.; Reddy, V.D.; Bulle, S.; Marthadu, S.B.; Maturu, P.; Varadacharyulu, N.C. Chronic cigarette smoking-induced oxidative/nitrosative stress in human erythrocytes and platelets. Mol. Cell Toxicol. 2018, 14, 27–34.
- Ho, J.C.; Chan-Yeung, M.; Ho, S.P.; Mak, J.C.; Ip, M.C.; Ooi, G.C.; Wong, M.P.; Tsang, K.W.; Lam, W.K. Disturbance of systemic antioxidant profile in nonsmall cell lung carcinoma. Eur. Respir. J. 2007, 29, 273–278.
- Kulikowska-Karpińska, E.; Czerw, K. Estimation of 8-hydroxy-2’-deoxyguanosine (8-OHdG) concentration in the urine of cigarette smokers. Wiad. Lek. 2015, 68, 32–38.
- Ito, K.; Yano, T.; Morodomi, Y.; Yoshida, T.; Kohno, M.; Haro, A.; Shikada, Y.; Okamoto, T.; Maruyama, R.; Maehara, Y. Serum antioxidant capacity and oxidative injury to pulmonary DNA in never-smokers with primary lung cancer. Anticancer Res. 2012, 32, 1063–1067.
- An, A.R.; Kim, K.M.; Park, H.S.; Jang, K.Y.; Moon, W.S.; Kang, M.J.; Lee, Y.C.; Kim, J.H.; Chae, H.J.; Chung, M.J. Association between expression of 8-OHdG and cigarette smoking in non-small cell lung cancer. J. Pathol. Transl. Med. 2019, 53, 217–224.
- Lu, C.-Y.; Ma, Y.-C.; Lin, J.-M.; Chuang, C.-Y.; Sung, F.-C. Oxidative DNA damage estimated by urinary 8-hydroxydeoxyguanosine and indoor air pollution among non-smoking office employees. Environ. Res. 2007, 103, 331–337.
- Isik, B.; Ceylan, A.; Isik, R. Oxidative stress in smokers and non-smokers. Inhal. Toxicol. 2007, 19, 767–769.
- Narita, S.; Saito, E.; Sawada, N.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Ishihara, J.; Takachi, R.; Shibuya, K.; Inoue, M.; et al. Dietary consumption of antioxidant vitamins and subsequent lung cancer risk: The Japan Public Health Center-based prospective study. Int. J. Cancer 2018, 142, 2441–2460.
- Van Helden, Y.G.J.; Keijer, J.; Knaapen, A.M.; Heil, S.G.; Briedé, J.J.; van Schooten, F.J.; Godschalk, R.W.L. Beta-carotene metabolites enhance inflammation-induced oxidative DNA damage in lung epithelial cells. Free Radic. Biol. Med. 2009, 46, 299–304.
- Chen, G.-Q.; Lin, B.; Dawson, M.I.; Zhang, X.-K. Nicotine modulates the effects of retinoids on growth inhibition and RAR beta expression in lung cancer cells. Int. J. Cancer 2002, 99, 171–178.
- Tripathi, S.K.; Pandey, K.; Panda, M.; Spinella, M.J.; Rengasamy, K.R.; Biswal, B.K. The potential of retinoids for combination therapy of lung cancer: Updates and future directions. Pharm. Res. 2019, 147, 104331.
- Lian, F.; Hu, F.-Q.; Russell, R.M.; Wang, X.-D. Beta-cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor beta expression. Int. J. Cancer 2006, 119, 2084–2089.
- Iskandar, A.R.; Miao, B.; Li, X.; Hu, K.-Q.; Liu, C.; Wang, X.-D. β-Cryptoxanthin reduced lung tumor multiplicity and inhibited lung cancer cell motility by downregulating nicotinic ccetylcholine receptor α7 signaling. Cancer Prev. Res. 2016, 9, 875–886.
- Liu, C.; Bronson, R.T.; Russell, R.M.; Wang, X.-D. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev. Res. 2011, 4, 1255–1266.
- Iskandar, A.R.; Liu, C.; Smith, D.E.; Hu, K.-Q.; Choi, S.-W.; Ausman, L.M.; Wang, X.-D. β-Cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev. Res. 2013, 6, 309–320.
- Cheng, J.; Miller, B.; Balbuena, E.; Eroglu, A. Lycopene protects against smoking-induced lung cancer by inducing base excision repair. Antioxidants 2020, 9, 643.
- Aizawa, K.; Liu, C.; Tang, S.; Veeramachaneni, S.; Hu, K.-Q.; Smith, D.E.; Wang, X.-D. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int. J. Cancer 2016, 139, 1171–1181.
- Campos, K.K.D.; de Oliveira Ramos, C.; Martins, T.L.; de Paula Costa, G.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Cangussú, S.D.; Costa, D.C.; Bezerra, F.S. Lycopene mitigates pulmonary emphysema induced by cigarette smoke in a murine model. J. Nutr. Biochem. 2019, 65, 93–100.
- Rakic, J.M.; Liu, C.; Veeramachaneni, S.; Wu, D.; Paul, L.; Chen, C.-Y.O.; Ausman, L.M.; Wang, X.-D. Lycopene inhibits smoke-induced chronic Obstructive pulmonary disease and lung carcinogenesis by modulating reverse cholesterol transport in ferrets. Cancer Prev. Res. 2019, 12, 421–432.
- Alsharairi, N.A. The effects of dietary supplements on asthma and lung cancer risk in smokers and non-smokers: A review of the literature. Nutrients 2019, 11, 725.
- Palozza, P.; Simone, R.; Mele, M.C. Interplay of carotenoids with cigarette smoking: Implications in lung cancer. Curr. Med. Chem. 2008, 15, 844–854.
- Hurst, J.S.; Contreras, J.E.; Siems, W.G.; van Kuijk, F.J.G.M. Oxidation of carotenoids by heat and tobacco smoke. Biofactors 2004, 20, 23–35.
- Baker, D.L.; Krol, E.S.; Jacobsen, N.; Liebler, D.C. Reactions of beta-carotene with cigarette smoke oxidants. Identification of carotenoid oxidation products and evaluation of the prooxidant/antioxidant effect. Chem. Res. Toxicol. 1999, 12, 535–543.
- Arora, A.; Willhite, C.A.; Liebler, D.C. Interactions of beta-carotene and cigarette smoke in human bronchial epithelial cells. Carcinogenesis 2001, 22, 1173–1178.
- Haider, C.; Ferk, F.; Bojaxhi, E.; Martano, G.; Stutz, H.; Bresgen, N.; Knasmüller, S.; Alija, A.; Eckl, P.M. Effects of β-Carotene and its cleavage products in primary pneumocyte type II cells. Antioxidants 2017, 6, 37.
- Eiserich, J.P.; van der Vliet, A.; Handelman, G.J.; Halliwell, B.; Cross, C.E. Dietary antioxidants and cigarette smoke-induced biomolecular damage: A complex interaction. Am. J. Clin. Nutr. 1995, 62, 1490S–1500S.
- Guénégou, A.; Leynaert, B.; Pin, I.; Le Moël, G.; Zureik, M.; Neukirch, F. Serum carotenoids, vitamins A and E, and 8 year lung function decline in a general population. Thorax 2006, 61, 320–326.
- Bruno, R.S.; Ramakrishnan, R.; Montine, T.J.; Bray, T.M.; Traber, M.G. -Tocopherol disappearance is faster in cigarette smokers and is inversely related to their ascorbic acid status. Am. J. Clin. Nutr. 2005, 81, 95–103.
- Dey, N.; Chattopadhyay, D.J.; Chatterjee, I.B. Molecular mechanisms of cigarette smoke-induced proliferation of lung cells and prevention by vitamin C. J. Oncol. 2011, 2011, 561862.
- Lee, B.M.; Lee, S.K.; Kim, H.S. Inhibition of oxidative DNA damage, 8-OHdG, and carbonyl contents in smokers treated with antioxidants (vitamin E, vitamin C, beta-carotene and red ginseng). Cancer Lett. 1998, 132, 219–227.
- Dietrich, M.; Block, G.; Hudes, M.; Morrow, J.D.; Norkus, E.P.; Traber, M.G.; Cross, C.E.; Packer, L. Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokers. Cancer Epidemiol. Biomark. Prev. 2002, 11, 7–13.
- Guertin, K.A.; Grant, R.K.; Arnold, K.B.; Burwell, L.; Hartline, J.; Goodman, P.J.; Minasian, L.M.; Lippman, S.M.; Klein, E.; Cassano, P.A. Effect of long-term vitamin E and selenium supplementation on urine F2-isoprostanes, a biomarker of oxidative stress. Free Radic. Biol. Med. 2016, 95, 349–356.
- Kim, H.S.; Lee, B.M. Protective effects of antioxidant supplementation on plasma lipid peroxidation in smokers. J. Toxicol. Env. Health A 2001, 63, 583–598.
- Ismail, N.M.; Harun, A.; Yusof, A.A.; Zaiton, Z.; Marzuki, A. Role of vitamin e on oxidative stress in smokers. Malays J. Med. Sci. 2002, 9, 34–42.
- Howard, D.J.; Ota, R.B.; Briggs, L.A.; Hampton, M.; Pritsos, C.A. Oxidative stress induced by environmental tobacco smoke in the workplace is mitigated by antioxidant supplementation. Cancer Epidemiol. Biomark. Prev. 1998, 7, 981–988.
- Woodson, K.; Tangrea, J.A.; Barrett, M.J.; Virtamo, J.; Taylor, P.R.; Albanes, D. Serum alpha-tocopherol and subsequent risk of lung cancer among male smokers. J. Natl. Cancer Inst. 1999, 91, 1738–1743.
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. A prospective study of serum vitamin E and 28-year risk of lung cancer. J. Natl. Cancer Inst. 2020, 112, 191–199.
- Traber, M.G.; Winklhofer-Roob, B.M.; Roob, J.M.; Khoschsorur, G.; Aigner, R.; Cross, C.; Ramakrishnan, R.; Brigelius-Flohé, R. Vitamin E kinetics in smokers and nonsmokers. Free Radic. Biol. Med. 2001, 31, 1368–1374.
- Bruno, R.S.; Leonard, S.W.; Atkinson, J.; Montine, T.J.; Ramakrishnan, R.; Bray, T.M.; Traber, M.G. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic. Biol. Med. 2006, 40, 689–697.
- Møller, P.; Viscovich, M.; Lykkesfeldt, J.; Loft, S.; Jensen, A.; Poulsen, H.E. Vitamin C supplementation decreases oxidative DNA damage in mononuclear blood cells of smokers. Eur. J. Nutr. 2004, 43, 267–274.
- Lykkesfeldt, J.; Christen, S.; Wallock, L.M.; Chang, H.H.; Jacob, R.A.; Ames, B.N. Ascorbate is depleted by smoking and repleted by moderate supplementation: A study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am. J. Clin. Nutr. 2000, 71, 530–536.
- Pathak, A.K.; Bhutani, M.; Guleria, R.; Bal, S.; Mohan, A.; Mohanti, B.K.; Sharma, A.; Pathak, R.; Bhardwaj, N.K.; Prasad, K.N.; et al. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non-small cell lung cancer. J. Am. Coll. Nutr. 2005, 24, 16–21.
- Lim, S.-J.; Choi, M.K.; Kim, M.J.; Kim, J.K. Alpha-tocopheryl succinate potentiates the paclitaxel-induced apoptosis through enforced caspase 8 activation in human H460 lung cancer cells. Exp. Mol. Med. 2009, 41, 737–745.
- Yang, L.; Zhao, H.; Liu, K.; Wang, Y.; Liu, Q.; Sun, T.; Chen, S.; Ren, L. Smoking behavior and circulating vitamin D levels in adults: A meta-analysis. Food Sci. Nutr. 2021, 9, 5820–5832.
- Lange, N.E.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am. J. Respir. Crit. Care Med. 2012, 186, 616–621.
- Zheng, X.; Qu, N.; Wang, L.; Wang, G.; Jiao, R.; Deng, H.; Li, S.; Qin, Y. Effect of vitamin D3 on lung damage induced by cigarette smoke in mice. Open Med. 2019, 14, 827–832.
- Akiba, T.; Morikawa, T.; Odaka, M.; Nakada, T.; Kamiya, N.; Yamashita, M.; Yabe, M.; Inagaki, T.; Asano, H.; Mori, S.; et al. Vitamin D supplementation and survival of patients with non-small cell lung cancer: A Randomized, double-blind, placebo-controlled trial. Clin. Cancer Res. 2018, 24, 4089–4097.
- Songyang, Y.; Song, T.; Shi, Z.; Li, W.; Yang, S.; Li, D. Effect of vitamin D on malignant behavior of non-small cell lung cancer cells. Gene 2021, 768, 145309.
- Ma, K.; Xu, W.; Wang, C.; Li, B.; Su, K.; Li, W. Vitamin D deficiency is associated with a poor prognosis in advanced non-small cell lung cancer patients treated with platinum-based first-line chemotherapy. Cancer Biomark. 2017, 18, 297–303.
- Wang, Q.; Cui, Q.; Gao, J.-P.; Xing, R. Role of iron biomarkers and iron intakes in lung cancer risk: A systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2022, 74, 127060.
- Park, E.; Ha, E.; Leem, J.; Lee, K.; Chung, J.; Hong, Y. Dietary iron uptake increases lipid peroxidation by exposure to smoking. Epidemiology 2007, 18, pS180.
- Oba, S.; Inaba, Y.; Shibuya, T.; Oshima, J.; Seyama, K.; Kobayashi, T.; Kunugita, N.; Ino, T. Changes in oxidative stress levels during two weeks of smoking cessation treatment and their association with nutritional characteristics in Japanese smokers. Exp. Med. 2019, 17, 2757–2764.
- Wu, S.; Zhu, C.; Tang, D.; Dou, Q.P.; Shen, J.; Chen, X. The role of ferroptosis in lung cancer. Biomark. Res. 2021, 9, 82.
- Fukano, Y.; Yoshimura, H.; Yoshida, T. Heme oxygenase-1 gene expression in human alveolar epithelial cells (A549) following exposure to whole cigarette smoke on a direct in vitro exposure system. Exp. Toxicol. Pathol. 2006, 57, 411–418.
- Baglole, C.J.; Sime, P.J.; Phipps, R.P. Cigarette smoke-induced expression of heme oxygenase-1 in human lung fibroblasts is regulated by intracellular glutathione. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L624–L636.
- Ge, G.-Z.; Xu, T.R.; Chen, C. Tobacco carcinogen NNK-induced lung cancer animal models and associated carcinogenic mechanisms. Acta Biochim. Biophys. Sin. 2015, 47, 477–487.
- Yang, R.; Liu, N.; Chen, L.; Jiang, Y.; Shi, Y.; Mao, C.; Liu, Y.; Wang, M.; Lai, W.; Tang, H.; et al. LSH interacts with and stabilizes GINS4 transcript that promotes tumourigenesis in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2019, 38, 280.
- Ma, T.-L.; Zhou, Y.; Wang, C.; Wang, L.; Chen, J.-X.; Yang, H.-H.; Zhang, C.-Y.; Zhou, Y.; Guan, C.-X. Targeting ferroptosis for lung diseases: Exploring novel strategies in ferroptosis-associated mechanisms. Oxid. Med. Cell Longev. 2021, 2021, 1098970.
- Scaglia, N.; Igal, R.A. Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int. J. Oncol. 2008, 33, 839–850.
- Zhang, X.; Yang, Q. Association between serum copper levels and lung cancer risk: A meta-analysis. J. Int. Med. Res. 2018, 46, 4863–4873.
- Wang, Y.; Sun, Z.; Li, A.; Zhang, Y. Association between serum zinc levels and lung cancer: A meta-analysis of observational studies. World J. Surg. Oncol. 2019, 17, 78.
- Sarkar, C.; Mitra, P.K.; Saha, S.; Nayak, C.; Chakraborty, R. Effect of copper-hydroquinone complex on oxidative stress-related parameters in human erythrocytes (in vitro). Toxicol. Mech. Methods 2009, 19, 86–93.
- Lapenna, D.; Mezzetti, A.; de Gioia, S.; Pierdomenico, S.D.; Daniele, F.; Cuccurullo, F. Plasma copper and lipid peroxidation in cigarette smokers. Free Radic. Biol. Med. 1995, 19, 849–852.
- Thounaojam, M.C.; Jadeja, R.N.; Valodkar, M.; Nagar, P.S.; Devkar, R.V.; Thakore, S. Oxidative stress induced apoptosis of human lung carcinoma (A549) cells by a novel copper nanorod formulation. Food Chem. Toxicol. 2011, 49, 2990–2996.
- Zabłocka-Słowińska, K.; Płaczkowska, S.; Prescha, A.; Pawełczyk, K.; Porębska, I.; Kosacka, M.; Pawlik-Sobecka, L.; Grajeta, H. Serum and whole blood Zn, Cu and Mn profiles and their relation to redox status in lung cancer patients. J. Trace Elem. Med. Biol. 2018, 45, 78–84.
- Kocdor, H.; Ates, H.; Aydin, S.; Cehreli, R.; Soyarat, F.; Kemanli, P.; Harmanci, D.; Cengiz, H.; Kocdor, M.A. Zinc supplementation induces apoptosis and enhances antitumor efficacy of docetaxel in non-small-cell lung cancer. Drug Des. Devel. Ther. 2015, 9, 3899–3909.
- Kocyigit, A.; Erel, Q.; Gur, S. Effects of tobacco smoking on plasma selenium, zinc, copper and iron concentrations and related antioxidative enzyme activities. Clin. Biochem. 2001, 34, 629–633.
- Foronjy, R.F.; Mirochnitchenko, O.; Propokenko, O.; Lemaitre, V.; Jia, Y.; Inouye, M.; Okada, Y.; D’Armiento, J.M. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am. J. Respir. Crit. Care Med. 2006, 173, 623–631.
- Kim, S.H.; Kim, J.S.; Shin, H.S.; Keen, C.L. Influence of smoking on markers of oxidative stress and serum mineral concentrations in teenage girls in Korea. Nutrition 2003, 19, 240–243.
- Cay, M.; Naziroğlu, M.; Köylü, H. Selenium and vitamin E modulates cigarette smoke exposure-induced oxidative stress in blood of rats. Biol. Trace Elem. Res. 2009, 131, 62–70.
- Duan, L.; Shen, H.; Zhao, G.; Yang, R.; Cai, X.; Zhang, L.; Jin, C.; Huang, Y. Inhibitory effect of Disulfiram/copper complex on non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2014, 446, 1010–1016.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
21 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




