
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcella Bini | -- | 1063 | 2022-12-19 09:45:47 | | | |
| 2 | Camila Xu | + 3 word(s) | 1066 | 2022-12-22 05:48:42 | | |
Video Upload Options
The brunogeierite GeFe2O4 is a rare mineral of germanium, with a normal spinel structure and amazing functional properties. Its spectroscopic, optical, magnetic and electronic properties are known. For many years it was left behind, but recently a renewed interest in this oxide has arisen, particularly for its application in the electrochemical field, as anode in Lithium Ion and Sodium Ion Batteries and as electrocatalyst for urea oxidation reaction.
1. Introduction
2. Crystal Structure
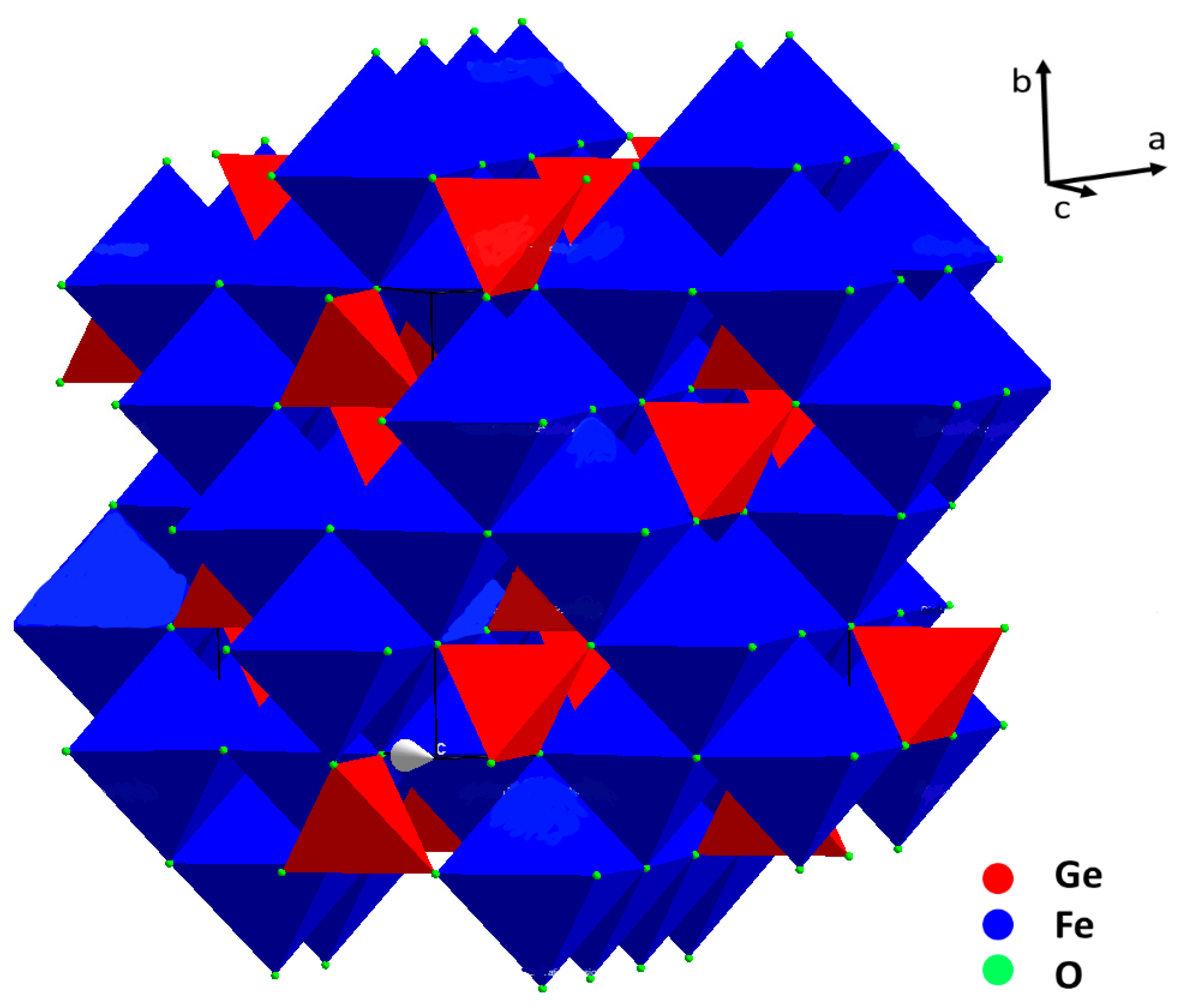
As previously discussed, GeFe2O4 can be found in nature in peculiar locations in the form of single crystals, which were usually employed for crystal structure determination. However, single crystals were also synthesized in laboratory and were used, for example, for high pressure Raman studies and for the determination of optical and electrical properties. For electrochemical and electrocatalytic applications, GFO was used instead in the form of nano-powders, which are more easily synthesized, by using solid state, mechanochemistry, hydrothermal and freeze drying synthetic routes.
GFO single crystals and powders were characterized by many spectroscopic techniques, such as Mössbauer, X-ray Photoelectron Spectroscopy (XPS), Infrared Spectroscopy (IR) and Raman, as well as with X-ray diffraction. Mössbauer and XPS spectroscopies allowed to determine the cation oxidation states, that were definitely established as Ge4+ and Fe2+. In some cases, traces of Fe3+ were found, suggesting the formation of solid solutions with Fe3O4. XPS was also useful to characterize the possible carbon coating, particularly for electrochemical applications. The IR spectrum was not conclusively interpreted, diffently from Raman spectra that were collected as a function of pressure to study the structure stability. It was demonstrated that the changes in the collected spectra were not due to sample decomposition (at least below 30 GPa) and/or to polymorphs formation, but possibly to partial spinel inversion. The difference in ionic radii of Fe2+ and Ge4+ ions inhibited the inversion in single crystals but not in nanocrystalline GFO that, when obtained from mechano-synthesis showed an inversion degree of 0.67. The new bands and the band splitting observed in Raman spectra can be due also to structure distortion towards the tetragonal structure (I41/amd), the most probable distortion of the cubic spinel containing Fe2+ ions. The magnetic properties of GFO were determined in the past and newly measured more recently[7]. Its behaviour was unusual in non-metallic materials: the reason was attributed to a frustration wave order in GFO derived from exchange interactions between the ordered spin components in a sublattice via the dynamic components of their neighbours in the other. The peculiar magnetic features of GFO were not shared by all the Ge based spinels, because being Ge4+an inactive ion, they were determined by the B2+ sublattice, so a variety of different behaviours could be identified. The positive value of the Hall constant suggested a p-type conduction. Conductivity and Hall effect measurements showed that the acceptor ionization energy was 0.39 eV and that the mobility was low and independent of temperature, probably due to a narrow valence band and/or large polaron formation. GFO differed, however, with respect to other hopping-type spinels, because its electronic properties were similar to those of NiO at high temperatures[9].
References
- Höll, R.; Kling, M.; Schroll, E.; Metallogenesis of germanium—A review. Ore Geol. Rev. 2007, 30, 145.
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N.; The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; Walter De Gruyter: Berlin, Germany, 2015, -, 1-30.
- 3. Cempírek, J.; Groat, L.A.; Note on the formula of brunogeierite and the first bond-valence parameters for Ge2+. J. Geosci. 2013, 58, 71.
- Gao, H.; Liu, S.; Li, Y.; Conte, E.; Cao, Y.; A Critical Review of Spinel Structured Iron Cobalt Oxides Based Materials for Electrochemical Energy Storage and Conver-sion. Energies 2017, 10, 1787.
- Bini, M.; Ambrosetti, M.; Spada, D.; ZnFe2O4, a green and high-capacity anode material for lithium-ion batteries: A review. Appl. Sci. 2021, 11, 11713.
- Welch, M.D.; Cooper, M.A.; Hawthorne, F.C.; The crystal structure of brunogeierite, Fe2GeO4 spinel. Mineral. Mag. 2001, 65, 441.
- Perversi, G.; Arevalo-Lopez, A.M.; Ritter, C.; Attfield, J.P.; . Frustration wave order in iron(II) oxide spinels. Commun. Phys 2018, 1, 69.
- Setkova, T.V.; Spivak, A.V.; Borovikova, E.Y.; Voronin, M.V.; Zakharchenko, E.S.; Balitsky, V.S.; Kuzmin, A.V.; Sipavina, L.V.; Iskrina, A.V.; Khasanov, S.S.; et al. . Synthetic brunogeierite Fe2GeO4: XRD, Mössbauer and Raman high-pressure study. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2022, 267, 120597.
- Strobel, P.; Koffyberg, F.P.; Wold, A.; Electrical and optical properties of high-purity p-type single crystals of GeFe2O4. J. Solid State Chem. 1980, 31, 209.




