Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | William de Oliveira e Sousa Salvador | -- | 2886 | 2022-12-17 14:05:38 | | | |
| 2 | Catherine Yang | Meta information modification | 2886 | 2022-12-19 02:48:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Salvador, W.O.S.; Ribeiro, I.A.B.; Nogueira, D.E.S.; Ferreira, F.C.; Cabral, J.M.S.; Rodrigues, C.A.V. Bioprocess Economic Modeling for Stem Cell Therapy Products. Encyclopedia. Available online: https://encyclopedia.pub/entry/38919 (accessed on 04 March 2026).
Salvador WOS, Ribeiro IAB, Nogueira DES, Ferreira FC, Cabral JMS, Rodrigues CAV. Bioprocess Economic Modeling for Stem Cell Therapy Products. Encyclopedia. Available at: https://encyclopedia.pub/entry/38919. Accessed March 04, 2026.
Salvador, William O. S., Inês A. B. Ribeiro, Diogo E. S. Nogueira, Frederico C. Ferreira, Joaquim M. S. Cabral, Carlos A. V. Rodrigues. "Bioprocess Economic Modeling for Stem Cell Therapy Products" Encyclopedia, https://encyclopedia.pub/entry/38919 (accessed March 04, 2026).
Salvador, W.O.S., Ribeiro, I.A.B., Nogueira, D.E.S., Ferreira, F.C., Cabral, J.M.S., & Rodrigues, C.A.V. (2022, December 17). Bioprocess Economic Modeling for Stem Cell Therapy Products. In Encyclopedia. https://encyclopedia.pub/entry/38919
Salvador, William O. S., et al. "Bioprocess Economic Modeling for Stem Cell Therapy Products." Encyclopedia. Web. 17 December, 2022.
Copy Citation
Bioprocess economic models (BEMs) are fundamental tools for guiding decision-making in bioprocess design, being capable of supporting process optimization and helping to reduce production costs. These tools are particularly important when it comes to guiding manufacturing decisions and increasing the likelihood of market acceptance of cell-based therapies, which are often cost-prohibitive because of high resource and quality control costs. Not only this, but the inherent biological variability of their underlying bioprocesses makes them particularly susceptible to unforeseen costs arising from failed or delayed production batches.
bioprocess economic modeling
cell and gene therapy
stem cell manufacturing
1. Development of Bioprocesses for Stem Cell Therapy Products
Bioprocesses which aim to manufacture cell therapy products (CTPs) involve highly variable cell-based raw materials and often fragile final products with short shelf lives [1]. At the same time, they must adhere to rigorous guidelines in order to obtain regulatory approval, which is frequently the source of costly setbacks. Naturally, this leads to laborious and arduous development lifecycles, and there is no assurance that these will result in a viable product suitable for commercialization. As such, it is imperative to follow a strategic framework for cell therapy process development, preferably based on the concept of quality by design (QbD) and its emphasis on continued innovation and iterative refinement, to ensure the success of such ventures.
When developing a CTP with a QbD approach in mind, the first step that must be taken is to carefully define the desired properties of the CTP in question. These are known collectively as the target product profile (TPP) and encompass properties such as cellular identity, potency, purity and quantity [2]. It is equally important to identify which of these properties are absolutely essential for the efficacy and safety of the product, without which it would not exert its function or become a liability to the patient. These are termed critical quality attributes (CQAs). Finally, it is also necessary to ascertain which parameters play a part in determining these attributes, with these being designated as critical process parameters (CPPs). These encompass cellular and noncellular features that range from the specific growth kinetics and gene expression of a cell population to the environmental conditions which influence cell behavior.
As an illustrative example, one of the earliest therapeutic applications of human induced pluripotent stem cells (hiPSCs), i.e., the transplantation of autologous hiPSC-derived retinal pigment epithelium (RPE) cell sheets to treat age-related macular degeneration, can be considered [3][4]. In this case, the TPP of the RPE sheets might be said to contemplate the following properties: the expression of typical RPE markers must be above a certain level (identity); the polarized secretion of growth factors associated with native RPE, such as pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF), should be significant (potency); the absence of undifferentiated hiPSCs in the final product must be ensured (purity); a sufficient quantity of RPE cells should be produced to form the sheets (quantity). The adequate secretion of PEDF and VEGF clearly constitutes a CQA, since without this, the RPE sheets would not be capable of exerting their biological function. Associated with this CQA is the degree of pigmentation of the hiPSC-derived RPE, which is influenced by a number of CPPs, such as the concentration of the growth factors used to induce the differentiation of hiPSCs toward RPE cells and the duration of the differentiation process.
Evidently, the TPP of a CTP cannot be fully defined during the early stages of its development, given that there is limited knowledge available regarding how the product will really act in a clinical setting [1]. This is unavoidable, and thus the concept of iterative refinement is at the core of QbD. The idea is that, after roughly outlining the TPP and CQAs, a process design space should be defined, representing the many parameters which interact in such a way as to affect the CQAs. The TPP should then be iteratively refined through the accrual of understanding about the product’s mode of action, derived from clinical trials, and the CPPs, derived from extensive monitoring and collection of experimental data. This refinement is reflected in the gradual narrowing of the design space as process optimization becomes focused on a smaller number of parameters.
Iterative refinement can best be guided by taking advantage of two complementary strategies, namely design of experiments (DOE) and computational modeling [1][2]. DOE comprises a set of methodologies that attempt to evaluate multiple factors simultaneously so as to understand the impact of their interactions on the output of a given bioprocess [5]. The data obtained from these meticulously designed experiments can afterwards be used to build a computational model which describes the expected behavior of the bioprocess as a function of certain input parameters. Such a model might be limited to technical aspects or expanded to also include economic considerations, as is the case of bioprocess economic models (BEMs). The model can then be employed to explore and fine-tune the design space in silico, identifying which parameters are most promising for optimization and streamlining further experimental process development [1]. The QbD framework, evidencing the roles which DOE and bioprocess economic modeling play in its implementation, is represented schematically in Figure 1.
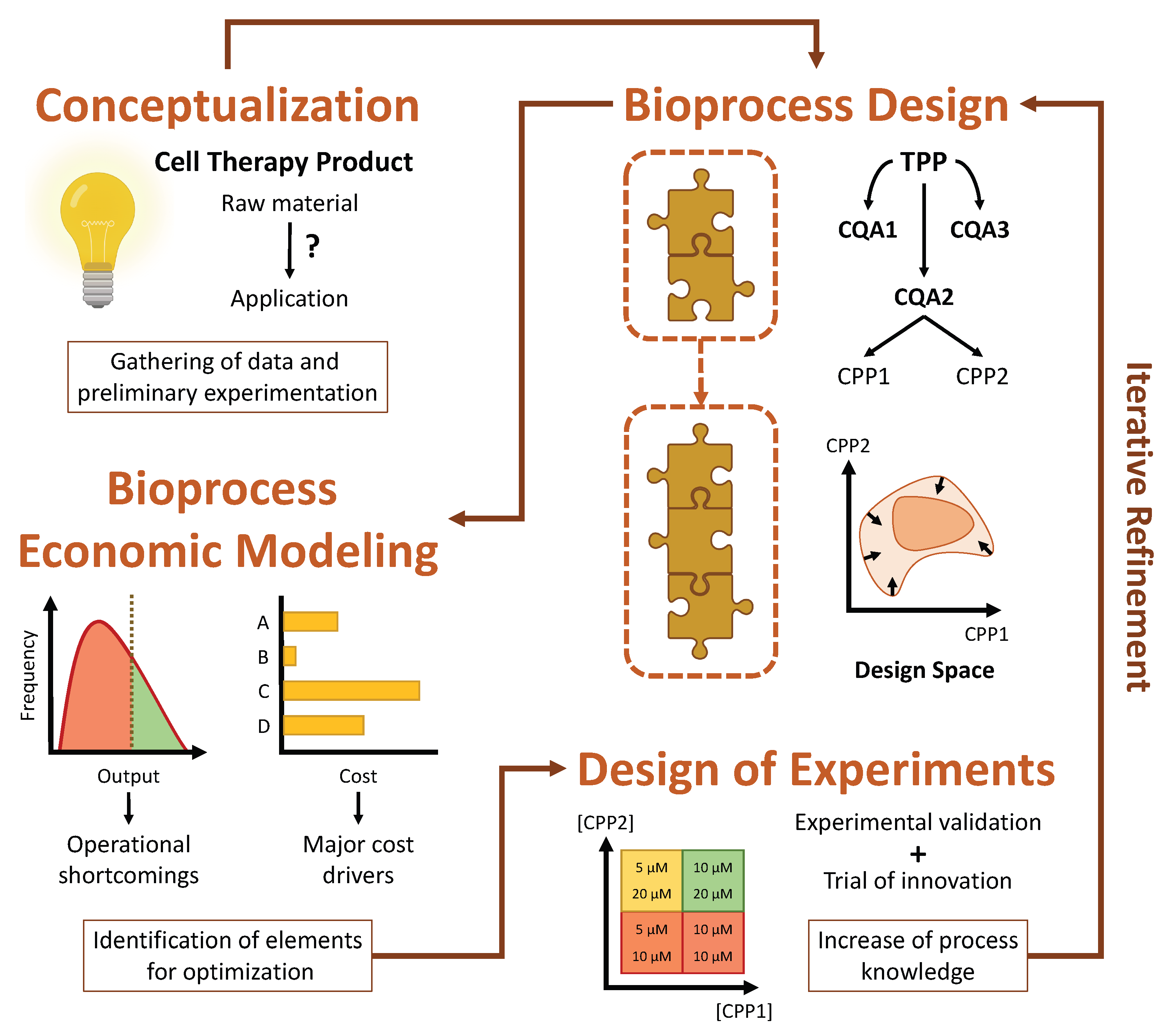
Figure 1. In the context of the quality by design framework, the development of a cell therapy product (CTP) begins with the conceptualization of a bioprocess that leads from cell-based raw materials to a product capable of fulfilling its envisaged application. It then moves on to the creation of a rough draft of the bioprocess, defining the CTP in terms of its target product profile (TPP), critical quality attributes (CQAs) and critical process parameters (CPPs). Based on the CPPs, a process design space is also delineated. Naturally, the initial rough draft is limited by a lack of process knowledge, but it provides a blueprint for future development. So as to guide the accrual of process knowledge, a bioprocess economic model (BEM) can be established to simulate the outlined bioprocess, serving as a useful tool for swiftly and inexpensively identifying those elements whose optimization would bring the most benefit to the bioprocess in terms of quality or cost. Once these have been identified, directed experimentation should be carried out following a methodical design of experiments approach. The results of this experimentation effectively increase process knowledge, which can then be used to improve the initial bioprocess design, clarifying its TPP and narrowing its design space. The established BEM should then also be improved, increasing its fidelity, and used to identify further elements for optimization. This process, termed iterative refinement, is cyclically repeated, until a robust and economically viable bioprocess is achieved.
Returning to the example of the RPE cell sheets, it is easy to see how a BEM could help in the optimization of this CTP. As was mentioned, the purity of the final product, i.e., the absence of undifferentiated hiPSCs that could prove to be tumorigenic, is a CQA. In the initial production strategy, this purity was guaranteed solely through a series of manual “pick up and expand” procedures, whereby cells which visually resembled the desired RPE cells were selectively expanded over several passages before the formulation of the final product [3]. Not only is this procedure extremely time-consuming, but it also requires highly specialized personnel. Therefore, a manufacturer would likely consider improving this step of the bioprocess by implementing an affinity purification strategy. After some consideration, he may come to the conclusion that he should choose either fluorescent-activated cell sorting (FACS), magnetic-activated cell sorting (MACS) or buoyancy-activated cell sorting (BACS). But which would be best? After some preliminary experiments, carried out with the objective of determining the yield and purity of the strategies under consideration, the manufacturer might arrive at the conclusion that FACS and MACS allow for a somewhat higher purity than BACS, but that BACS is significantly cheaper to implement due to reduced labor and equipment costs. So the question remains, which should he choose? This decision would ideally be made by employing a BEM to compare the cost-effectiveness of each strategy when integrated within the overall bioprocess. The BEM could estimate, for example, that executing two or three consecutive BACS purification steps would lead to a purity comparable to that of FACS and MACS while still being less expensive, thus giving the manufacturer a solid reason for choosing BACS in detriment of the other strategies. Similar observations could be made about the many other decisions the manufacturer would be confronted with, such as selecting the ideal hiPSC reprogramming strategy or the optimal retinal culture medium.
Interestingly, computational models should undergo iterative refinement alongside their respective bioprocesses, since, as more becomes known about an underlying bioprocess, the robustness and fidelity of its respective model can be progressively increased. Recent work by Manstein et al. presents an excellent example of this [6][7]. In their work, after establishing an initial mechanistic model based on experimental data, they cyclically challenged the model by altering input parameters and verifying if the model’s outputs corresponded with reality. When they did not, the necessary adjustments were made to bring the model closer in line with the observed experimental results. This allowed a model with strong predictive capability to be built, which was then successfully used to optimize culture conditions and maximize hiPSC density in stirred-tank bioreactors.
Another fundamental aspect heavily encouraged by QbD is continuous process innovation. This means that, whenever favorable, novel manufacturing strategies and elements with the potential to either reduce bioprocess complexity or increase product quality should be adopted [1]. A prime example of this is the transition from planar culture formats, ubiquitous in laboratory settings and thus likely to be used in the early stages of development, to automated bioreactor culture, so as to benefit from improved scalability, integrated manufacturing and increased cell quality [8][9]. Other sources of innovation include the development of less costly and more rigorously defined culture media [10], ingenious cell purification methods [11] and streamlined cell characterization techniques [12]. As will be seen further in this entry, BEMs are particularly well suited to identifying whether a given innovation should or not be implemented, both from a technical and economic perspective. This is important in order to justify the large investment of time and monetary resources which normally accompanies innovation.
As a last note concerning QbD, the development of bioprocesses which are composed of multiple sequential stages should give rise to modular manufacturing processes [1]. In other words, these bioprocesses can be organized as several modular blocks, each encompassing a certain set of unit operations at the end of which an intermediate cell population is obtained, with its own distinct TPP and CQAs. This allows for a much simpler optimization of the overall bioprocess, given that each modular block can be individually optimized without interfering with another. Notably, this same modularity should be reflected in any eventual BEM, so that it can be used to independently explore the design space of each modular block.
Once more looking to the RPE cell sheets, the applicability of this modular approach is clearly evident [3][4]. Fibroblasts from a patient must first be reprogrammed into hiPSCs, followed by their differentiation into RPE cells and then their expansion until enough cells are obtained to form sheets. At the end of the reprogramming block, an intermediate cell population of fully characterized, pluripotent and genetically stable hiPSCs is desired. On the other hand, upon completion of the differentiation block, an intermediate cell population of aptly differentiated, RPE marker expressing and PEDF and VEGF secreting cells is expected. The CPPs that influence the CQAs of the cell populations of each of these blocks are indubitably distinct, and can therefore be iteratively refined separately within their own specific design spaces, decreasing the complexity of this endeavor.
2. Bioprocess Economics
Insofar as bioprocess economics are concerned, two key cost-metrics must be reckoned with for the estimation of manufacturing costs, namely fixed capital investment (FCI) and cost of goods (CoG) [13]. A firm grasp of these metrics is indispensable in order to integrate the appropriate cost equations within a bioprocess model. Another concept that is particularly important for determining the expenses incurred by a bioprocess whose end product is intended for therapeutic applications is cost of quality (CoQ). This is because the quality of these products must be categorically assured in accordance with strict cGMP guidelines, which are naturally associated with hefty costs [8].
2.1. Fixed Capital Investment and Cost of Goods
The FCI represents the total initial investment required to build a manufacturing facility with all of the necessary installations and equipment to execute a given bioprocess. Aside from depending directly on the manufacturing structure of the bioprocess itself, the value of the FCI can be influenced by several factors, such as the ratio between open and closed cleanroom areas, the choice between automated versus manual processing and the use of disposable or nondisposable process platforms [13]. To determine the total initial investment involved in the establishment of a manufacturing facility for stem cell processing, two models can be implemented: a cost-per-area-model or a Lang factor model [14]. The first model consists in defining the cleanroom area of a facility along with its noncleanroom area, summing both areas multiplied by their respective price-per-square-meter (or another unit of area) and then adding the total equipment acquisition cost [13]. The Lang factor model determines the FCI by multiplying the total equipment acquisition cost by a cost factor, which should be based on the relationship between the FCI and equipment acquisition cost observed for past projects of a similar nature [13][14].
The CoG incorporates all direct and indirect costs that arise from the manufacture of a CTP [13]. Generally speaking, the CoG is expressed in relation to a single therapeutic dose. Direct costs can be further broken down into three categories, namely consumable, reagent and quality control costs [14]. Consumables include all disposable lab equipment, such as single-use vessels, serological pipettes, gloves, etc. Reagents comprise all of the chemical or biological substances used throughout a bioprocess, such as cell culture medium, dissociation enzymes, buffers for product storage and final formulation, etc. Quality controls encompass any procedure carried out for intermediate or final CTP testing, which seek to ensure that it satisfactorily fulfills its CQAs. These procedures are thus highly specific to each bioprocess.
Indirect costs can broadly be subdivided into infrastructure and labor costs [14]. Infrastructure costs cover a wide variety of expenses, including facility and equipment depreciation. Depreciation denotes the loss of an asset’s value with time, which is often considered to occur in a linear manner [15]. In the case of a facility and its equipment, the depreciation rate of each constituent is equivalent to its associated portion of the FCI divided by its respective lifetime, with the lifetime of the facility generally being longer than that of its equipment. Lengthy bioprocesses that occupy a facility and its equipment for a long time during each production batch will naturally originate higher depreciation costs than shorter bioprocesses. Infrastructure costs also encompass operating costs, such as those incurred by the supply of gases (O2, CO2, N2), cleaning and maintenance of the facility, requalification procedures, etc., storage costs and transportation costs. These last two are heavily influenced by the final formulation of the product in question, with cryogenically preserved products almost always being cheaper alternatives to fresh-preserved products, due to their prolonged shelf life [16]. This means that in some cases it may be advantageous to preserve a CTP at an intermediate stage if this allows it to be cryopreserved, with its final formulation being completed before its delivery to the site of care. Labor costs include salaries, benefits and expenses associated with the specialized training of personnel.
2.2. Cost of Quality
As has been repeatedly mentioned, CTP bioprocess design and development is a challenging affair that carries many risks. Manufacturing failures can arise from a multitude of factors, such as the biological variability inherent to cell-based raw materials, the high sensitivity of cells to their physiochemical environment, which might lead to spontaneous differentiation or unpredicted cell death, and culture contaminations. These manufacturing failures can have a large financial impact, preventing products from reaching the market. Furthermore, regulatory agencies have stringent requirements in terms of process manufacturing and control, which, when not met, can result in products being held back from regulatory approval. Therefore, bioprocesses should be carefully designed to achieve the desired high productivity and product quality, while ensuring their economic viability [2][8][17][18].
CoQ models are of key importance for identifying and measuring the costs associated with quality assurance, helping to minimize these costs while maintaining a certain level of quality [19]. CoQ takes into account the costs of preventing poor quality along with the costs incurred by the nonconformance with quality criteria that causes manufacturing failures. The majority of CoQ models follow a prevention-appraisal-failure (P-A-F) scheme. These models divide quality costs into prevention costs, which are associated with actions taken to ensure the quality of a product, appraisal costs, related to measuring the level of quality of a product, and failure costs, corresponding to the costs of correcting the quality of a product. The main principle of P-A-F models is that investing more in prevention and appraisal activities frequently proves to be beneficial by significantly decreasing failure costs. This is particularly true when talking about bioprocesses since their end product’s quality is rarely correctable, meaning that when a batch failure occurs the resources invested towards its production are entirely wasted and a new batch must be started to replace the lost product.
References
- Csaszar, E.; Mills, S.; Zandstra, P.W. Process evolution in cell and gene therapy from discovery to commercialization. Can. J. Chem. Eng. 2021, 99, 2517–2524.
- Lipsitz, Y.Y.; Timmins, N.E.; Zandstra, P.W. Quality cell therapy manufacturing by design. Nat. Biotechnol. 2016, 34, 393–400.
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014, 2, 205–218.
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 376, 1038–1046.
- Toms, D.; Deardon, R.; Ungrin, M. Climbing the mountain: Experimental design for the efficient optimization of stem cell bioprocessing. J. Biol. Eng. 2017, 11, 35.
- Manstein, F.; Ullmann, K.; Kropp, C.; Halloin, C.; Triebert, W.; Franke, A.; Farr, C.M.; Sahabian, A.; Haase, A.; Breitkreuz, Y.; et al. High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Stem Cells Transl. Med. 2021, 10, 1063–1080.
- Manstein, F.; Ullmann, K.; Triebert, W.; Zweigerdt, R. Process control and in silico modeling strategies for enabling high density culture of human pluripotent stem cells in stirred tank bioreactors. STAR Protoc. 2021, 2, 100988.
- Nath, S.C.; Harper, L.; Rancourt, D.E. Cell-Based Therapy Manufacturing in Stirred Suspension Bioreactor: Thoughts for cGMP Compliance. Front. Bioeng. Biotechnol. 2020, 8, 599674.
- Nogueira, D.E.; Cabral, J.; Rodrigues, C.A. Single-Use Bioreactors for Human Pluripotent and Adult Stem Cells: Towards Regenerative Medicine Applications. Bioengineering 2021, 8, 68.
- Kuo, H.H.; Gao, X.; DeKeyser, J.M.; Fetterman, K.A.; Pinheiro, E.A.; Weddle, C.J.; Fonoudi, H.; Orman, M.V.; Romero-Tejeda, M.; Jouni, M.; et al. Negligible-cost and weekend-free chemically defined human iPSC culture. Stem Cell Rep. 2020, 14, 256–270.
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013, 12, 127–137.
- D’Antonio, M.; Woodruff, G.; Nathanson, J.L.; D’Antonio-Chronowska, A.; Arias, A.; Matsui, H.; Williams, R.; Herrera, C.; Reyna, S.M.; Yeo, G.W.; et al. High-throughput and cost-effective characterization of induced pluripotent stem cells. Stem Cell Rep. 2013, 8, 1101–1111.
- Jenkins, M.J.; Farid, S.S. Human pluripotent stem cell-derived products: Advances towards robust, scalable and cost-effective manufacturing strategies. Biotechnol. J. 2015, 10, 83–95.
- Bandeiras, C. TESSEE-Tool for Early Stem Cells Economic Evaluation. Ph.D. Thesis, IST, Lisbon, Portugal, 2019.
- Liapis, K.J.; Kantianis, D.D. Depreciation methods and life-cycle costing (LCC) methodology. Procedia Econ. Financ. 2015, 19, 314–324.
- Harrison, R.P.; Medcalf, N.; Rafiq, Q.A. Cell therapy-processing economics: Small-scale microfactories as a stepping stone toward large-scale macrofactories. Regen. Med. 2018, 13, 159–173.
- Luo, Y.; Kurian, V.; Ogunnaike, B.A. Bioprocess systems analysis, modeling, estimation, and control. Curr. Opin. Chem. Eng. 2021, 33, 100705.
- Lipsitz, Y.Y.; Milligan, W.D.; Fitzpatrick, I.; Stalmeijer, E.; Farid, S.S.; Tan, K.Y.; Smith, D.; Perry, R.; Carmen, J.; Chen, A.; et al. A roadmap for cost-of-goods planning to guide economic production of cell therapy products. Cytotherapy 2017, 19, 1383–1391.
- Schiffauerova, A.; Thomson, V. A review of research on cost of quality models and best practices. Int. J. Qual. Reliab. Manag. 2006, 23, 647–669.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
924
Revisions:
2 times
(View History)
Update Date:
19 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





