Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andreas Till | -- | 1926 | 2022-12-16 14:29:12 | | | |
| 2 | Camila Xu | -18 word(s) | 1908 | 2022-12-19 03:10:59 | | | | |
| 3 | Camila Xu | + 3 word(s) | 1911 | 2022-12-19 12:36:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Till, A.; Fries, C.; Fenske, W.K. Brown Adipose Tissue Architecture and Thermogenic Function. Encyclopedia. Available online: https://encyclopedia.pub/entry/38895 (accessed on 08 February 2026).
Till A, Fries C, Fenske WK. Brown Adipose Tissue Architecture and Thermogenic Function. Encyclopedia. Available at: https://encyclopedia.pub/entry/38895. Accessed February 08, 2026.
Till, Andreas, Charlotte Fries, Wiebke K. Fenske. "Brown Adipose Tissue Architecture and Thermogenic Function" Encyclopedia, https://encyclopedia.pub/entry/38895 (accessed February 08, 2026).
Till, A., Fries, C., & Fenske, W.K. (2022, December 16). Brown Adipose Tissue Architecture and Thermogenic Function. In Encyclopedia. https://encyclopedia.pub/entry/38895
Till, Andreas, et al. "Brown Adipose Tissue Architecture and Thermogenic Function." Encyclopedia. Web. 16 December, 2022.
Copy Citation
The body of mammals harbors two distinct types of adipose tissue: While cells within the white adipose tissue (WAT) store surplus energy as lipids, brown adipose tissue (BAT) is nowadays recognized as the main tissue for transforming chemical energy into heat. This process, referred to as ‘non-shivering thermogenesis’, is facilitated by the uncoupling of the electron transport across mitochondrial membranes from ATP production. BAT-dependent thermogenesis acts as a safeguarding mechanism under reduced ambient temperature but also plays a critical role in metabolic and energy homeostasis in health and disease.
obesity
adipocytes
UCP1
Brown Adipose Tissue
BAT
thermogenesis
mitochondria
type 2 diabetes
1. Introduction: Metabolism Matters!
The year 2016 represents a dramatic and alarming turning point in the history of humankind: According to the World Health Organisation (WHO), for the first time there are more people killed by overweight than by underweight. With an incredible 2 billion adults being overweight and 650 million obese (as defined by Body Mass Index/BMI ≥ 30 kg/m2), the world is likely to harbor more than one billion obese adults by 2025, a health burden which is a dramatic menace for the world-wide health systems. While this alarming trend is global (with the exception of sub-Saharan Africa and Asia), obesity itself and the entirety of associated comorbidities is, of course, preventable. Understanding how the human body orchestrates the input of dietary fuels and output of energy and thus facilitates homeostasis (and why this obviously does not work efficiently in obese patients) will be key to face the upcoming obesity pandemic and to design smart prevention and treatment strategies. Luckily, groundbreaking basic and translational research in the area of metabolism and metabolic diseases has sparked the scientific interest in one peculiar (and often overlooked) tissue which eventually could turn into the most attractive target to fight the imminent health crisis. This research focusses on the function and complex regulation of this promising and exciting metabolic organ.
2. Overview of BAT Discovery and Architecture
Homoeothermic animals require tight control of their core body temperature in order to keep up organismal functions and avoid systemic damage by hypothermia. With the intention to investigate the cellular basis for this vital function, a peculiar tissue consisting of fat cells (adipocytes) with numerous small lipid droplets and an unusually high number of mitochondria was discovered in the interscapular region at the back of marmots. Due to the high iron content of the mitochondria and the distribution of lipid droplets throughout the cytoplasm, this tissue appears histologically dark red to brown why it was referred to as ‘Brown Adipose Tissue’ (BAT). This designation contrasts with the White Adipose Tissue (WAT, located at the abdomen, around the waist and thighs), which usually contains only limited mitochondrial mass and one big liposome conveying its typical yellowish appearance. While WAT is well recognized as the main tissue for storing excess calories in the form of lipids (i.e., triglycerides/TG), BAT was long thought to be exclusively devoted to the regulation of body temperature during hibernation. It took until the beginning of the 20th century to discover that the BAT is not only a heat-producing organ in hibernating mammals but is also regulating thermogenic function in non-hibernating organisms in response to external stress conditions, i.e., acute cold stress and long-term cold acclimation (for review see [1]). How far BAT thermogenic activity also contributes to hyperthermia during fever is still a subject of ongoing debate (see below).
Technical advances in the field of imaging technology, particularly hybrid positron emission tomography/computed tomography (PET-CT), and functional analyses using traceable glucose analogs (such as 18F-fluoro-2-deoxy-d-glucose/FDG), have greatly contributed to unraveling more important details on BAT morphology and function. The relative mass and distribution of BAT dramatically change during early development and across the entire life span. Neonatal mammals and infants are largely protected against cold stress due to dense BAT depots within their interscapular regions. In contrast, adult mammals (in addition to hibernating species) were long time believed to harbor only minor remnants of BAT without physiological relevance. In this regard, the years 2007–2009 represent a major turning point since several groups independently demonstrated the presence, functional activity and metabolic plasticity (i.e., the ability to adjust metabolically to external conditions) of BAT conserved in adult humans [2][3][4] with six main anatomical storage sites in the human organism: cervical, supraclavicular, mediastinal, paravertebral, axiallary and abdominal [5] (Figure 1).
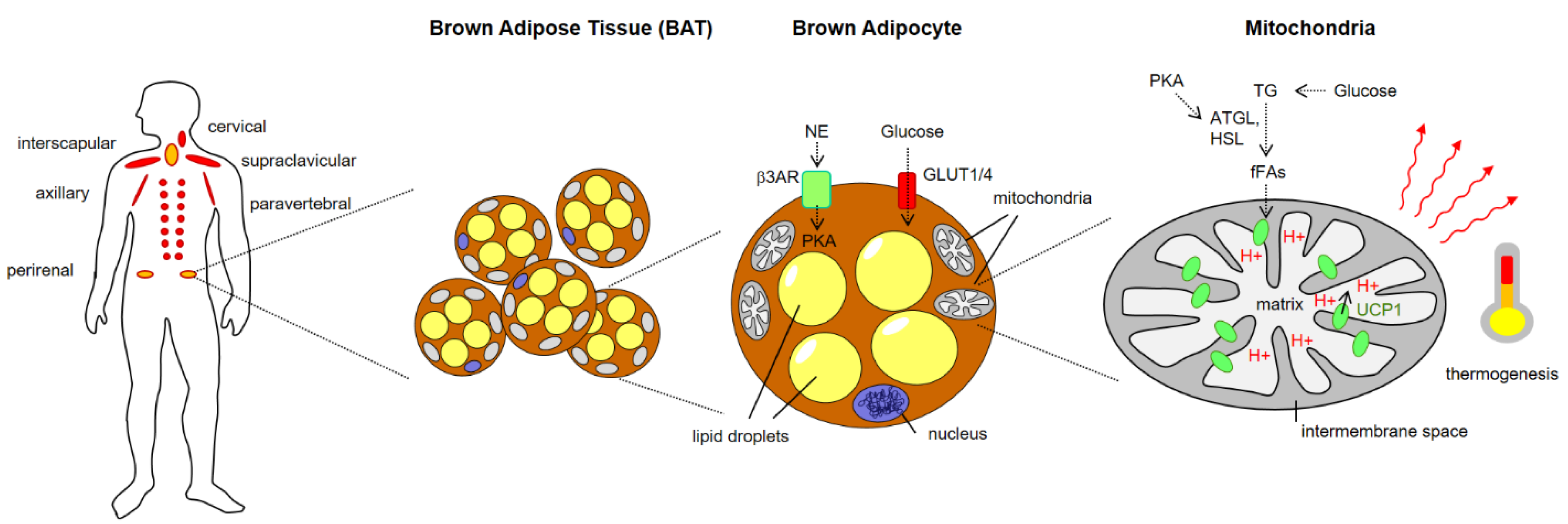
Figure 1. Brown Adipose Tissue (BAT) localization, architecture and role in thermogenesis. ATGL, adipose triglyceride lipase; fFAs, free fatty acids; GLUT, Glucose transporter; HSL, hormone-sensitive lipase; NE norepinephrine, PKA, protein kinase A, TG, triglycerides.
3. Metabolic Function of BAT
BAT is now recognized as the main tissue in the organism for transforming chemical energy (stored in the form of lipids) into thermic energy (i.e., heat). This process, referred to as ‘non-shivering thermogenesis’ (as opposed to the shivering of muscles that generates heat as a byproduct of muscle activity), fulfills at least two important functions: First, it represents a safeguarding mechanism both under short-term cold stress and long-term cold acclimation conditions to protect key organs from hypothermic damage by warming the blood flow. Second, BAT thermogenesis is a major regulatory process ensuring systemic metabolic homeostasis by increasing glucose uptake and combusting energy in response to surplus nutrient conditions (reviews: [6][7]). In addition, recent research has shown that the physiological role of BAT is not only to produce heat but also to act as an endocrine/paracrine organ, secreting molecules that affect systemic physiology and thus shape whole-body metabolism. The group of these secretory factors (collectively named ‘batokines’ [8][9]) comprises various types of signaling molecules, including peptides (such as FGF-21, IGF-1, follistatin, IL-6 and RBP-4), lipid-based metabolites (e.g., 12,13-dihydroxy-9Z-octadecenoic acid) and even exosomal microRNAs (e.g., miR-99b). Batokines enhance the thermogenic capacity by promoting BAT hypertrophy, adipose tissue vascularization and WAT beiging, but they also exert long-distance control of metabolism—particularly whole-body glucose and lipid disposal—by conveying systemic signaling cues to metabolic organs. Moreover, they mediate the general metabolic activity of the liver, heart and muscle, and affect vascularization, WAT/BAT innervation and immune functions [10][11][12].
Given this central role of BAT for both energy dissipation and endocrine/paracrine control of metabolic balance, significant attention has been directed within the last years toward exploiting BAT thermogenic function for the treatment of obesity and associated comorbidities. In fact, there are several interesting arguments for targeting BAT activity in the development of novel therapies for weight management and metabolic diseases. First, concerning the overall metabolic function of BAT, it was demonstrated that cold-induced BAT activity as determined by (18)FDG-PET correlates with human leanness [3] and that BAT-positive persons at (18)FDG-PET had lower visceral and subcutaneous abdominal adipose tissue and liver fat content than BAT-negative persons [13]. Moreover, subjects with more active BAT exhibit better metabolic profiles [14], including lower fasting glucose levels [15] and higher insulin-stimulated glucose disposal [16], as well as reduced arterial inflammation and resulting arteriosclerosis [17]. Notably, a recent largescale epidemiological study indicates that BAT presence inversely correlates with hyperlipidemia, type 2 diabetes (T2D) and major cardiometabolic diseases [18]. Overall, it was reported that a fully-activated BAT organ could dissipate energy equivalent to approximately 4 kg of WAT over one year [4][15]. Consequently, current evidence suggests that BAT-dependent energy combustion significantly contributes to whole-body energy expenditure and that organ activity is linked with multiple beneficial effects on energy homeostasis and metabolic outcome parameters. As a consequence, recruiting BAT volume and boosting its activity may represent an attractive target for the development of novel pharmacological drugs against obesity and its comorbidities such as cardiovascular disease, diabetes and cognitive decline.
4. Molecular Mechanisms of BAT Thermogenesis
However, how exactly do brown adipocytes accomplish their job? At the cellular level, the greatest energy transformation in humans and other eukaryotes occur in the mitochondria. Here, the electrochemical gradient of protons (H+) across the inner membrane of the respiratory chain usually drives the phosphorylation of ADP to ATP via the activity of the F0/F1-ATP synthase. Therefore, under physiological conditions, respiration is directly coupled to ATP production. In BAT adipocytes, this fundamental mechanism is hijacked and exploited to translate chemical energy into thermic energy by bypassing (‘uncoupling’) the electron transport from ATP production (see [19] for an excellent review). The most prominent and best-characterized effector of this process is a protein termed cold-inducible mitochondrial uncoupling protein 1 (UCP1)[19][20][21].
UCP1 (also known as thermogenin) represents a member of the large mitochondrial anion carrier family whose members facilitate the shuttling of ions across mitochondrial membranes. It is a multi-pass transmembrane protein spanning the inner membrane within mitochondria. In the presence of free fatty acids (derived from TG breakdown by lipolysis), UCP1-channels mediate the leakage of H+ ions from the intramembrane space to the mitochondrial matrix, thereby uncoupling the electron transport from ATP synthesis [20][22] (Figure 1). As a consequence of this mitochondrial short-circuit, heat is produced within mitochondria and distributed across cell and tissue barriers via the blood stream to increase the body core temperature.
The canonical activation pathway for cold- and diet-induced BAT thermogenesis relies on neurotransmitter-mediated activation of β-adrenergic receptors (mainly of the β3 subtype, β3ARs) on brown adipocytes [23]. Engagement of these G-protein coupled receptors leads to cAMP-mediated activation of protein kinase A (PKA), which activates adipocytic lipases (such as adipose triglyceride lipase/ATGL and hormone-sensitive lipase/HSL) that convert triglycerides (TG) into free fatty acids (fFAs). Synthesis of fFAs from TG in BAT is further fueled by glucose uptake and generation of TG from glucose catabolism via pyruvate and acetyl-CoA. After the import into the mitochondria, fFAs bind to UCP1 and trigger steric changes of UCP1 binding domains that affect its tridimensional conformation, eventually resulting in leakage of protons into the matrix and subsequent heat production.
Importantly, while UCP1-dependent mitochondrial uncoupling appears to be the most prominent pathway for thermogenesis in BAT (mainly induced by cold and nutrient surplus), recent reports have identified interesting alternative pathways that act either in concert or entirely independent of UCP1. Adipocytes within the WAT that acquire brown-like features upon appropriate stimulation (sometimes referred to as beige or brown-in-white/‘brite’ adipocytes) have been shown to exploit a calcium cycling mechanism selectively for heat production. This mechanism relies on Ca2+ ATPase 2b, an ATPase that represents the pivotal pump for sequestering calcium from the cytosol into the endoplasmatic reticulum (ER) and thus is involved in ER stress responses [24]. Moreover, it has repeatedly been demonstrated in both beige and brown adipocytes that the mitochondrial Creatine Kinase and its substrate Creatine elicit an ATP → ADP turnover cycle that results in thermogenesis by dissipation of stored energy and that this mechanism is downstream of canonical β3-adrenergic signaling, thus acting in parallel to UCP1 (for review, see [25]). Finally, a UCP1-independent but fatty acid-activated pathway has been described by Bertholet and colleagues that utilizes the ADP/ATP carrier (AAC) located at the mitochondrial inner membrane. Patch-clamp measurements of isolated mitochondria could demonstrate pronounced proton leaks dependent on AAC in the absence of UCP1 [26].
In general, the concept is emerging that molecular mechanisms that result in a decrease in the cellular ATP/ADP ratio convene on exerting thermogenic activity, either via UCP1 or in a UCP1-independent fashion.
One major task for future research will be the identification of feedback mechanisms, e.g., how body weight and the energy storage state are monitored within the body and integrated to orchestrate energy expenditure and homeostasis. Further research in this area, leading to a detailed understanding of this ‘Baristat’ and its regulatory mechanisms, will help us to identify promising targets and open novel avenues for therapies against the imminent obesity pandemic.
References
- Smith, R.E.; Horwitz, B.A.; Brown fat and thermogenesis. Physiol. Rev. 1969, 49, 330–425.
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al.et al. Identification and importance of brown adipose tissue in adult humans.. N. Engl. J. Med. 2009, 360, 1509–1517, 10.1056.
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J.; Cold-activated brown adipose tissue in healthy men. . N. Engl. J. Med. 2009, 360, 1500–1508, 10.1056.
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerbäck, S.; et al. Functional brown adipose tissue in healthy adults. . N. Engl. J. Med. 2009, 360, 1518–1525, 10.1056.
- Peirce, V.; Carobbio, S.; Vidal-Puig, A.; The different shades of fat.. Nature 2014, 510, 76–83., 10.1038.
- Betz, M.J.; Enerbäck, S.; Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease.. Nat. Rev. Endocrinol. 2018, 14, 77–87, 10.1038.
- Alcalá, M.; Calderon-Dominguez, M.; Serra, D.; Herrero, L.; Viana, M.; Mechanisms of Impaired Brown Adipose Tissue Recruitment in Obesity. . Front. Physiol. 2019, 10, 94, 10.3389.
- Yang, F.T.; Stanford, K.I.; Batokines: Mediators of Inter-Tissue Communication (a Mini-Review). . Curr. Obes. Rep. 2022, 11, 1–9, 10.1007.
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Brown adipose tissue as a secretory organ. . Nat. Rev. Endocrinol. 2017, 13, 26–35, 10.1038.
- Svensson, K.J.; Long, J.Z.; Jedrychowski, M.P.; Cohen, P.; Lo, J.C.; Serag, S.; Kir, S.; Shinoda, K.; Tartaglia, J.A.; Rao, R.R.; et al.et al. A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. . Cell Metab. 2016, 23, 454–466, 10.1016.
- Long, J.Z.; Svensson, K.J.; Bateman, L.A.; Lin, H.; Kamenecka, T.; Lokurkar, I.A.; Lou, J.; Rao, R.R.; Chang, M.R.; Jedrychowski, M.P.; et al.et al. The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria.. Cell 2016, 166, 424–435, 10.1016.
- Wang, G.-X.; Zhao, X.-Y.; Meng, Z.-X.; Kern, M.; Dietrich, A.; Chen, Z.; Cozacov, Z.; Zhou, D.; Okunade, A.L.; Su, X.; et al.et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. . Nat. Med. 2014, 20, 1436–1443.
- Brendle, C.; Werner, M.K.; Schmadl, M.; La Fougère, C.; Nikolaou, K.; Stefan, N.; Pfannenberg, C.; Correlation of Brown Adipose Tissue with Other Body Fat Compartments and Patient Characteristics: A Retrospective Analysis in a Large Patient Cohort Using PET/CT. . Acad. Radiol. 2018, 25, 102–110.
- Franssens, B.T.; Hoogduin, H.; Leiner, T.; van der Graaf, Y.; Visseren, F.L.J.; Relation between brown adipose tissue and measures of obesity and metabolic dysfunction in patients with cardiovascular disease.. J. Magn. Reson. Imaging 2017, 46, 497–504.
- Lee, P.; Greenfield, J.R.; Ho, K.K.Y.; Fulham, M.J.; A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. . Am. J. Physiol. Endocrinol. Metab. 2010, 299, E601–E606.
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Annamalai, P.; Enerbäck, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al.et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. . Diabetes 2014, 63, 4089–4099.
- Nam, H.-Y.; Jun, S.; Association between active brown adipose tissue and coronary artery calcification in healthy men. . Nuklearmedizin 2017, 56, 184–190.
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al.et al. Brown adipose tissue is associated with cardiometabolic health. . Nat. Med. 2021, 27, 58–65.
- Ricquier, D.; Kader, J.C.; Mitochondrial protein alteration in active brown fat: A soidum dodecyl sulfate-polyacrylamide gel electrophoretic study.. Biochem. Biophys. Res. Commun. 1976, 73, 577–583.
- Nicholls, D.G.; Locke, R.M.; Thermogenic mechanisms in brown fat. . Physiol. Rev. 1984, 64, 1–64.
- Ricquier, D.; UCP1, the mitochondrial uncoupling protein of brown adipocyte: A personal contribution and a historical perspective. . Biochimie 2017, 134, 3–8.
- Crichton, P.G.; Lee, Y.; Kunji, E.R.S.; The molecular features of uncoupling protein 1 support a conventional mitochondrial carrier-like mechanism.. Biochimie 2017, 134, 35–50.
- Himms-Hagen, J.; Cui, J.; Danforth, E.; Taatjes, D.J.; Lang, S.S.; Waters, B.L.; Claus, T.H.; Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. . Am. J. Physiol. 1994, 266, R1371–R1382.
- Park, S.W.; Zhou, Y.; Lee, J.; Lee, J.; Ozcan, U.; Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. . Proc. Natl. Acad. Sci. USA. 2010, 107, 19320–19325.
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M.; New Advances in Adaptive Thermogenesis: UCP1 and Beyond. . Cell Metab. 2019, 29, 27–37.
- Bertholet, A.M.; Chouchani, E.T.; Kazak, L.; Angelin, A.; Fedorenko, A.; Long, J.Z.; Vidoni, S.; Garrity, R.; Cho, J.; Terada, N.; et al.et al. H+ transport is an integral function of the mitochondrial ADP/ATP carrier. . Nature 2019, 571, 515–520.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
3 times
(View History)
Update Date:
19 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




