Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Jankowska | -- | 1978 | 2022-12-15 14:10:14 | | | |
| 2 | Conner Chen | Meta information modification | 1978 | 2022-12-19 06:11:36 | | | | |
| 3 | Conner Chen | Meta information modification | 1978 | 2022-12-19 06:13:12 | | | | |
| 4 | Conner Chen | + 4 word(s) | 1982 | 2022-12-22 04:51:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Szczerba, A.; Śliwa, A.; Pieta, P.P.; Jankowska, A. Biology of Circulating Tumor Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/38839 (accessed on 08 February 2026).
Szczerba A, Śliwa A, Pieta PP, Jankowska A. Biology of Circulating Tumor Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/38839. Accessed February 08, 2026.
Szczerba, Anna, Aleksandra Śliwa, Pawel P. Pieta, Anna Jankowska. "Biology of Circulating Tumor Cells" Encyclopedia, https://encyclopedia.pub/entry/38839 (accessed February 08, 2026).
Szczerba, A., Śliwa, A., Pieta, P.P., & Jankowska, A. (2022, December 15). Biology of Circulating Tumor Cells. In Encyclopedia. https://encyclopedia.pub/entry/38839
Szczerba, Anna, et al. "Biology of Circulating Tumor Cells." Encyclopedia. Web. 15 December, 2022.
Copy Citation
Tumor cells circulating in the bloodstream of cancer patients are thought to have the potential to reach and settle in new niches and develop metastasis. Thus, their presence, which shows tumor dissemination from the primary site to distant organs, might be an indicator of the disease progression.
ovarian cancer
invasiveness
metastasis
CTC
CSC
1. Introduction
Despite the advance in diagnostic and treatment methods, ovarian cancer (OC) remains the most lethal type among all gynecological cancers [1][2].
The leading causes of treatment failures and consequent deaths of ovarian cancer patients are late diagnosis, disease progression, and metastasis, defined by the spread of invasive cancer cells. Dissemination of ovarian cancer is one of its characteristic features; about 80% of ovarian cancer patients have disseminated disease at the time of diagnosis [3]. Even though metastasis is the leading cause of ovarian cancer-related fatalities, the understanding of the mechanisms that regulate the process remains limited.
Ovarian cancer cells can spread via three main routes: transcoelomic, hematogenous, and lymphatic (Figure 1).
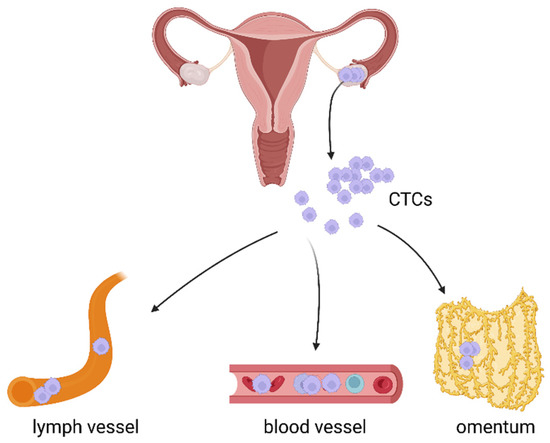
Figure 1. Three main routes of cancer cell dissemination: transcoelomic, hematogenous, and lymphatic. Created with https://biorender.com/, (accessed on 31 October 2022).
The most common and best known route of OC spread is the transcoelomic route. It is associated with metastasis within the peritoneal cavity and affects the surrounding peritoneal organs [4][5]. In this type of cancer, the dissemination of single cells, multicellular aggregates, and spheroids seed into the mesothelial layer and organs of the peritoneal cavity [6].
Compared to the transcoelomic route, distant metastasis via lymph vessels and blood is less common and the mechanisms related to these two modes of cancer dissemination are poorly understood, and merit detailed investigations.
However, numerous studies confirm that lymphatic and haematogenous spread of ovarian cancer is associated with the presence of cells that can detach from the tumor mass and persist in biological fluids, mainly in blood [7][8][9][10][11][12][13][14][15][16][17]. These cells are known as circulating tumor cells—CTCs.
2. Biology of Circulating Tumor Cells
Tumor cells circulating in the bloodstream of cancer patients are thought to have the potential to reach and settle in new niches and develop metastasis [11][18]. Thus, their presence, which shows tumor dissemination from the primary site to distant organs, might be an indicator of the disease progression.
The significance of CTCs and hematogenous spread in ovarian cancer is just starting to be recognized. One reason for such negligence is the lack of easily available models of vascular ovarian cancer metastasis. The mechanisms of haematogenous metastasis are studied using a few animal models, including the parabiosis model, traditional murine xenograft models, genetically modified mouse models, as well as in vitro experiments: 3D spheroids, and organoids [13][19]. However, a growing body of research suggests that CTCs play an important role in ovarian cancer metastasis [7][8][9][10][11][12][13][14][15][16][17].
The results of CTCs studies rely on the accessibility of CTCs detection methods. The difficulty in detecting and isolating rare and heterogeneous CTCs in ovarian cancer therefore remains the main limitation. In fact, depending on the techniques used for the CTCs detection, the positivity rates documented in different studies varied from 12% to 90%. The detection rate might be even higher and reach 95%, as it was recently presented using the subtraction enrichment of the cells followed by immunostaining and fluorescence in situ hybridization [20]. It points to the importance of proper isolation methods allowing successful evaluation of ovarian CTCs.
CTCs detection and identification in blood of OC patients is usually based on cell population enrichment using different biomarkers, followed by CTCs molecular profiling. The most popular approaches include: (i) PCR-based methods analyzing tumor-specific transcripts, (ii) immunological assays using monoclonal antibodies specific for tumor (usually epithelial) markers, (iii) isolation by the size of the tumor cells [21].
Markers used in the identification of CTCs include epithelial antigens (EpCAM, WT1, MUC16, MUC1, KRT7, KRT18, and KRT19), mesenchymal and epithelial–mesenchymal transition (EMT)-related factors (vimentin, N-cadherin, Snai2, CD117, CD146, and PI3Kα, Akt-2, TIMP1, CXCR4, and Twist) as well as stem cell markers (CD44, ALDH1A1, Oct4, and Nanog) [22][23][24]. Recently, the clinical significance of tumor-specific markers, such as CEA, CA125, and HE4 (better than epithelial-specific markers: EPCAM and MUC1) for CTCs isolation before and after adjuvant chemotherapy was shown [25]. Confirming the presence of CTCs in the blood of cancer patients and determining the cells’ phenotype have been indicated to be of diagnostic importance [7][8][9][11][12][13][14][15][16][18][24][26].
Combining CTCs profiling with other biomarkers assessment currently used for diagnosis and monitoring of OC patients may help find new combinations of markers with improved sensitivity and specificity.
However, it needs to be emphasized that none of the markers is specific and sensitive enough to identify all types of CTCs, especially in ovarian cancer patients, where CTCs number is rather low and the cells present with high heterogeneity. In fact, the only FDA-approved CTCs detection platform—CellSearch, detects epithelial CTCs, expressing both epithelial cell adhesion molecule (EpCAM) and cytokeratin, and might miss CTCs undergoing epithelial-to-mesenchymal transition.
Thus, only sensitive diagnostic techniques based on detailed analysis of CTCs-specific genetic profiles might allow the identification and isolation of the cells. This in turn, should increase the chances of metastasis detection.
2.1. The Ever-Changing Phenotype of CTCs
It has been demonstrated that even tumors without clinically confirmed metastasis can shed CTCs into the vascular or lymphatic system [12]. Still, a significant number of CTCs die before they reach a new niche. To increase their chances of survival and protect themselves from cell death CTCs may use different strategies. This includes changing their phenotype from epithelial to mesenchymal, clustering and/or acquiring cancer stem cell (CSC) features (Figure 2). CTCs are heterogeneous in nature. They consist of cell populations with different morphology, molecular characteristics, metastatic potential, and ability to survive chemotherapy. CTCs able to form metastases are known as invasive CTCs (iCTC) [11][19][21][22][25][27][28].
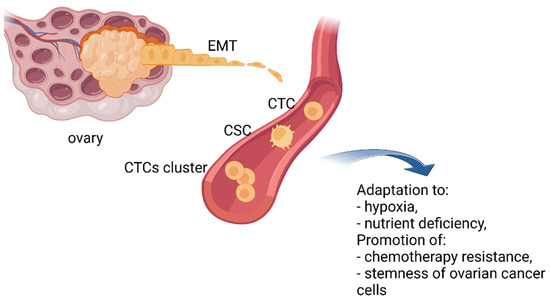
Figure 2. The ever-changing phenotype of CTCs. To increase their chances of survival, CTCs may change their phenotype from epithelial to mesenchymal, by clustering and/or acquiring cancer stem cell (CSC) properties. Created with https://biorender.com/, (accessed on 31 October 2022).
CTCs are believed to disseminate to distant sites thanks to epithelial–mesenchymal transition. This process includes a series of molecular, morphological, functional, and consequently, phenotypical changes of cells leading to the transition of polarized epithelial cells into mobile mesenchymal cells. Epithelial–mesenchymal transition may also generate hybrid phenotypes with an increased ability to survive in the circulation and adapt to various microenvironments [27].
A growing body of evidence demonstrates that EMT allows ovarian cancer cells to adapt to adverse conditions, such as hypoxia and nutrient deficiency, and promote chemotherapy resistance to therapeutic agents as well as activate the stemness of ovarian cancer cells [28]. Sharing some common features with cancer stem cells permits CTCs to increase their tumorigenicity and resist anoikis, chemo- and radiotherapy [29].
All this increases the heterogeneity of ovarian CTCs population and points to the significance of detailed molecular analysis of the cells’ expression profiles, especially in terms of their detection and clinical utility.
2.2. CTCs Clusters
Strong evidence suggests that CTCs can be organized in clusters. CTCs clusters may have up to 100-fold increased metastatic potential in comparison with the same number of isolated single CTCs. Clustering supports the collective migration of cells increasing their chances of survival, but also promotes specific changes such as stemness, drug resistance, and metastasis [29][30][31].
It has recently been proved that stemness and metastasis are promoted by specific changes in DNA methylation induced by the cells’ clustering. CTCs clustering leads to hypomethylation of binding sites for stemness and proliferation regulators, including OCT4, NANOG, SOX2, and SIN3A, and hypermethylation of Polycomb target genes [32].
Most cancers manifesting the presence of CTCs clusters are solid cancers and the clusters were detected in 16% to 75% of patients [31]. A higher number of clusters in patients’ blood was confirmed to be associated with shorter progression-free survival (PFS). This points to a possible link between CTCs clusters presence in peripheral blood and metastatic disease [15][31].
In ovarian cancer, CTCs clusters and their clinical relevance have not been extensively studied. Only a few studies demonstrate the presence of CTCs clusters in blood of OC patients and only a single research group provides information about their clinical significance [15][17][33]. One study describes CTCs clusters consisting of 2–30 cells. Such clusters are associated with platinum resistance, shorter time to progression (TTP), and PFS. Out of 24 OC patients with the primary disease and 30 patients with recurrences, CTCs were detected in 98.1%. Nevertheless, in women with the primary disease median counts of single CTCs and CTCs clusters were 4 and 1, and in those patients with recurrences, median counts were 3 and 1, respectively. Even though CTCs presence did not correlate with tumor stage and serum CA125 level, still CTCs counts ≥3 as well as CTCs clusters positivity correlated with platinum resistance and shortened overall survival in patients with recurrent disease. In the case of two patients CTCs isolation was followed by a successful in vitro culture. The results of ex vivo experiments indicated that CTCs can be more sensitive to anticancer drugs and proliferated more rapidly than established cell lines [15].
CTCs clusters in OC patients were also identified by Pearl et al. They demonstrated the invasive CTCs (iCTCs) isolated by functional cell adhesion matrix (CAM) uptake followed by microscopy and flow cytometry analysis using antibodies against epithelial/tumor antigens and negative selection with antibodies against hematopoietic lineage markers. These iCTCs tended to be heterogeneous in size and exhibited solitary cells and clusters. The changes in iCTCs and CA125 levels as well as changes in the intervals associated with no evidence of disease were noted. Additionally, an increased number of iCTCs (79.5%) was showed to be more sensitive than the increased CA125 level (67.6%) when it comes to predicting progressive disease (PD) or relapse. Finally iCTCs, but not CA125, preceded changes in the clinical status from PD to no evidence of disease during and after chemotherapy [17]. Thus, iCTCs in OC patients may help to predict the disease outcome and therapeutic responsiveness.
The presence of CTCs clusters isolated with the ALS CellCelector™ in ovarian cancers was also confirmed with liquid biopsy [33]. However, the authors of this study do not provide any information regarding the biology and/or clinical relevance of detected CTCs clusters.
Therefore, the development of efficient and reliable methods of CTCs clusters identification, together with cohort studies are needed to determine their suitability for clinical use.
2.3. CTCs and Cancer Stem Cells
In ovarian cancer, tumor cells are known to display cancer stem cells (CSCs) features such as self-renewal, differentiation, and tumorigenicity. CSCs are believed to support tumor growth and metastasis [29]. Due to the fact that they are also resistant to anoikis, CSCs may easily spread and survive within the lymphatic and vascular systems where they are considered to be stem CTCs [34][35][36][37][38].
Ovarian CSCs are characterized by the expression of specific markers. The best described include: CD44, CD133, CD24, CD117, Nestin, Nanog, and Oct3/4, as well as ALDH1A1 and ABC transporters. These markers allow CSCs detection and indicate tumor invasiveness, chemoresistance, and poor prognosis [29][33][38][39][40][41][42][43][44].
Some ovarian CSCs markers were reported to correlate with distinct metastasis via the haematogenous route. Recently, CD44 variant 6 was demonstrated to be a central player in the development of distant metastasis in parenchymal organs. A high number of CD44v6-positive ovarian cancer cells was associated with a high rate of distant metastasis at the time of diagnosis and distant metastasis-free survival varied significantly between CD44v6-high and -low patients [45]. Distant metastasis was also linked with the stem cell regulatory factor—EGFL6. EGFL6 induces cell division and migration of ALDH-positive ovarian CSCs, consequently promoting tumor growth and metastasis. Silencing of EGFL6 expression proved effective in reducing the haematogenous spread of ovarian cancer cells [46]. Thus, both CD44v6 and EGFL6 are involved in distant metastatic relapse and could be predictive biomarkers for distant parenchymal metastasis as well as a novel therapeutic target [45][46]. Their inhibition, in a similar way to blocking signal transduction pathways active in ovarian CSCs (e.g. Wnt, Hedgehog Notch, PI3K/PTEN/AKT) [47][48][49][50], seems to be a promising treatment alternative that should help overcome therapy resistance and reduce the mortality of ovarian cancer patients.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30.
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Womens Health 2019, 11, 287–299.
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M. SEER Cancer Statistics Review, 1975-2018, National Cancer Institute. Bethesda. Available online: https://seer.cancer.gov/csr/1975_2018/, (accessed on 31 October 2022).
- Rose, P.G.; Piver, M.S.; Tsukada, Y.; Lau, T. Metastatic Patterns in Histologic Variants of Ovarian Cancer. An Autopsy Study. Cancer 1989, 64, 1508–1513.
- Güth, U.; Arndt, V.; Stadlmann, S.; Huang, D.J.; Singer, G. Epidemiology in Ovarian Carcinoma: Lessons from Autopsy. Gynecol. Oncol. 2015, 138, 417–420.
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064.
- Obermayr, E.; Reiner, A.; Brandt, B.; Braicu, E.I.; Reinthaller, A.; Loverix, L.; Concin, N.; Woelber, L.; Mahner, S.; Sehouli, J.; et al. The Long-Term Prognostic Significance of Circulating Tumor Cells in Ovarian Cancer—A Study of the OVCAD Consortium. Cancers 2021, 13, 2613.
- Kolostova, K.; Pinkas, M.; Jakabova, A.; Pospisilova, E.; Svobodova, P.; Spicka, J.; Cegan, M.; Matkowski, R.; Bobek, V. Molecular Characterization of Circulating Tumor Cells in Ovarian Cancer. Am. J. Cancer Res. 2016, 6, 973–980.
- Pearl, M.L.; Dong, H.; Tulley, S.; Zhao, Q.; Golightly, M.; Zucker, S.; Chen, W.-T. Treatment Monitoring of Patients with Epithelial Ovarian Cancer Using Invasive Circulating Tumor Cells (ICTCs). Gynecol. Oncol. 2015, 137, 229–238.
- Tarin, D.; Price, J.E.; Kettlewell, M.G.; Souter, R.G.; Vass, A.C.; Crossley, B. Mechanisms of Human Tumor Metastasis Studied in Patients with Peritoneovenous Shunts. Cancer Res. 1984, 44, 3584–3592.
- Lou, E.; Vogel, R.I.; Teoh, D.; Hoostal, S.; Grad, A.; Gerber, M.; Monu, M.; Łukaszewski, T.; Deshpande, J.; Linden, M.A.; et al. Assessment of Circulating Tumor Cells as a Predictive Biomarker of Histology in Women With Suspected Ovarian Cancer. Lab. Med. 2018, 49, 134–139.
- Banys-Paluchowski, M.; Fehm, T.; Neubauer, H.; Paluchowski, P.; Krawczyk, N.; Meier-Stiegen, F.; Wallach, C.; Kaczerowsky, A.; Gebauer, G. Clinical Relevance of Circulating Tumor Cells in Ovarian, Fallopian Tube and Peritoneal Cancer. Arch. Gynecol. Obstet. 2020, 301, 1027–1035.
- Yousefi, M.; Dehghani, S.; Nosrati, R.; Ghanei, M.; Salmaninejad, A.; Rajaie, S.; Hasanzadeh, M.; Pasdar, A. Current Insights into the Metastasis of Epithelial Ovarian Cancer—Hopes and Hurdles. Cell. Oncol. 2020, 43, 515–538.
- Zeng, L.; Liang, X.; Liu, Q.; Yang, Z. The Predictive Value of Circulating Tumor Cells in Ovarian Cancer: A Meta Analysis. Int. J. Gynecol. Cancer 2017, 27, 1109–1117.
- Lee, M.; Kim, E.J.; Cho, Y.; Kim, S.; Chung, H.H.; Park, N.H.; Song, Y.-S. Predictive Value of Circulating Tumor Cells (CTCs) Captured by Microfluidic Device in Patients with Epithelial Ovarian Cancer. Gynecol. Oncol. 2017, 145, 361–365.
- Poveda, A.; Kaye, S.B.; McCormack, R.; Wang, S.; Parekh, T.; Ricci, D.; Lebedinsky, C.A.; Tercero, J.C.; Zintl, P.; Monk, B.J. Circulating Tumor Cells Predict Progression Free Survival and Overall Survival in Patients with Relapsed/Recurrent Advanced Ovarian Cancer. Gynecol. Oncol. 2011, 122, 567–572.
- Pearl, M.L.; Zhao, Q.; Yang, J.; Dong, H.; Tulley, S.; Zhang, Q.; Golightly, M.; Zucker, S.; Chen, W.-T. Prognostic Analysis of Invasive Circulating Tumor Cells (ICTCs) in Epithelial Ovarian Cancer. Gynecol. Oncol. 2014, 134, 581–590.
- Chebouti, I.; Kasimir-Bauer, S.; Buderath, P.; Wimberger, P.; Hauch, S.; Kimmig, R.; Kuhlmann, J.D. EMT-like Circulating Tumor Cells in Ovarian Cancer Patients Are Enriched by Platinum-Based Chemotherapy. Oncotarget 2017, 8, 48820–48831.
- Bregenzer, M.E.; Horst, E.N.; Mehta, P.; Novak, C.M.; Repetto, T.; Snyder, C.S.; Mehta, G. Tumor Modeling Maintains Diverse Pathology in Vitro. Ann. Transl. Med. 2019, 7, S262.
- Cheng, H.; Wang, S.; Luan, W.; Ye, X.; Dou, S.; Tang, Z.; Zhu, H.; Lin, P.P.; Li, Y.; Cui, H.; et al. Combined Detection and Subclass Characteristics Analysis of CTCs and CTECs by SE-IFISH in Ovarian Cancer. Chin. J. Cancer Res. 2021, 33, 256–270.
- Bankó, P.; Lee, S.Y.; Nagygyörgy, V.; Zrínyi, M.; Chae, C.H.; Cho, D.H.; Telekes, A. Technologies for Circulating Tumor Cell Separation from Whole Blood. J. Hematol. Oncol. 2019, 12, 48.
- Kolostova, K.; Matkowski, R.; Jędryka, M.; Soter, K.; Cegan, M.; Pinkas, M.; Jakabova, A.; Pavlasek, J.; Spicka, J.; Bobek, V. The Added Value of Circulating Tumor Cells Examination in Ovarian Cancer Staging. Am. J. Cancer Res. 2015, 5, 3363–3375.
- Chebouti, I.; Kuhlmann, J.D.; Buderath, P.; Weber, S.; Wimberger, P.; Bokeloh, Y.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. ERCC1-Expressing Circulating Tumor Cells as a Potential Diagnostic Tool for Monitoring Response to Platinum-Based Chemotherapy and for Predicting Post-Therapeutic Outcome of Ovarian Cancer. Oncotarget 2017, 8, 24303–24313.
- Blassl, C.; Kuhlmann, J.D.; Webers, A.; Wimberger, P.; Fehm, T.; Neubauer, H. Gene Expression Profiling of Single Circulating Tumor Cells in Ovarian Cancer—Establishment of a Multi-Marker Gene Panel. Mol. Oncol. 2016, 10, 1030–1042.
- Yousefi, M.; Rajaie, S.; Keyvani, V.; Bolandi, S.; Hasanzadeh, M.; Pasdar, A. Clinical Significance of Circulating Tumor Cell Related Markers in Patients with Epithelial Ovarian Cancer before and after Adjuvant Chemotherapy. Sci. Rep. 2021, 11, 10524.
- Asante, D.-B.; Calapre, L.; Ziman, M.; Meniawy, T.M.; Gray, E.S. Liquid Biopsy in Ovarian Cancer Using Circulating Tumor DNA and Cells: Ready for Prime Time? Cancer Lett. 2020, 468, 59–71.
- Genna, A.; Vanwynsberghe, A.M.; Villard, A.V.; Pottier, C.; Ancel, J.; Polette, M.; Gilles, C. EMT-Associated Heterogeneity in Circulating Tumor Cells: Sticky Friends on the Road to Metastasis. Cancers 2020, 12, 1632.
- Loret, N.; Denys, H.; Tummers, P.; Berx, G. The Role of Epithelial-to-Mesenchymal Plasticity in Ovarian Cancer Progression and Therapy Resistance. Cancers 2019, 11, 838.
- Thankamony, A.P.; Saxena, K.; Murali, R.; Jolly, M.K.; Nair, R. Cancer Stem Cell Plasticity—A Deadly Deal. Front. Mol. Biosci. 2020, 7, 79.
- Schuster, E.; Taftaf, R.; Reduzzi, C.; Albert, M.K.; Romero-Calvo, I.; Liu, H. Better Together: Circulating Tumor Cell Clustering in Metastatic Cancer. Trends Cancer 2021, 7, 1020–1032.
- Amintas, S.; Bedel, A.; Moreau-Gaudry, F.; Boutin, J.; Buscail, L.; Merlio, J.-P.; Vendrely, V.; Dabernat, S.; Buscail, E. Circulating Tumor Cell Clusters: United We Stand Divided We Fall. Int. J. Mol. Sci. 2020, 21, 2653.
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e14.
- Nelep, C.; Eberhardt, J. Automated Rare Single Cell Picking with the ALS CellcelectorTM. Cytom. Part A 2018, 93, 1267–1270.
- Virant-Klun, I.; Zech, N.; Rožman, P.; Vogler, A.; Cvjetičanin, B.; Klemenc, P.; Maličev, E.; Meden-Vrtovec, H. Putative Stem Cells with an Embryonic Character Isolated from the Ovarian Surface Epithelium of Women with No Naturally Present Follicles and Oocytes. Differentiation 2008, 76, 843–856.
- Parte, S.; Bhartiya, D.; Telang, J.; Daithankar, V.; Salvi, V.; Zaveri, K.; Hinduja, I. Detection, Characterization, and Spontaneous Differentiation In Vitro of Very Small Embryonic-Like Putative Stem Cells in Adult Mammalian Ovary. Stem Cells Dev. 2011, 20, 1451–1464.
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian Cancer Spheroid Cells with Stem Cell-like Properties Contribute to Tumor Generation, Metastasis and Chemotherapy Resistance through Hypoxia-Resistant Metabolism. PLoS ONE 2014, 9, e84941.
- Keyvani, V.; Farshchian, M.; Esmaeili, S.-A.; Yari, H.; Moghbeli, M.; Nezhad, S.-R.K.; Abbaszadegan, M.R. Ovarian Cancer Stem Cells and Targeted Therapy. J. Ovarian Res. 2019, 12, 120.
- Auersperg, N. The Stem-Cell Profile of Ovarian Surface Epithelium Is Reproduced in the Oviductal Fimbriae, with Increased Stem-Cell Marker Density in Distal Parts of the Fimbriae. Int. J. Gynecol. Pathol. 2013, 32, 444–453.
- Bapat, S.A.; Mali, A.M.; Koppikar, C.B.; Kurrey, N.K. Stem and Progenitor-Like Cells Contribute to the Aggressive Behavior of Human Epithelial Ovarian Cancer. Cancer Res. 2005, 65, 3025–3029.
- Hu, L.; McArthur, C.; Jaffe, R.B. Ovarian Cancer Stem-like Side-Population Cells Are Tumourigenic and Chemoresistant. Br. J. Cancer 2010, 102, 1276–1283.
- Muñoz-Galván, S.; Carnero, A. Targeting Cancer Stem Cells to Overcome Therapy Resistance in Ovarian Cancer. Cells 2020, 9, 1402.
- Kim, M.; Suh, D.H.; Choi, J.Y.; Bu, J.; Kang, Y.-T.; Kim, K.; No, J.H.; Kim, Y.B.; Cho, Y.-H. Post-Debulking Circulating Tumor Cell as a Poor Prognostic Marker in Advanced Stage Ovarian Cancer. Medicine 2019, 98, e15354.
- Aktas, B.; Kasimir-Bauer, S.; Heubner, M.; Kimmig, R.; Wimberger, P. Molecular Profiling and Prognostic Relevance of Circulating Tumor Cells in the Blood of Ovarian Cancer Patients at Primary Diagnosis and after Platinum-Based Chemotherapy. Int. J. Gynecol. Cancer 2011, 21, 822–830.
- Ma, J.; Yang, J.; Jin, Y.; Cheng, S.; Huang, S.; Zhang, N.; Wang, Y. Artificial Intelligence Based on Blood Biomarkers Including CTCs Predicts Outcomes in Epithelial Ovarian Cancer: A Prospective Study. Onco. Targets. Ther. 2021, 14, 3267–3280.
- Motohara, T.; Fujimoto, K.; Tayama, S.; Narantuya, D.; Sakaguchi, I.; Tashiro, H.; Katabuchi, H. CD44 Variant 6 as a Predictive Biomarker for Distant Metastasis in Patients With Epithelial Ovarian Cancer. Obstet. Gynecol. 2016, 127, 1003–1011.
- Bai, S.; Ingrfam, P.; Chen, Y.-C.; Deng, N.; Pearson, A.; Niknafs, Y.S.; O’Hayer, P.; Wang, Y.; Zhang, Z.-Y.; Boscolo, E.; et al. EGFL6 Regulates the Asymmetric Division, Maintenance, and Metastasis of ALDH+ Ovarian Cancer Cells. Cancer Res. 2016, 76, 6396–6409.
- Zhang, X.; Li, H.; Yu, X.; Li, S.; Lei, Z.; Li, C.; Zhang, Q.; Han, Q.; Li, Y.; Zhang, K.; et al. Analysis of Circulating Tumor Cells in Ovarian Cancer and Their Clinical Value as a Biomarker. Cell. Physiol. Biochem. 2018, 48, 1983–1994.
- Zuo, L.; Li, X.; Zhu, H.; Li, A.; Wang, Y. Expression of MiR-181a in Circulating Tumor Cells of Ovarian Cancer and Its Clinical Application. ACS Omega 2021, 6, 22011–22019.
- Yang, J.; Ma, J.; Jin, Y.; Cheng, S.; Huang, S.; Zhang, N.; Wang, Y. Development and Validation for Prognostic Nomogram of Epithelial Ovarian Cancer Recurrence Based on Circulating Tumor Cells and Epithelial-Mesenchymal Transition. Sci. Rep. 2021, 11, 6540.
- Marth, C.; Kisic, J.; Kaern, J.; Tropé, C.; Fodstad, Ø. Circulating Tumor Cells in the Peripheral Blood and Bone Marrow of Patients with Ovarian Carcinoma Do Not Predict Prognosis. Cancer 2002, 94, 707–712.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
910
Revisions:
4 times
(View History)
Update Date:
22 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




