
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thomas Liehr | -- | 3302 | 2022-12-14 10:13:01 | | | |
| 2 | Sirius Huang | -1079 word(s) | 2223 | 2022-12-16 08:59:03 | | |
Video Upload Options
There are two major principle modalities to perform prenatal genetic diagnostics: by invasive or non-invasive means. Invasive prenatal genetic diagnostics depends on fetal or placental tissues, which can only be acquired by approaching the unborn with a needle to gain this material for further in vitro studies. Conversely, non-invasive tests do not disturb the fetal or placental tissues at all. The newest non-invasive prenatal diagnostic test approach is the so-called non-invasive prenatal testing (NIPT). Here an overview on real advantages and still critical issues of this approach are put together.
1. Introduction
| Type of Test | Invasive | |
|---|---|---|
| Can be carried out from w.o.g.: | Is the fetus studied? | |
| (cyto)genetics from CVS | ~11 | No |
| (cyto)genetics from AC | ~14 | Yes |
| (cyto)genetics from UCB | ~20 | Yes |
| Type of test | Non-invasive | |
| Can be carried out from w.o.g.: | Is the fetus studied? | |
| Early sonography | ~6 | (Yes) 1 |
| Late fine sonography | ~19 | (Yes) 1 |
| First trimester-screening (FTS) | 11–14 | (No) 2 |
| Tests for protein markers (e.g., alpha fetoprotein, etc.) from maternal blood serum | ~11 | No |
| Non-invasive prenatal testing (NIPT) from free placenta-derived DNA in maternal blood serum | ~10 | No |
| Molecular genetic/molecular cytogenetic tests on fetal nucleated erythrocytes from maternal blood | before 10 | Yes |
2. Technical Bases of NIPT
- (a)
-
trisomy 13, 18 and 21, only;
- (b)
-
aforementioned trisomies and for changes in the copy numbers of sex chromosomes;
- (c)
-
all mentioned in b) plus only DiGeorge syndrome [20];
- (d)
-
all mentioned in b) plus other selected microdeletion or microduplication syndromes [21];
- (e)
-
all mentioned in (b), (c) or (d) plus all other copy number changes of all other autosomes;
- (f)
-
whole genome, for any kind of copy number alteration.
- (g)
-
Finally, some NIPT providers have started to also offer screening for point mutations of specific genes, such as the Rhesus factor and blood groups.
3. NIPT and Its Advantages
- I.
-
can be applied earlier than other tests during pregnancy;
- II.
-
has the potential to reach populations with no access to centers offering invasive diagnostics [24];
- III.
-
can exclude and detect a trisomy 21 with the highest probability of all non-invasive approaches—the positive predictive value (PPV) in cases of a NIPT suggesting trisomy 21 is >99%;
- IV.
-
can bring a psychological relief to an anxious pregnant woman in the case of a normal NIPT result; and
- V.
4. NIPT and Its Practical Restrictions and Shortcuts
- (a)
-
The term NIPT suggests to the uninformed public that the platform studies cell free fetal DNA (cffDNA); however, all cell free DNA in the pregnant individual’s blood (not being of maternal origin) derives from the placenta (and not the fetus); thus, in 1–2% of the cases studied by NIPT, a placenta confined mosaic condition must be expected. They constitute a part of the false positive and negative NIPT results [15][19].
- (b)
-
Each abnormal NIPT result has to be checked by an invasive prenatal test, optimally an AC, as indicated by each commercial NIPT provider in the package leaflet [9]. However, one major motivation to do NIPT is to avoid any invasive procedure, due to the previously suggested high abortion risk [3][5][6][7].
- (c)
-
A normal NIPT result can maximally exclude (to a certain extent) genetic conditions, which are covered by the NIPT platform used. A normal NIPT is never synonymous with the statement: ‘a healthy child will be born’ [26]. As recently shown, NIPT can at best detect 5–10% of all cases potentially born with birth defects [19] (Figure 1).
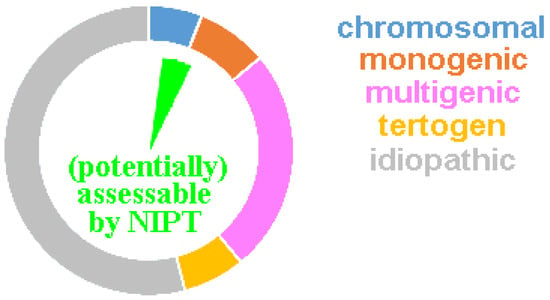 Figure 1. The ring diagram includes the 3–6% of newborns with major inborn abnormalities. A chromosomal disorder is present in ~6%, teratogenic damage in ~7%, and a monogenetic or multigenetic disease in ~8% or ~25%, respectively. For the remaining ~54%, the diagnosis usually remains a lifelong suffering attributed to an “idiopathic disorder”, i.e., the cause remains unclear. The green inner pie diagram shows the ~5 to max 10% of cases potentially assessable by NIPT.
Figure 1. The ring diagram includes the 3–6% of newborns with major inborn abnormalities. A chromosomal disorder is present in ~6%, teratogenic damage in ~7%, and a monogenetic or multigenetic disease in ~8% or ~25%, respectively. For the remaining ~54%, the diagnosis usually remains a lifelong suffering attributed to an “idiopathic disorder”, i.e., the cause remains unclear. The green inner pie diagram shows the ~5 to max 10% of cases potentially assessable by NIPT. - (d)
-
90% of aneuploid fetuses detected in NIPT would be aborted naturally and do not survive to birth [19]. Thus, 90% of pregnant women with a true abnormal NIPT result would not have to undergo an induced abortion.
- (e)
-
5–10% of NIPTs provide no result at the first attempt—this is most often due to the low fraction of cell-free placenta derived DNA. The greater the obesity severity in pregnant women, the more likely a ‘no-call’ result will occur [15][19]. The number of cases repeated is not extensively reported in the literature, which could yield data on how many of these cases ultimately yield an informative result. However, it should be noted that within this population, there is overrepresentation of cases ending in an early abortion, largely attributed to a placenta that is too small for the gestational age and preeclampsia.
- (f)
- (g)
- (h)
-
As aforementioned, the PPV for trisomy 21 is >99% in all available NIPTs; however, it is neither known nor studied why screening for trisomies 13 and 18, is less reliable. PPVs for all other tested copy number changes (including sex chromosomes, other autosomes, and microdeletion/-duplications) remain substantially lower between 5 and 60—which means 40 to 95% of women receiving an abnormal NIPT result have indeed received a false positive result [19].
- (i)
-
In a worldwide perspective, there are hints in the literature that in ~20% of cases, there is a strong tendency to trust an abnormal NIPT result so much that a second test is skipped and termination of the pregnancy is simply based on a single screening test [19][30]. Such policies are also reinforced by the cost-effectiveness of NIPT analyses, which inappropriately encourage NIPT use as a diagnostic versus a screening tool [31].
(1) “The high specificity, efficiency and safety (non-invasiveness) of NIPT can effectively improve the detection rate of common chromosomal aneuploidy, thereby reducing the occurrence of birth defects” [33]; or (2) “NIPT has revolutionized the approach to prenatal diagnosis and, to date, it is the most superior screening method for the common autosomal aneuploidies”. [34]
5. Conclusions
While the theoretical possibilities of NIPT are promising problems and limitations must also be considered. Overall, NIPT results are not well understood and the reliability of the obtained data is, at the least, also not communicated well to the MDs ordering the test nor to the pregnant women taking the test. As stated by Hessel and Henn, recently: “Fact is, that the NIPT is perceived by many pregnant as a standard measure for pregnant women and is carried out unquestioningly” (translated from [35]). This attitude may lead to a rude awakening [26]. Thus, appropriate training and continuing education of the physicians providing NIPT counseling [14] are urgently necessary. As outlined elsewhere [15] this can only be achieved by unbiased offers of ongoing education—not from NIPT providers, but from medical associations or independent researchers.
References
- Darouich, A.A.; Liehr, T.; Weise, A.; Schlembach, D.; Schleußner, E.; Kiehntopf, M.; Schreyer, I. Alpha-fetoprotein and its value for predicting pregnancy outcomes—A re-evaluation. Prenat. Med. 2015, 9, 18–23.
- Benn, K.N.; Benn, P.; Campbell, W.A.; Moaddab, A.; Shamshira, A.A. Genetic sonogram: Components and role in the era of prenatal screening. Fetal Matern. Med. Rev. 2014, 25, 214–231.
- Liehr, T.; Lauten, A.; Schneider, U.; Schleussner, E.; Weise, A. Noninvasive prenatal testing (NIPT)—When is it advantageous to apply? Hub. 2017, 2, 458432.
- Liehr, T.; Harutyunyan, T.; Williams, H.; Weise, A. Non-invasive prenatal testing in Germany. Diagnostics 2022, 12, 2816.
- Wulff, C.B.; Gerds, T.A.; Rode, L.; Ekelund, C.K.; Petersen, O.B.; Tabor, A.; Danish Fetal Medicine Study Group. Risk of fetal loss associated with invasive testing following combined first-trimester screening for Down syndrome: A national cohort of 147,987 singleton pregnancies. Ultrasound Obstet. Gynecol. 2016, 47, 38–44.
- Tabor, A.; Alfirevic, Z. Update on procedure-related risks for prenatal diagnosis techniques. Fetal Diagn. Ther. 2010, 27, 1–7.
- Akolekar, R.; Beta, J.; Picciarelli, G.; Ogilvie, C.; D’Antonio, F. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2015, 45, 16–26.
- Available online: https://www.google.com/search?q=invasive+pr%C3%A4nataldiagnostik+risiken&client=firefox-b-d&biw=1876&bih=970&ei=Wu8_Y-i6OLuBxc8PlsyV0AY&oq=invasive+pr%C3%A4nataldiagnsotik+&gs_lcp=Cgdnd3Mtd2l6EAMYADIHCAAQgAQQDTIHCAAQgAQQDTIHCAAQgAQQDTIHCAAQgAQQDTIICAAQHhAWEAoyBggAEB4QFjIGCAAQHhAWMgYIABAeEBYyBggAEB4QFjIGCAAQHhAWOgoIABBHENYEELADOg0IABBHENYEELADEMkDOgQIABBDOgsIABCABBCxAxCDAToRCC4QgAQQsQMQgwEQxwEQ0QM6CAgAELEDEIMBOgsILhCABBCxAxCDAToLCC4QgAQQxwEQ0QM6CAguEIAEENQCOggIABCABBCxAzoLCC4QgAQQsQMQ1AI6BQgAEIAEOgcIABCxAxBDOggIABAeEA0QBUoECEEYAEoECEYYAFC0BFj8J2DsPmgBcAF4AIABSogB4wySAQIyOJgBAKABAcgBCMABAQ&sclient=gws-wiz (accessed on 16 September 2022).
- Kypri, E.; Ioannides, M.; Achilleos, A.; Koumbaris, G.; Patsalis, P.; Stumm, M. Non-invasive prenatal screening tests—Update. Med. 2022, 46, 311–320.
- Kuo, P.L.; Guo, H.R. Nucleated red blood cells in maternal blood during pregnancy. Gynecol. 1999, 94, 464–468.
- Zhang, Q.; Zhang, K.; Guo, Y.; Wei, X.; Sun, Y.; Cai, B.; Shi, Y.; Du, Y.; Liu, Y.; Fan, C.; et al. The isolation and analysis of fetal nucleated red blood cells using multifunctional microbeads with a nanostructured coating toward early noninvasive prenatal diagnostics. Mater. Chem. B 2021, 9, 3047–3054.
- Cheng, L.; Wei, X.; Wang, Z.; Feng, C.; Gong, Q.; Fu, Y.; Zhao, X.; Zhang, Y. Silica microbeads capture fetal nucleated red blood cells for noninvasive prenatal testing of fetal ABO genotype. Electrophoresis 2020, 41, 966–972.
- Seppo, A.; Frisova, V.; Ichetovkin, I.; Kim, Y.; Evans, M.I.; Antsaklis, A.; Nicolaides, K.H.; Tafas, T.; Tsipouras, P.; Kilpatrick, M.W. Detection of circulating fetal cells utilizing automated microscopy: Potential for noninvasive prenatal diagnosis of chromosomal aneuploidies. Diagn. 2008, 28, 815–821.
- Levine, R.J.; Qian, C.; Leshane, E.S.; Yu, K.F.; England, L.J.; Schisterman, E.F.; Wataganara, T.; Romero, R.; Bianchi, D.W. Two-stage elevation of cell-free fetal DNA in maternal sera before onset of preeclampsia. J. Obstet. Gynecol. 2004, 190, 707–713.
- Liehr, T. Non-invasive prenatal testing, what patients do not learn, may be due to lack of specialist genetic training by gynecologists and obstetricians? Genet. 2021, 12, 682980.
- Chen, S.; Lau, T.K.; Zhang, C.; Xu, C.; Xu, Z.; Hu, P.; Xu, J.; Huang, H.; Pan, L.; Jiang, F.; et al. A method for noninvasive detection of fetal large deletions/duplications by low coverage massively parallel sequencing. Diagn. 2013, 33, 584–590.
- Koumbaris, G.; Kypri, E.; Tsangaras, K.; Achilleos, A.; Mina, P.; Neofytou, M.; Velissariou, V.; Christopoulou, G.; Kallikas, I.; González-Liñán, A.; et al. Cell-free DNA analysis of targeted genomic regions in maternal plasma for non-invasive prenatal testing of trisomy 21, trisomy 18, trisomy 13, and fetal sex. Chem. 2016, 62, 848–855.
- Dahl, F.; Ericsson, O.; Karlberg, O.; Karlsson, F.; Howell, M.; Persson, F.; Roos, F.; Stenberg, J.; Ahola, T.; Alftrén, I.; et al. Imaging single DNA molecules for high precision NIPT. Rep. 2018, 8, 4549.
- Liehr, T. False-positives and false-negatives in non-invasive prenatal testing (NIPT): What can we learn from a meta-analyses on > 750,000 tests? Cytogenet. 2022, 15, 36.
- Cortés-Martín, J.; Peñuela, N.L.; Sánchez-García, J.C.; Montiel-Troya, M.; Díaz-Rodríguez, L.; Rodríguez-Blanque, R. Deletion syndrome 22q11.2: A systematic review. Children 2022, 9, 1168.
- Weise, A.; Mrasek, K.; Klein, E.; Mulatinho, M.; Llerena, J.C., Jr.; Hardekopf, D.; Pekova, S.; Bhatt, S.; Kosyakova, N.; Liehr, T. Microdeletion and microduplication syndromes. Histochem. Cytochem. 2012, 60, 346–358.
- Zaninović, L.; Bašković, M.; Ježek, D.; Katušić Bojanac, A. Validity and utility of non-invasive prenatal testing for copy number variations and microdeletions: A systematic review. Clin. Med. 2022, 11, 3350.
- Weise, A.; Liehr, T. Fluorescence in situ hybridization for prenatal screening of chromosomal aneuploidies. Expert Rev. Mol. Diagn. 2008, 8, 355–357.
- Vossaert, L.; Chakchouk, I.; Zemet, R.; Van den Veyver, I.B. Overview and recent developments in cell-based noninvasive prenatal testing. Diagn. 2021, 41, 1202–1214.
- Lewit-Mendes, M.F.; Robson, H.; Kelley, J.; Elliott, J.; Brown, E.; Menezes, M.; Archibald, A.D. Experiences of receiving an increased chance of sex chromosome aneuploidy result from non-invasive prenatal testing in Australia: “A more complicated scenario than what I had ever realized”. Genet. Couns. 2022, 1–11. https://doi.org/10.1002/jgc4.1635.
- Kraft E. Geschäft mit der Falschen Sicherheit [Business with the Wrong Security]. Available online: https://www.pressreader.com/germany/ostthuringer-zeitung-stadtroda/20200831/282200833311280 (accessed on 16 September 2022).
- Lau, T.K.; Jiang, F.M.; Stevenson, R.J.; Lo, T.K.; Chan, L.W.; Chan, M.K.; Lo, P.S.; Wang, W.; Zhang, H.Y.; Chen, F.; et al. Secondary findings from non-invasive prenatal testing for common fetal aneuploidies by whole genome sequencing as a clinical service. Diagn. 2013, 33, 602–608.
- Tolva, G.; Silipigni, R.; Quarenghi, A.; Vergani, P.; Guerneri, S.; Milani, D. Tetrasomy 18p: The challenges of noninvasive prenatal testing and combined test. Obstet. Gynaecol. Res. 2019, 45, 705–708.
- Tamaki, Y.; Katagiri, Y.; Umemura, N.; Takeshita, N.; Morita, M. Noninvasive prenatal testing aids identification of tetrasomy 18p: A case report. Case Rep. Womens Health 2020, 27, e00236.
- Zelig, C.M.; Knutzen, D.M.; Ennen, C.S.; Dolinsky, B.M.; Napolitano, P.G. Chorionic villus sampling, early amniocentesis, and termination of pregnancy without diagnostic testing: Comparison of fetal risk following positive non-invasive prenatal testing. Obstet. Gynaecol. Can. 2016, 38, 441–445.e2.
- Ohno, M.; Caughey, A. The role of noninvasive prenatal testing as a diagnostic versus a screening tool—A cost-effectiveness analysis. Diagn. 2013, 33, 630–635.
- Haidar, H.; Birko, S.; Laberge, A.M.; Le Clerc-Blain, J.; Ravitsky, V. Views of Canadian healthcare professionals on the future uses of non-invasive prenatal testing: A mixed method study. J. Hum. Genet. 2022, 30, 1269–1275. https://doi.org/10.1038/s41431-022-01151-5.
- Zhang, Y.; Xu, H.; Zhang, W.; Liu, K. Non-invasive prenatal testing for the detection of trisomy 13, 18, and 21 and sex chromosome aneuploidies in 68,763 cases. Genet. 2022, 13, 864076.
- D’Ambrosio, V.; Squarcella, A.; Vena, F.; Di Mascio, D.; Corno, S.; Pajno, C.; Piccioni, M.G.; Brunelli, R.; Pizzuti, A.; Benedetti Panici, P.; et al. Update in non-invasive prenatal testing. Minerva Ginecol. 2019, 71, 44–53.
- Hessel, L.; Henn, W. Nichtinvasive Pränataltests—Fragwürdige Parameterauswahl (translation: Non-invasive prenatal testing—Questionable parameter selection). Ärztebl. 2022, 24, 1076–1077.




