1. Catalysis
The carbons synthesized by R.B.N Prasad et al.
[1][2][3][4][5], using the procedure previously described, were tested as a solid-acid catalyst for a diversity of one-pot reactions (described in the following paragraphs) showing very good performance with their activity maintained during several cycles. Yet, no investigation was carried out about leaching into the reaction medium and only one catalyst concentration (10 wt%) was tested.
The first carbon obtained by partial carbonization and sulfonation of glycerol
[1] was tested as a catalyst in the esterification of palmitic acid with methanol at 65 °C. It revealed a high activity (99% conversion in 4 h). The carbon obtained from pitch glycerol using the same experimental procedure was tested as catalyst for tetrahydropyranylation (THP ether synthesis) and dehydropyranylation using a wide variety of alcohols and phenols (17 total)
[2]. The tetrahydropyranylation reactions were performed in dichloromethane at room temperature. The THP ethers were obtained in 80–98% yield, the yield being dependent on the alcohol structure, the lowest yields being observed for phenols due to the lower nucleophilic character of the phenolic oxygen. On the other hand, for the dehydropyranylation reactions, the structure of the substrate had no influence, and reactions yields of 95–99% were obtained using methanol as a solvent. Other reactions were also investigated. For instance, highly substituted imidazole derivatives were obtained from 1,2-diketones, aldehydes, NH
4OAc, and amines
[3]. A study on the reaction temperature and solvents showed the use of acetonitrile at 50–55 °C gives the highest yields (70–84%). The catalyst was also effective for the synthesis of diverse dihydropyrimidinones in refluxing acetonitrile (yields 80–92%)
[5]. The introduction of a halogen onto the aromatic ring yields or replaces urea with thiourea originated the lowest yields. Additionally, the same authors explored the use of the catalysts in the obtention of substituted benzamides (71–78% yield) using different aldehydes and amines as substrates
[4]. Many parameters were optimized, and the best condition was attained using acetonitrile at 60–65 °C and Cs
2CO
3 as a base. Finally, using slightly different conditions, two other reactions were investigated using the same type of catalysts
[6]. First, the acetylation of alcohols, phenols, and aromatic amines using acetic anhydride at 65 °C (no-solvent and 15% catalyst) was investigated. The acetylation reactions of the alcohols and phenols had a 75–96% yield, while for aromatic amines had a 95–97% yield. The yields and reaction time of the acetylation of alcohol and phenol were related to their structure. The primary and secondary alcohols had higher yields and rapid reaction times, while the phenols showed slow reactions. Interesting, selectivity for the acetylation of the amine group was observed, a fact that may be explained by more nucleophilicity of amines than phenols. Secondly, using different aldehydes and 2,2-bis (hydroxymethyl) propane-1,3-diol different pentaerythritol diacetals were obtained
[7]. Among the investigated reaction conditions (catalyst content and reaction temperature), the best results were obtained using 5 wt% catalyst in toluene at 80 °C. Aliphatic aldehydes showed no reactivity. The catalyst showed selectivity towards aromatic aldehydes. Among the aromatic aldehydes, the presence of the electron-donor group showed less reactivity, while the presence of electron-withdrawing groups enhanced the reaction rate. The catalyst was also effective in the deprotection of the diacetals in methanol in reflux within 30 min.
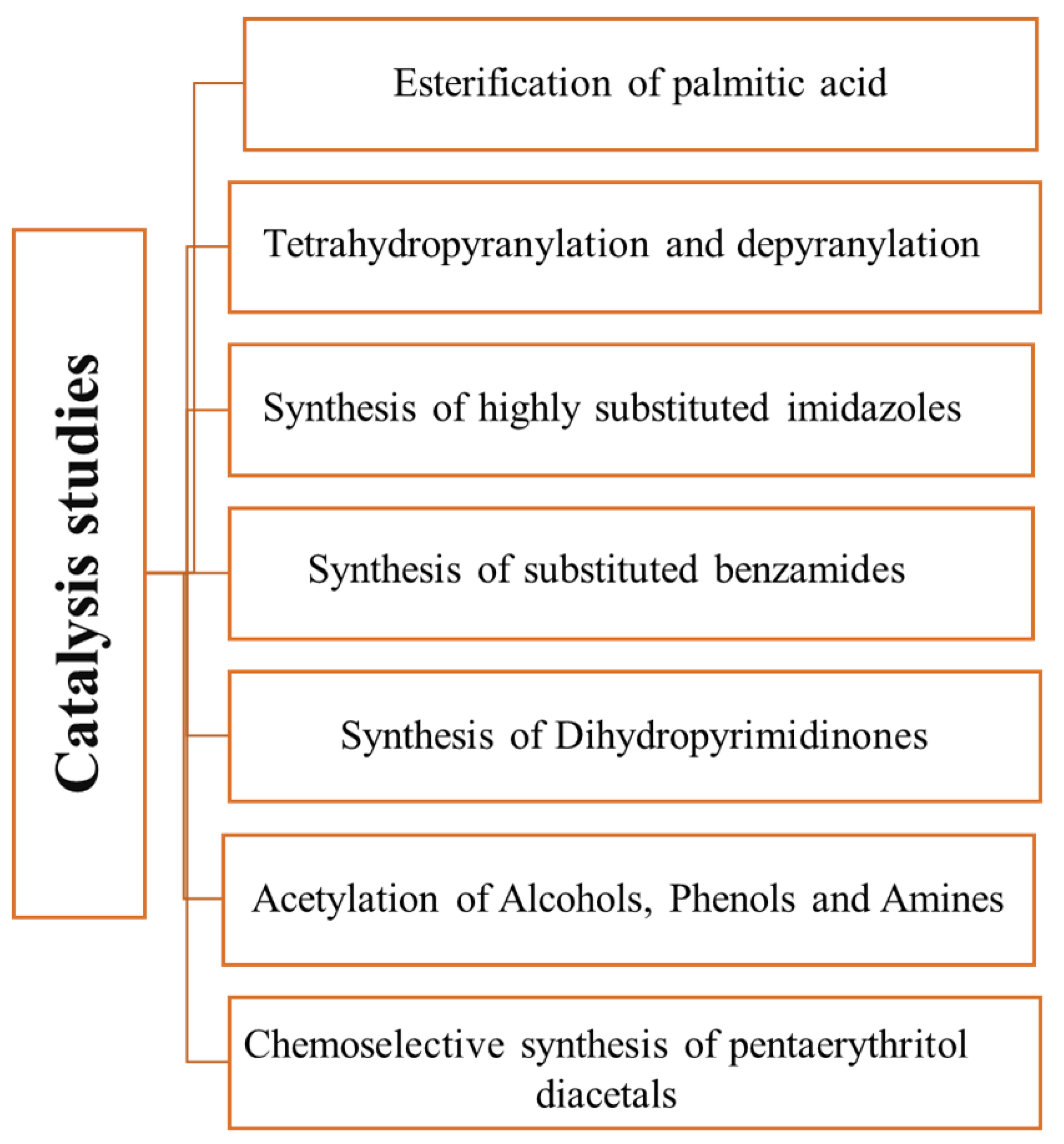
Figure 1 summarizes the studies carried out up to this point.
Figure 1. Reactions studied
[1][2][3][4][5][6][7][8] using carbons obtained from glycerol as catalysts.
The group of Gonçalves et al.
[9][10] used a glycerin-based carbon obtained by hydrothermal carbonization of a mixture of glycerol waste and sulfuric acid as a catalyst in the glycerol acetalization reaction. The acid character attributed to the –SO
3H and –COOH groups at the material surface and the excess of sulfuric acid used during the synthetic procedure affected the final surface chemistry of the material by increasing the surface acidity of these catalysts. The catalyst activity of the carbons through the glycerol acetalization with acetone was dependent on the carbon used as a catalyst. For instance, the 3:1 showed no activity, probably due to the low concentration and absence of –COOH and –SO
3H groups, respectively. The importance of the sulfonic groups on the surface was once more confirmed and was highly related to the high catalytic activity of the carbon. For instance, the 3:1 and 2:1 carbon (the ones with higher sulfonic groups) had an 82% conversion of glycerol with almost complete selectivity for solketal. Further studies, using the 2:1 carbon, were conducted to evaluate the effect of different variables in the catalytic activity. They revealed that an increase in the glycerol: acetone molar ratio provided a relevant increase in the glycerol conversion; additionally, an increase in the glycerol conversion was observed. Increasing the amount of catalyst by more than 3% brought no advantage. Regards the temperature reaction, it was observed that at room temperature, the reaction was slightly slower than at 40 °C and 65 °C. However, the process reaches equilibrium at the same level of conversion independent of temperature. The leaching tests showed no appreciable leaching of any active groups present over the surface of the solids.
The same group used carbons obtained by hydrothermal carbonization of a mixture of glycerol waste and sulfuric acid and post-synthesis modified as catalysts for the glycerol etherification reaction
[11]. The catalytic activity was evaluated through glycerol etherification with tert-butyl alcohol using a 5 wt% catalyst at 120 °C. The 10:1 carbon showed negligible catalytic activity while the 1:3 carbon had a high catalytic activity (better than Amberlyst resin), a fact that may be attributed to the lower concentration of acidic sites on the 10:1 carbon surface, which is essential for etherification reactions. Interestingly, the post-synthesis modification of the 10:1 carbon led to a substantial improvement in the catalytic (similar to the 1:3 carbon). This improvement was attributed to the introduction of sulfonic acid groups but also to other surface functional groups, such as carboxylic acids.
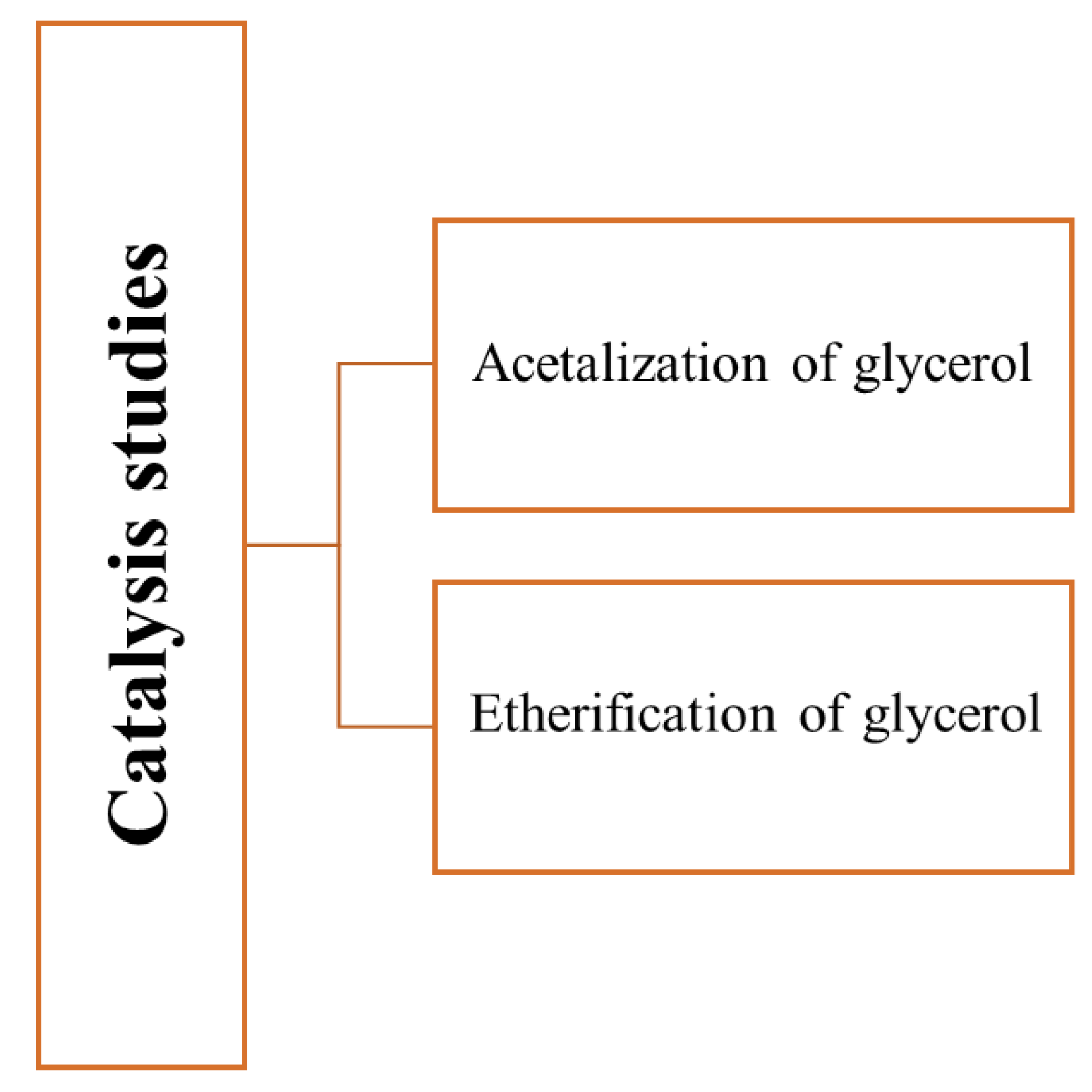
Figure 2 shows the reactions studied by Gonçalves et al.
[9][10][11].
Figure 2. Reactions studied
[9][10][11] using carbons obtained from glycerol as catalysts.
The different reactions in which carbons from glycerol may be used as catalysts are shown in Table 1.
Table 1. Review of the most relevant data for the indicated reactions using glycerol-based non-porous carbon as catalysts.
The previously described work concerns the use of carbons without activation for the use as catalysts. As already referred, the use of activated carbon is not so usual, and only one work exists that effectively used activated carbons as a catalyst
[12]. The catalytic activity of carbons was investigated in the catalytic wet peroxide oxidation (CWPO) of 2-nitrophenol. Adsorption studies revealed that some adsorption on the material surface occurred, yet catalytic activity of the carbons was observed, especially to carbon activated at 300 °C. The characteristics of these materials (developed porosity allied to high basicity and lower oxygen content) seemed to explain their catalytic activity. On the other hand, the higher removal of 2-nitrophenol by the material activated at 350 °C may be explained by a high contribution of the adsorption process. Further studies with the carbon activated at 300 °C revealed its catalytic efficiency was increased when the CWPO process was conducted under intensified conditions (
T = 50 °C, pH = 3, stoichiometric amount of H
2O
2 and a pollutant/catalyst mass ratio = 2). However, the recyclability of the catalyst was studied. The catalyst lost activity after the first cycle due to the adsorption process and the deactivation of the carbon active sites responsible for hydrogen peroxide decomposition, yet its activity may be restored by a simple oxidative thermal regeneration.
2. Adsorption
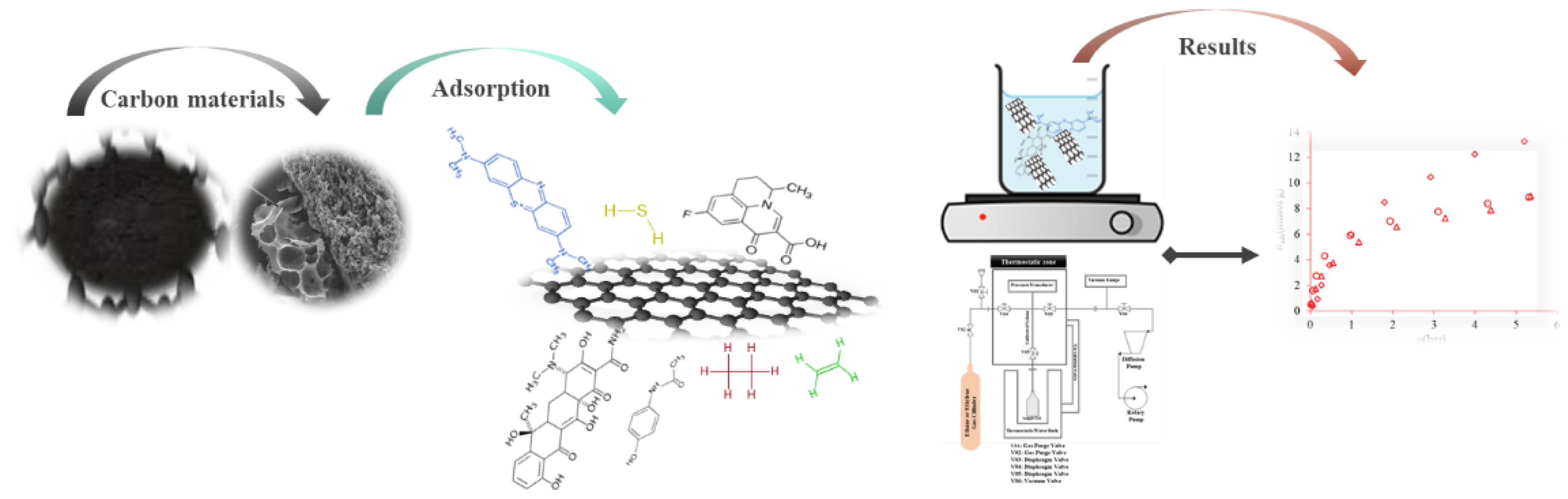
As mentioned, glycerol can be converted into a material that has promising properties for application as adsorbent materials. Their adsorbent capacity was examined to different adsorbates such as medicines (flumequine, tetracycline and paracetamol)
[11][13], aqueous phase chromium Cr(VI), dyes (methylene blue and indigo carmine), VOCs (toluene and hexane), and ethene, ethylene. The adsorption studies (
Figure 3) in aqueous solutions are different from the gas adsorption, which normally requires special equipment based on gravimetric or volumetric methods.
Figure 3. Representation of the adsorption studies.
The adsorption of flumequine was found to be dependent on the textural properties of the glycerol-based activated carbon materials. The maximum adsorption capacity (41.5 mg·g−1) was verified onto sample GBCM350. The sequence of flumequine adsorption capacity in the glycerol-activated carbon series was the following: GBCM350 > GBCM300 > GBCM200 with adsorption capacities of 41.5 mg·g−1, 33.7 mg·g−1 and 0.9 mg·g−1, respectively. For tetracycline this sequence was GBCM350 > GBCM200 > GBCM300 (58.1 mg·g−1, 53.9 mg·g−1 and 51.3 mg·g−1, respectively). The activated carbons showed a higher adsorption capacity for tetracycline and its adsorption was almost the same for all three activated carbons, showing that the adsorption of this antibiotic was not dependent on the structural differences obtained at the different activation temperatures used. Additionally, no relation between the antibiotic structure and activated carbon properties was found.
In 2021, Batista et al.
[14] prepared a series of glycerin-activated carbons from crude glycerin (82% glycerol) for gas separation by adsorption processes. The glycerin-activated carbons were evaluated as adsorbents for the adsorption of ethane and ethylene. All the adsorbents were shown to be ethane selective. The materials exhibited a higher adsorption capacity of ethane (8.92–14.81 mmol·g
−1) than ethylene (8.27–12.63 mmol·g
−1). The glycerin-activated carbons (except for the sample G@700/3) after two regeneration cycles presented ~100% of the adsorption capacity. In addition, in another work, M. Batista et al.
[15] used the glycerin-based activated carbon (Gta@600) and its chitosan-based carbon (Gta@600Chi) as H
2S adsorbents. The chitosan-based carbon (Gta@600Chi) presented a H
2S insignificant release due to its chemical adsorption. However, the Gta@600 adsorbed a significant amount of H
2S and it could be investigated for other applications such as natural gas purification.
The results already available in the literature clearly show activated carbons obtained from glycerol can adsorb compounds with different structures and properties, and therefore indicate the importance to extend the research to the adsorption of other class of chemicals. Most of the studies may have applications in environmental problems. However, as was shown they may also be used for separation processes. Nevertheless, and despite their potential, more studies should be conducted, namely regeneration studies. At this moment no commercial products exist, and their development is dependent on more research to be possible obtain high effective products at low production cost.
3. Capacitors
The ability that activated carbons obtained from glycerol may have as capacitors has been investigated very recently and a brief overview of the work done so far is presented. The first reference to this possibility was in 2019 by Gonçalves et al. and is a small part of the work concerning the adsorption capacity of activated carbon described before
[16]. The authors selected three activated carbons: two with larger surface areas activated differently and one with the higher micropores/mesopores ratio. Electrodes were prepared by pressing a mixture of activated, multiwalled carbon nanotubes. The more suitable activated carbon was obtained from using ZnCl
2 as the activating agent. It presented the higher micropores/mesopores ratio and not the largest surface. This characteristic was attributed to the more suitable pore distribution in this carbon and the higher micropores/mesopores ratio. More recently, Narvekar et al.
[17] synthesized carbon from glycerol which was chemically activated with KOH at 800 °C under N
2 atmosphere for 2 h. Cyclic voltammetry studies showed the activated carbon had a much higher capacitance than the commercial carbons (Vulcan XC-72 or CNT), a fact that was attributed to the carbonyl and sulphonyl surface functionalities and large surface area with favorable pore size distribution wherein the pores are accessible to form an extended electrical double layer. More recently, Juchen et al.
[18] synthesized KOH activated carbon from crude glycerol. The electrodes were prepared by mixing 90 wt% of chemically activated carbon and 10 wt% of Polyvinylidene fluoride (PVDF) in n-methyl-pyrrolidone (NMP) solvent and used for the desalination of brackish water.