Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jeong Hee Hong | -- | 1557 | 2022-12-14 03:01:25 | | | |
| 2 | Catherine Yang | Meta information modification | 1557 | 2022-12-14 03:12:37 | | | | |
| 3 | Catherine Yang | -3 word(s) | 1554 | 2022-12-14 03:13:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lee, D.; Hong, J. PyK2-Associated Molecules in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/38735 (accessed on 07 February 2026).
Lee D, Hong J. PyK2-Associated Molecules in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/38735. Accessed February 07, 2026.
Lee, Dongun, Jeong-Hee Hong. "PyK2-Associated Molecules in Cancer" Encyclopedia, https://encyclopedia.pub/entry/38735 (accessed February 07, 2026).
Lee, D., & Hong, J. (2022, December 14). PyK2-Associated Molecules in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/38735
Lee, Dongun and Jeong-Hee Hong. "PyK2-Associated Molecules in Cancer." Encyclopedia. Web. 14 December, 2022.
Copy Citation
PyK2 is a member of the proline-rich tyrosine kinase and focal adhesion kinase families and is ubiquitously expressed. PyK2 is mainly activated by stimuli, such as activated Src kinases and intracellular acidic pH. The mechanism of PyK2 activation in cancer cells has been addressed extensively. The acidic milieu is a favorable condition in cancer systems. Various evidence has shown that the activation of PyK2 regulated cancer progression and migration. Thus, the mechanism of molecular interaction in regulating PyK2 activity in cancer and PyK2-associated strategies against cancer was summarized.
PyK2
migration
metastasis
acidic milieu
PyK2-interactive proteins
1. Chemical Reagents
Kinase inhibitors, which decrease the phosphorylation of PyK2, suppress cancer viability and migration. Mitoxantrone, which targets the ATP-binding site of FAK and decreases the auto-phosphorylation of FAK, decreased PyK2 kinase activity in BT474 breast carcinoma cells [1]. Moreover, the tyrosine kinase inhibitor, SAR103168, decreased PyK2 phosphorylation by the downstream inhibition of Src in human myeloid leukemia cells (KG1) [2]. SKI-606, which is an Src inhibitor, decreased the phosphorylation of PyK2 and the migration and invasion of MDA-MB-468 breast cancer cells without affecting proliferation, suggesting that PyK2 induced the migration of breast cancer cells by activating Src [3]. The reactive oxygen species (ROS) inducer eicosapentaenoic acid (EPA), which dephosphorylates PyK2, exhibited anti-cancer effects by decreasing the proliferation and migration of PC3 prostate cancer cells [4]. PyK2 regulation ameliorated drug resistance to cisplatin and doxorubicin. The overexpression of PyK2 increased the effect of cisplatin in human hepatocellular carcinoma cells to decrease proliferation [5]. Alpha-naphthoflavone (ANF) decreased the phosphorylation of PyK2 in MCF-7 cells, and the combination of doxorubicin and ANF reduced breast cancer volume compared with a single treatment of doxorubicin or ANF in breast cancer-xenografted mice [6].
2. Interaction of Protein with PyK2 in Cancers
PyK2 interacts with various proteins, and its interactions with PyK2 have been developed in cancer systems. For example, the Csk homologous kinase (CHK), which inhibits the activation of Src family kinases, physically binds to PyK2 in T47D breast cancer cells [7]. A deficiency of heat shock cognate protein 70 (hsc70), which promotes the proliferation and migration of human glioma cells (U251 and U87), attenuated the phosphorylation of Src, FAK, and PyK2 [8]. Rb1-inducible coiled-coil 1 (RB1CC1) is a tumor suppressor that is considered to be a therapeutic target in renal carcinoma [9]. The overexpression of RB1CC1 decreased the phosphorylation of PyK2 and doxorubicin, which increased RB1CC1 expression and reduced the size of xenografted renal cell carcinoma tumors [10]. A decrease in PyK2 phosphorylation decreased cancer progression, and cancer migration and invasion were affected by PyK2 and its interactive proteins. Melatonin exerted an anti-cancer effect on brain tumor cells [11], and treatment with melatonin reduced the phosphorylation of PyK2 and the expression of alpha V beta 3 (αVβ3) integrin in U251 glioma cells [12]. The knockdown of αVβ3 decreased PyK2 phosphorylation and the migration of U251 cells [12].
3. Chemokine-Related Molecules
PyK2 is regulated by chemokine-related proteins, including the C-C motif chemokine ligand/receptor (CCL/CCR) and C-X-C motif chemokine ligand/receptor (CXCL/CXCR). CCL and CXCL recruit monocytes and neutrophils to the tumor site [13][14][15]. Thus CCL- and CXCL-related immune pathways have a close connection with cancer therapy. For example, CCL2 and CCL5, which are secreted by mesenchymal stem cells, induced PyK2-dependent chemoresistance in ovarian cancer cells (Skov3 and Ovcar3) [16]. CCL2- and CCL5-mediated chemoresistance was decreased through treatment with the PyK2 inhibitor PF-431396 [16]. PyK2 also plays a role in tumor viability and reactions with CCL2 and CCL5. ADP-ribosylation factor-GTPase activating protein (Arf-GAP), with an SH3 domain, ankyrin repeat, and PH domain-containing protein 1 (ASAP1, also called DDEF1 or AMAP1), is highly expressed on breast cancer cells and mediates breast cancer invasion and metastasis [17]. Treatment with CCL18 increased ASAP1 phosphorylation, and the knockdown of PyK2 prevented CCL18-induced increases in p-ASAP1 in MCF-7 cells [18]. p-ASAP1 trans-locates toward the plasma membrane to form a complex with PyK2 in the presence of CCL18 [18]. Treatment with CCL18 stimulated cellular adhesion, migration, and invasion, whereas the inhibition of ASAP1 through siRNA attenuated CCL18-induced cellular mobility features in MCF-7 cells [18]. CCR7 also plays a role in cancer migration and invasion. CCR7, which binds with CCL19, stimulated the phosphorylation of Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) in head and neck squamous cell carcinoma cell lines (PCI-4B and PCI-37B) [19]. The phosphorylation of JAK2 and STAT3 was attenuated by the PyK2 inhibitor A9 in PCI-4B and PCI-37B cells [19]. The inhibition of JAK2 and STAT3 decreased the migration and invasion of PCI-4B and PCI-37B cells [19], and treatment with CXCL12 induced the chemotaxis and chemoinvasion of MDA-MB-231 cells [20]. CXCL12, which binds with CXCR4, induced PyK2 phosphorylation in breast cancer cells (MDA-MB-231) [20]. The tyrosine phosphatase inhibitors vanadate and phenylarsine oxide attenuated the chemotaxis and chemo-invasion of MDA-MB-231 cells [20]. Although accumulating evidence has been reported, further verification of multiple chemokine/PyK2-associated mechanisms will provide potential strategies for treating cancer.
4. Ca2+ Channels and Transporters
PyK2 phosphorylation is also modulated by the signaling messenger, intracellular Ca2+. PyK2 senses Ca2+ signaling through calmodulin (CaM), and PyK2 has a CaM-binding motif [21]. In hypoxia, increases in the intracellular Ca2+ concentration ([Ca2+]i) induced PyK2 phosphorylation [22]. Treatment with the Ca2+ chelator BAPTA attenuated hydrogen peroxide (H2O2)-stimulated PyK2 phosphorylation [23]. Ca2+ signaling plays important roles in muscle contraction, neurotransmitter release, immune cell differentiation, fluid secretion, and cell proliferation [24][25][26][27]. Cancer progression and cancer cell death are especially affected by Ca2+ signaling [28][29][30][31][32][33][34]. In addition, the activation of Ca2+ channels and transporters regulates the interaction between PyK2 and cancer activity. [Ca2+]i is increased by the activation of various Ca2+ channels and transporters that are located on intracellular organelle and plasma membranes. Intracellular Ca2+ is stored in intracellular organelles, including the nucleus, mitochondria, and endoplasmic reticulum (ER), to maintain Ca2+ homeostasis. The mitochondrial protein Lon is involved in protein quality control and maintains mitochondrial homeostasis [35][36]. The overexpression of Lon induced the phosphorylation of PyK2, increased [Ca2+]i through the involvement of a mitochondrial Na2+/Ca2+ exchanger, and enhanced chemoresistance to cisplatin in human oral squamous carcinoma cells (OEC-M1) [37].
The ER, another intracellular Ca2+ store, contains a Ca2+ sensor protein called stromal interaction molecule 1 (STIM1) [38]. This Ca2+ sensor STIM1 recognizes depletions in ER Ca2+ by a STIM1-Orai1 complex on plasma membranes and mediates increases in [Ca2+]i in a process called store-operated Ca2+ (SOC) entry (SOCE) [38]. The down-regulation of STIM1 decreased the EGF-induced phosphorylation of PyK2 and enhanced the focal adhesion of cervical cancer cells (SiHa) [39]. The knockdown of STIM1 inhibited tumor progression in a cervical cancer mouse model [39]. Additionally, the inhibition of SOCE by the SOCE inhibitors shOrai1 and SKF96365 increased PyK2 dephosphorylation and focal adhesion in mouse glioma cells (C6), human glioma cells (U251 and SNB19), and human melanoma cells (WM793) [40][41][42]. Transient receptor potential melastatin 2 (TRPM2), which is located on plasma membranes, inhibited the effect of the anti-cancer drug doxorubicin in neuroblastoma [43]. The knockdown of TRPM2 enhanced the anti-cancer effects of doxorubicin to decrease PyK2 phosphorylation. Hirschler-Laszkiewicz et al. suggested the inhibition of TRPM2 as a target for cancer therapy in patients with doxorubicin chemoresistance [43]. Although the effect of modulating TRPM2 channels must be carefully verified because of conflicting views of TRPM2 (Ca2+ influx through TRPM2 induces apoptosis through goldnano-conjugated doxorubicin) [44], enhanced PyK2 phosphorylation through Ca2+ signaling presents further challenges in verifying the precise mechanism for cancer therapy.
5. Reactive Oxygen Species
In cancer cells, oxidative modification has pathological roles in protein alterations through the involvement of second messengers, including ROS, H2O2, reactive nitrogen species (RNS), and nitric oxide (NO) [45][46][47]. Oxidative stress has been considered a hallmark of cancer to increase cancer progression, including proliferation and invasion [48][49]. Oxidative stress also affects PyK2 activation in cancer cells. Treatment with estrogen produced ROS, and increased PyK2 phosphorylation in human breast cancer cells, including MCF-7, T47D, ZR75-1, and MDA-MB-468 cells [50]. Hypoxic conditions increased the phosphorylation of PyK2 in U251 glioma cells [12]. The migration and invasion of U251 cells were increased by hypoxic stimulation, and the knockdown of PyK2 inhibited hypoxia-induced U251 cell migration [12].
PyK2 was reported to bind with dihydronicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) in KySE30 and KySE410 esophageal squamous cell carcinoma (ESCC) [51]. Hypoxia induced the phosphorylation of PyK2 and the production of H2O2 in ESCC [51]. NOX5 shRNA and PyK2 mutation decreased H2O2 levels in ESCC cells under hypoxic conditions and decreased ESCC proliferation [51]. Oxidation also plays a critical role in cardiovascular functions and CTLs [23][52][53][54][55]. Treatment with H2O2 enhanced the phosphorylation of PyK2 in mouse left ventricular myocytes [23] and H9c2 cardiomyocytes [53]. The deletion of PyK2 attenuated the production of NO in primary cultured-mouse endothelial cells from the aorta [52]. Additionally, treatment with H2O2 stimulated PyK2 phosphorylation, and the activation of PyK2 phosphorylation increased the production of ROS in CTLs [54]. Overall, oxidative stress induces PyK2 phosphorylation with tumor progression. Thus, the development of antioxidants and modulation of PyK2 phosphorylation provide potential strategies for cancer treatment. The mechanism of the various molecules involved in regulating PyK2 activity in cancer systems is shown in Figure 1.
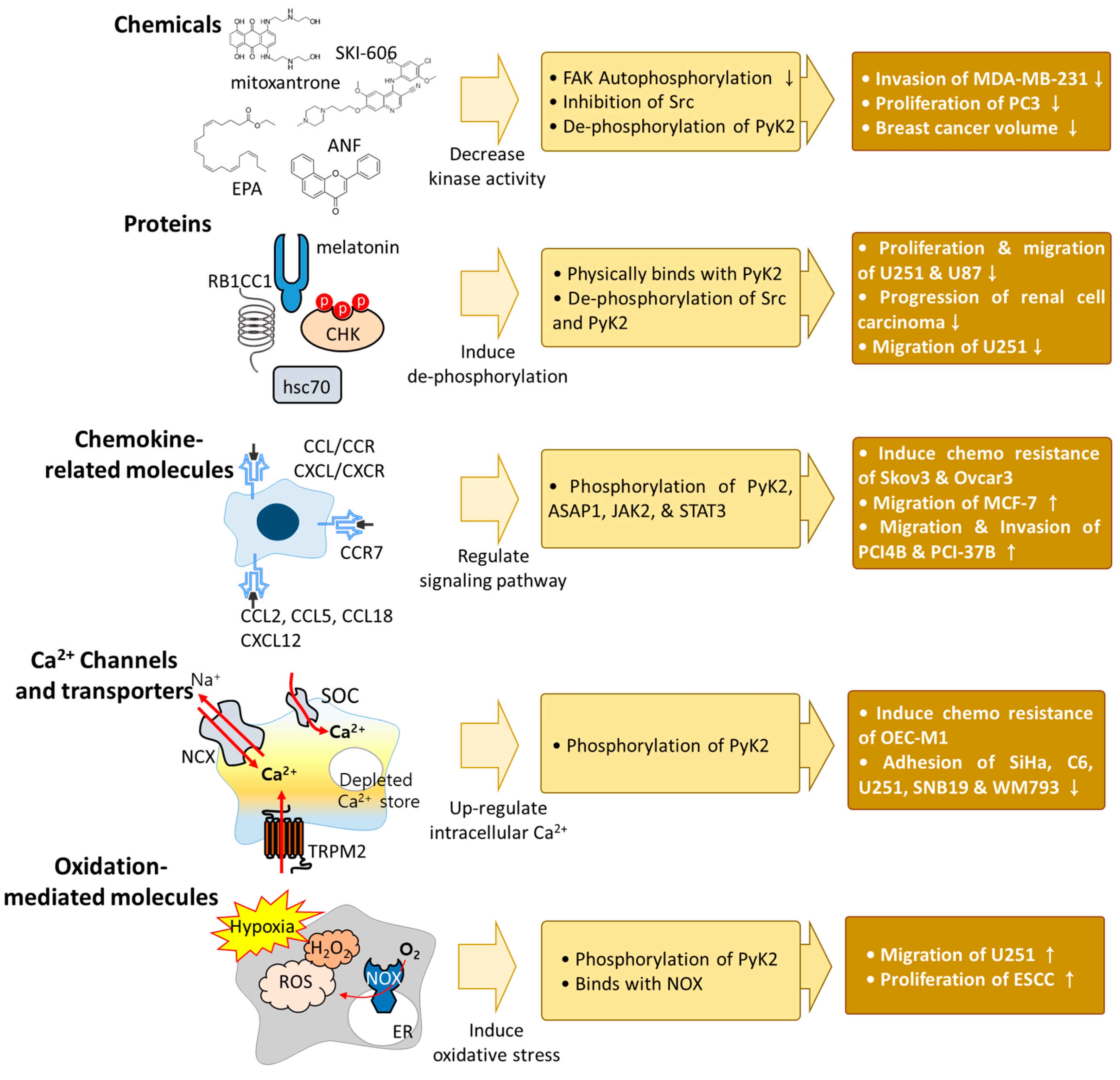
Figure 1. Schematic illustration of PyK2-associated molecules. Various molecules affect PyK2 activation, including chemical reagents, interactive proteins, chemokine-related molecules, Ca2+ channels, transporters, and oxidation-mediated molecules. The phosphorylation of PyK2 induces cancer cell migration and proliferation. Various effector signals and chemicals exert different phosphorylation effects on PyK2. Thus, verification of the phosphorylation status of PyK2 could be a prognostic marker for evaluating cancer progression.
References
- Golubovskaya, V.M.; Ho, B.; Zheng, M.; Magis, A.; Ostrov, D.; Cance, W.G. Mitoxantrone targets the ATP-binding site of FAK, binds the FAK kinase domain and decreases FAK, Pyk-2, c-Src, and IGF-1R in vitro kinase activities. Anticancer Agents Med. Chem. 2013, 13, 546–554.
- Bourrie, B.; Brassard, D.L.; Cosnier-Pucheu, S.; Zilberstein, A.; Yu, K.; Levit, M.; Morrison, J.G.; Perreaut, P.; Jegham, S.; Hilairet, S.; et al. SAR103168: A tyrosine kinase inhibitor with therapeutic potential in myeloid leukemias. Leuk. Lymphoma 2013, 54, 1488–1499.
- Vultur, A.; Buettner, R.; Kowolik, C.; Liang, W.; Smith, D.; Boschelli, F.; Jove, R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol. Cancer Ther. 2008, 7, 1185–1194.
- Oono, K.; Ohtake, K.; Watanabe, C.; Shiba, S.; Sekiya, T.; Kasono, K. Contribution of Pyk2 pathway and reactive oxygen species (ROS) to the anti-cancer effects of eicosapentaenoic acid (EPA) in PC3 prostate cancer cells. Lipids Health Dis. 2020, 19, 15.
- Geng, W.; Ng, K.T.; Sun, C.K.; Yau, W.L.; Liu, X.B.; Cheng, Q.; Poon, R.T.; Lo, C.M.; Man, K.; Fan, S.T. The role of proline rich tyrosine kinase 2 (Pyk2) on cisplatin resistance in hepatocellular carcinoma. PLoS ONE 2011, 6, e27362.
- Datta, A.; Bhasin, N.; Kim, H.; Ranjan, M.; Rider, B.; Abd Elmageed, Z.Y.; Mondal, D.; Agrawal, K.C.; Abdel-Mageed, A.B. Selective targeting of FAK-Pyk2 axis by alpha-naphthoflavone abrogates doxorubicin resistance in breast cancer cells. Cancer Lett. 2015, 362, 25–35.
- McShan, G.D.; Zagozdzon, R.; Park, S.Y.; Zrihan-Licht, S.; Fu, Y.; Avraham, S.; Avraham, H. Csk homologous kinase associates with RAFTK/Pyk2 in breast cancer cells and negatively regulates its activation and breast cancer cell migration. Int. J. Oncol. 2002, 21, 197–205.
- Sun, G.; Cao, Y.; Xu, Y.; Huai, D.; Chen, P.; Guo, J.; Li, M.; Dai, Y. Overexpression of Hsc70 promotes proliferation, migration, and invasion of human glioma cells. J. Cell. Biochem. 2019, 120, 10707–10714.
- Lebovitz, C.B.; Robertson, A.G.; Goya, R.; Jones, S.J.; Morin, R.D.; Marra, M.A.; Gorski, S.M. Cross-cancer profiling of molecular alterations within the human autophagy interaction network. Autophagy 2015, 11, 1668–1687.
- Chen, P.F.; Duan, Y.J.; Lu, X.S.; Chen, L.B.; Zhang, W.; Wang, H.; Hu, R.; Liu, S.M. RB1CC1 functions as a tumor-suppressing gene in renal cell carcinoma via suppression of PYK2 activity and disruption of TAZ-mediated PDL1 transcription activation. Cancer Immunol. Immun. 2021, 70, 3261–3275.
- Martin, V.; Sanchez-Sanchez, A.M.; Herrera, F.; Gomez-Manzano, C.; Fueyo, J.; Alvarez-Vega, M.A.; Antolin, I.; Rodriguez, C. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br. J. Cancer 2013, 108, 2005–2012.
- Xu, C.S.; Wang, Z.F.; Huang, X.D.; Dai, L.M.; Cao, C.J.; Li, Z.Q. Involvement of ROS-alpha v beta 3 integrin-FAK/Pyk2 in the inhibitory effect of melatonin on U251 glioma cell migration and invasion under hypoxia. J. Transl. Med. 2015, 13, 95.
- Balkwill, F.R. The chemokine system and cancer. J. Pathol. 2012, 226, 148–157.
- Caronni, N.; Savino, B.; Bonecchi, R. Myeloid cells in cancer-related inflammation. Immunobiology 2015, 220, 249–253.
- Bonavita, O.; Massara, M.; Bonecchi, R. Chemokine regulation of neutrophil function in tumors. Cytokine Growth Factor Rev. 2016, 30, 81–86.
- Pasquier, J.; Gosset, M.; Geyl, C.; Hoarau-Vechot, J.; Chevrot, A.; Pocard, M.; Mirshahi, M.; Lis, R.; Rafii, A.; Touboul, C. CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer. Mol. Cancer 2018, 17, 47.
- Hashimoto, S.; Hirose, M.; Hashimoto, A.; Morishige, M.; Yamada, A.; Hosaka, H.; Akagi, K.; Ogawa, E.; Oneyama, C.; Agatsuma, T.; et al. Targeting AMAP1 and cortactin binding bearing an atypical src homology 3/proline interface for prevention of breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA 2006, 103, 7036–7041.
- Li, H.Y.; Zhang, D.W.; Yu, J.D.; Liu, H.L.; Chen, Z.P.; Zhong, H.F.; Wan, Y.L. CCL18-dependent translocation of AMAP1 is critical for epithelial to mesenchymal transition in breast cancer. J. Cell. Physiol. 2018, 233, 3207–3217.
- Liu, F.Y.; Safdar, J.; Li, Z.N.; Fang, Q.G.; Zhang, X.; Xu, Z.F.; Sun, C.F. CCR7 Regulates Cell Migration and Invasion through JAK2/STAT3 in Metastatic Squamous Cell Carcinoma of the Head and Neck. Biomed. Res. Int. 2014, 2014, 415375.
- Fernandis, A.Z.; Prasad, A.; Band, H.; Klosel, R.; Ganju, R.K. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene 2004, 23, 157–167.
- Momin, A.A.; Mendes, T.; Barthe, P.; Faure, C.; Hong, S.; Yu, P.A.; Kadare, G.; Jaremko, M.; Girault, J.A.; Jaremko, L.; et al. PYK2 senses calcium through a disordered dimerization and calmodulin-binding element. Commun. Biol. 2022, 5, 800.
- Beitner-Johnson, D.; Ferguson, T.; Rust, R.T.; Kobayashi, S.; Millhorn, D.E. Calcium-dependent activation of Pyk2 by hypoxia. Cell. Signal. 2002, 14, 133–137.
- Miller, B.A.; Wang, J.; Song, J.; Zhang, X.Q.; Hirschler-Laszkiewicz, I.; Shanmughapriya, S.; Tomar, D.; Rajan, S.; Feldman, A.M.; Madesh, M.; et al. Trpm2 enhances physiological bioenergetics and protects against pathological oxidative cardiac injury: Role of Pyk2 phosphorylation. J. Cell. Physiol. 2019, 234, 15048–15060.
- Szent-Gyorgyi, A.G. Calcium regulation of muscle contraction. Biophys. J. 1975, 15, 707–723.
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814.
- Vig, M.; Kinet, J.P. Calcium signaling in immune cells. Nat. Immunol. 2009, 10, 21–27.
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058.
- Roberts-Thomson, S.J.; Chalmers, S.B.; Monteith, G.R. The Calcium-Signaling Toolkit in Cancer: Remodeling and Targeting. Cold Spring Harb. Perspect. Biol. 2019, 11, a035204.
- Tennakoon, S.; Aggarwal, A.; Kallay, E. The calcium-sensing receptor and the hallmarks of cancer. Biochim. Biophys. Acta 2016, 1863, 1398–1407.
- Bong, A.H.L.; Monteith, G.R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1786–1794.
- Varghese, E.; Samuel, S.M.; Sadiq, Z.; Kubatka, P.; Liskova, A.; Benacka, J.; Pazinka, P.; Kruzliak, P.; Busselberg, D. Anti-Cancer Agents in Proliferation and Cell Death: The Calcium Connection. Int. J. Mol. Sci. 2019, 20, 3017.
- Santoni, G.; Morelli, M.B.; Marinelli, O.; Nabissi, M.; Santoni, M.; Amantini, C. Calcium Signaling and the Regulation of Chemosensitivity in Cancer Cells: Role of the Transient Receptor Potential Channels. Adv. Exp. Med. Biol. 2020, 1131, 505–517.
- Romero-Garcia, S.; Prado-Garcia, H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review). Int. J. Oncol. 2019, 54, 1155–1167.
- Mundy, G.R. Calcium and cancer. Life Sci. 1978, 23, 1735–1744.
- Pinti, M.; Gibellini, L.; Nasi, M.; De Biasi, S.; Bortolotti, C.A.; Iannone, A.; Cossarizza, A. Emerging role of Lon protease as a master regulator of mitochondrial functions. BBA-Bioenergetics 2016, 1857, 1300–1306.
- Venkatesh, S.; Lee, J.; Singh, K.; Lee, I.; Suzuki, C.K. Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim. Biophys. Acta 2012, 1823, 56–66.
- Tangeda, V.; Lo, Y.K.; Babuharisankar, A.P.; Chou, H.Y.; Kuo, C.L.; Kao, Y.H.; Lee, A.Y.; Chang, J.Y. Lon upregulation contributes to cisplatin resistance by triggering NCLX-mediated mitochondrial Ca2+ release in cancer cells. Cell Death Dis. 2022, 13, 241.
- Zhang, S.Y.L.; Yu, Y.; Roos, J.; Kozak, J.A.; Deerinck, T.J.; Ellisman, M.H.; Stauderman, K.A.; Cahalan, M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005, 437, 902–905.
- Chen, Y.F.; Chiu, W.T.; Chen, Y.T.; Lin, P.Y.; Huang, H.J.; Chou, C.Y.; Chang, H.C.; Tang, M.J.; Shen, M.R. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15225–15230.
- Zhu, M.; Lv, B.; Ge, W.; Cui, Z.; Zhao, K.; Feng, Y.; Yang, X. Suppression of store-operated Ca2+ entry regulated by silencing Orai1 inhibits C6 glioma cell motility via decreasing Pyk2 activity and promoting focal adhesion. Cell Cycle 2020, 19, 3468–3479.
- Zhu, M.; Chen, L.; Zhao, P.F.; Zhou, H.; Zhang, C.; Yu, S.P.; Lin, Y.; Yang, X.J. Store-operated Ca2+ entry regulates glioma cell migration and invasion via modulation of Pyk2 phosphorylation. J. Exp. Clin. Cancer Res. 2014, 33, 98.
- Lu, F.; Sun, J.; Zheng, Q.; Li, J.; Hu, Y.; Yu, P.; He, H.; Zhao, Y.; Wang, X.; Yang, S.; et al. Imaging elemental events of store-operated Ca2+ entry in invading cancer cells with plasmalemmal targeted sensors. J. Cell Sci. 2019, 132, jcs224923.
- Hirschler-Laszkiewicz, I.; Chen, S.J.; Bao, L.; Wang, J.F.; Zhang, X.Q.; Shanmughapriya, S.; Keefer, K.; Madesh, M.; Cheung, J.Y.; Miller, B.A. The human ion channel TRPM2 modulates neuroblastoma cell survival and mitochondrial function through Pyk2, CREB, and MCU activation. Am. J. Physiol.-Cell Physiol. 2018, 315, C571–C586.
- Lee, D.U.; Park, J.Y.; Kwon, S.; Park, J.Y.; Kim, Y.H.; Khang, D.; Hong, J.H. Apoptotic lysosomal proton sponge effect in tumor tissue by cationic gold nanorods. Nanoscale 2019, 11, 19980–19993.
- Kang, S.W.; Lee, S.; Lee, E.K. ROS and energy metabolism in cancer cells: Alliance for fast growth. Arch. Pharm. Res. 2015, 38, 338–345.
- Miller, T.W.; Isenberg, J.S.; Roberts, D.D. Molecular regulation of tumor angiogenesis and perfusion via redox signaling. Chem. Rev. 2009, 109, 3099–3124.
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868.
- Fiaschi, T.; Chiarugi, P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: A diabolic liaison. Int. J. Cell Biol. 2012, 2012, 762825.
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616.
- Felty, Q.; Xiong, W.C.; Sun, D.M.; Sarkar, S.; Singh, K.P.; Parkash, J.; Roy, D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry 2005, 44, 6900–6909.
- Chen, J.; Wang, Y.; Zhang, W.; Zhao, D.; Zhang, L.; Fan, J.; Li, J.; Zhan, Q. Membranous NOX5-derived ROS oxidizes and activates local Src to promote malignancy of tumor cells. Signal Transduct. Target. Ther. 2020, 5, 139.
- Matsui, A.; Okigaki, M.; Amano, K.; Adachi, Y.; Jin, D.; Takai, S.; Yamashita, T.; Kawashima, S.; Kurihara, T.; Miyazaki, M.; et al. Central role of calcium-dependent tyrosine kinase PYK2 in endothelial nitric oxide synthase-mediated angiogenic response and vascular function. Circulation 2007, 116, 1041–1051.
- Bibli, S.I.; Szabo, C.; Chatzianastasiou, A.; Luck, B.; Zukunft, S.; Fleming, I.; Papapetropoulos, A. Hydrogen Sulfide Preserves Endothelial Nitric Oxide Synthase Function by Inhibiting Proline-Rich Kinase 2: Implications for Cardiomyocyte Survival and Cardioprotection. Mol. Pharmacol. 2017, 92, 718–730.
- Lysechko, T.L.; Cheung, S.M.S.; Ostergaard, H.L. Regulation of the Tyrosine Kinase Pyk2 by Calcium Is through Production of Reactive Oxygen Species in Cytotoxic T Lymphocytes. J. Biol. Chem. 2010, 285, 31174–31184.
- Martel-Gallegos, G.; Casas-Pruneda, G.; Ortega-Ortega, F.; Sanchez-Armass, S.; Olivares-Reyes, J.A.; Diebold, B.; Perez-Cornejo, P.; Arreola, J. Oxidative stress induced by P2X7 receptor stimulation in murine macrophages is mediated by c-Src/Pyk2 and ERK1/2. BBA-Gen. Subj. 2013, 1830, 4650–4659.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
908
Revisions:
3 times
(View History)
Update Date:
14 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




