Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Khairunisak Abdul Razak | -- | 2630 | 2022-12-13 04:07:57 | | | |
| 2 | Sirius Huang | Meta information modification | 2630 | 2022-12-14 02:12:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nor, N.M.; Ridhuan, N.S.; Razak, K.A. Generations of Glucose Biosensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/38722 (accessed on 07 March 2026).
Nor NM, Ridhuan NS, Razak KA. Generations of Glucose Biosensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/38722. Accessed March 07, 2026.
Nor, Noorhashimah Mohamad, Nur Syafinaz Ridhuan, Khairunisak Abdul Razak. "Generations of Glucose Biosensors" Encyclopedia, https://encyclopedia.pub/entry/38722 (accessed March 07, 2026).
Nor, N.M., Ridhuan, N.S., & Razak, K.A. (2022, December 14). Generations of Glucose Biosensors. In Encyclopedia. https://encyclopedia.pub/entry/38722
Nor, Noorhashimah Mohamad, et al. "Generations of Glucose Biosensors." Encyclopedia. Web. 14 December, 2022.
Copy Citation
Electrochemical glucose biosensors are widely applied for glucose monitoring due to their unbeatable sensitivity, selectivity, and simplicity. In general, there are four primary generations of glucose biosensor, which are classified according to the electron transfer mechanism. Three generations represent the enzymatic glucose biosensor, and one generation represents the non-enzymatic glucose biosensor.
glucose detection
electrochemical sensor
enzyme immobilization
glucose oxidation
metal and metal oxide nanomaterials
1. Introduction
Diabetes mellitus is a chronic disease that occurs when the pancreas fails to produce sufficient insulin to regulate blood sugar or when the body is unable to use the insulin produced effectively. If diabetes is not well treated, a number of other health problems may follow, such as eye complications, neuropathy, foot complications, kidney disease, hypertension, stroke, hyperglycemic nonketonic syndrome, gastroparesis, heart disease, and mental health disorders; it may also affect pregnancy [1][2]. Tight control of diabetes is critical to prevent or slow down the progress of diabetes complications. A normal blood glucose level in human serum before a meal is around 4–6 mM (70–110 mg/dL) and <7.8 mM (<140 mg/dL) after 2 h of mealtime [3][4]. In diabetic patients, the normal glucose concentration in serum is between 5.6 and 6.9 mM (100–125 mg/dL) before mealtime and 7.8 and 11 mM (140–199 mg/dL) after 2 h of mealtime [5]. For efficient therapy and to prevent any hyperglycemia or hypoglycemia, regular monitoring of physiological blood glucose levels is essential.
Clark and Lyons [6] were the first researchers who reported on glucose quantification by employing a dialysis membrane on the oxygen electrode surface based on glucose oxidase (GOx) entrapment via potentiometric measurements. The glucose concentration was analyzed based on the reduction of dissolved oxygen [2]. Since then, research on glucose biosensors has been conducted employing conductometric, impedimetric, potentiometric, and amperometric techniques based on the GOx enzyme, which catalyzes the oxidation of glucose into gluconic acid [7][8][9][10].
At present, the common method of blood glucose monitoring is invasive, which involves finger pricking, collecting a drop of blood on top of the glucose test strip, and analyzing the results by using a glucose meter. The blood sample undergoes an enzymatic chemical reaction at the test strip, followed by electrochemical detection with a glucose meter. Several commercial glucose test strips are available in the market for analysis of blood glucose levels, but these strips have varied performance. Normally, commercial glucose test strips have a glucose detection linearity of 0.5–33 mM and require 0.3–2 µL of blood and 4–5 s of assay time. The commercial glucose strip is able to maintain its stability at 4 °C for 35 to 50 days in an open vial container and 18–24 month in a sealed container. The failure time of a commercial glucose strip is determined as the concentration value at the time of measurement out of range by ±15% from the initial concentration value.
Nowadays, extensive efforts toward the non-invasive technique, which allows wearable [11][12], continuous [13][14], and point-of-care [15][16] blood glucose monitoring have drawn interest among researchers and users. Additionally, the applicability of different types of biofluids such as sweat, tear, urine, saliva, and interstitial fluid to replace blood in monitoring glucose levels is being considered [17][18][19]. Rapid and sensitive glucose biosensors are important not only for clinical chemistry, but also for food and industrial quality analysis [20][21][22]. Therefore, the fabrication of glucose biosensors to enhance sensitivity, accuracy, response time, reliability, long lifetime stability, and cost-effectiveness are important.
Electrochemical sensing strategies are versatile and powerful tools in providing real-time and on-site measurement in a variety of areas, including clinical diagnostic, environmental, agricultural, and food monitoring [23][24][25][26]. The electrochemical sensing provides advantages in offering high sensitivity, selectivity, accuracy, and cost effectiveness. Therefore, biosensors with electrochemical monitoring systems dominate the commercial glucometer market. Electrochemical glucose biosensors are widely applied for glucose monitoring due to their unbeatable sensitivity, selectivity, and simplicity. Electrochemical glucose biosensors can be further classified based on the output signal measuring techniques, namely, amperometric (measures the electrical current produced due to a redox reaction), potentiometric (measures the change in electrode potential), and conductometric (measures the change in charge transfer resistance). Amperometric glucose sensors are the most commonly employed glucose biosensors. Electrochemical glucose biosensors commonly comprise a three-electrode system: working, reference, and counter electrodes. Each type of electrode has a specific function. The working electrode is a sensor or transducer responding to the electrochemical reaction. The reference electrode is a steady and well-known electrode potential that is often based on a saturated calomel electrode (SCE) or silver-silver chloride Ag/AgCl electrode. The counter electrode completes the current circuit by providing a current connection in between the electrocatalytic solutions and the working electrode in electrochemical cell. The counter electrode is usually made of an inert material, such as platinum (Pt), gold (Au), graphite, or glassy carbon [27][28]. Among these three electrodes, the sensitivity and specificity of glucose detection are dependent on the working electrode.
Glucose biosensors are classified into two types: enzymatic and non-enzymatic. The enzymatic glucose biosensor is commonly employed because immobilized GOx enzyme provides excellent specificity and sensitivity to the glucose biosensor [29]. The immobilization of the GOx enzyme on the working electrode surface is an important factor to be considered in biosensor fabrication. The deep position of the active redox center of the GOx enzyme makes the electron exchange between GOx enzymes and the electrode surface difficult. The shape of GOx enzymes may shift after immobilization on the surface of the working electrode [30][31]. Another challenge is to prevent GOx enzyme denaturalization and deactivation, which ultimately reduces the lifetime of the biosensor. Therefore, the immobilization of GOx enzymes on the suitable matrix is crucial to maintain the catalytic properties and stability of the enzyme bioactivity.
The recent development in glucose biosensors involves modifying the working electrode with nanomaterials, such as metals, metal oxides, and carbon-based nanomaterials, as schematically shown in Figure 1 [32][33][34]. Nanomaterials serve as a matrix to modify the electrode surface and provide a biocompatible area for enzyme immobilization because nanomaterials have a large surface area for reaction activity, good catalytic efficiency, and strong adsorption ability [32][35]. The dependency of enzyme activity on temperature, pH, humidity, and toxic compounds has advanced research on non-enzymatic glucose biosensors [35][36][37]. Non-enzymatic glucose biosensors have excellent sensitivity, good stability, and ease of manufacture, and their current response is directly dependent on the oxidation of glucose on the modified electrode. The main restriction of non-enzymatic glucose biosensors is specificity. Recently, scholars reported high-sensitivity non-enzymatic glucose biosensors based on the modification of electrodes with metal [38][39], metal oxide [17][40], and composite nanomaterials [19][21][41].
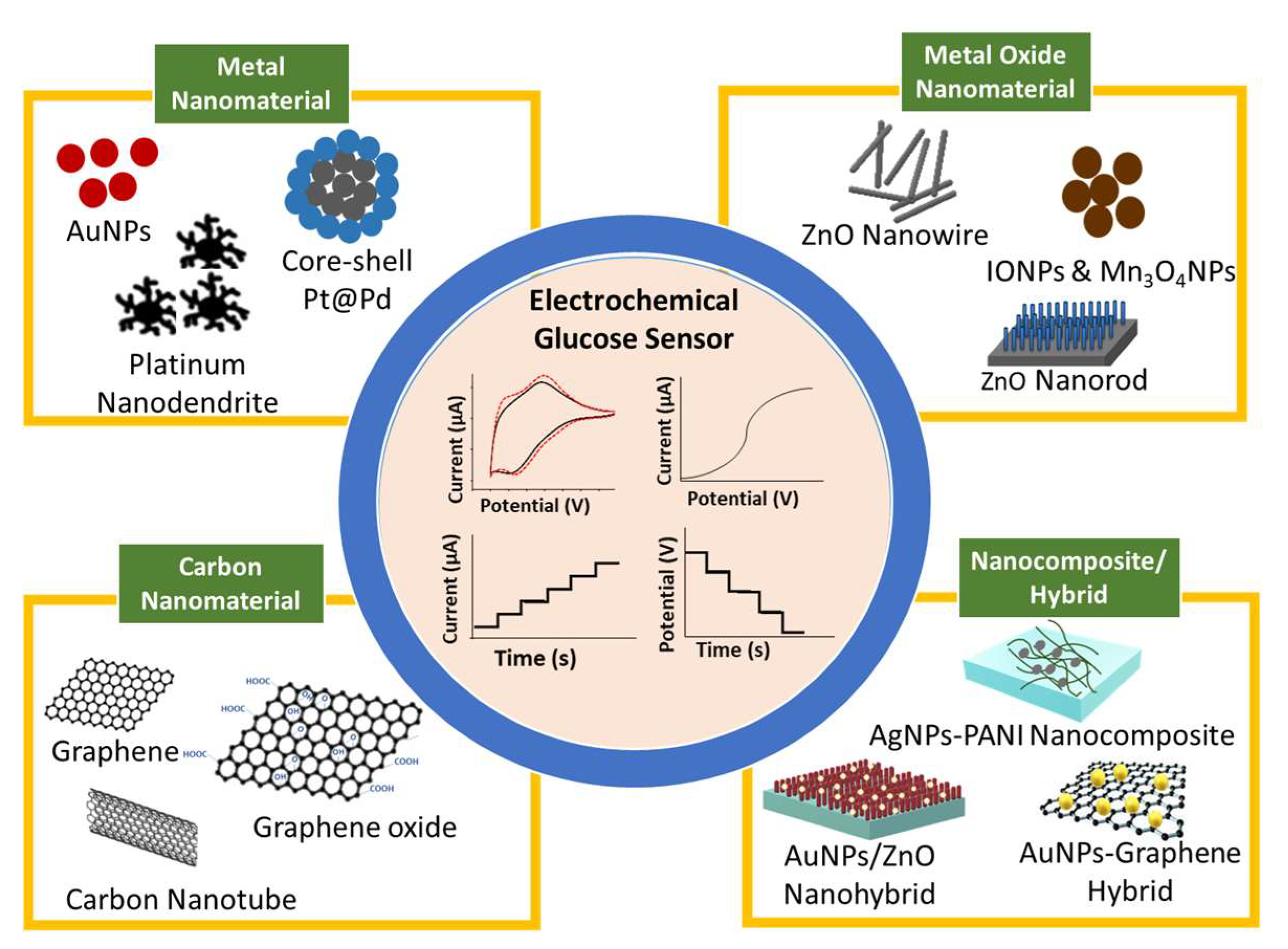
Figure 1. Schematic of nanomaterial-modified electrode for glucose biosensor.
2. Generations of Glucose Biosensor
In general, there are four primary generations of glucose biosensor, which are classified according to the electron transfer mechanism. Three generations represent the enzymatic glucose biosensor, and one generation represents the non-enzymatic glucose biosensor (Figure 2). The first-generation enzymatic glucose biosensors measure glucose concentration in the analyte sample based on H2O2 generation or by reduction in oxygen (O2) concentration as a natural co-substrate [42]. The immobilized GOx uses molecular O2 as an electron acceptor to catalyze the oxidation of D-glucose (C6H10O6) into gluconolactone (C6H12O6), yielding H2O2 and water as byproducts. As gluconolactone (C6H10O6) hydrolyzes further, gluconic acid (C6H12O7) is created [1][43]. As a catalyst, FAD, which is an active redox center of GOx, plays a role as the initial electron acceptor and is reduced to FADH2 in the presence of glucose. The re-oxidation of FADH2 with free oxygen generates the oxidized form of the enzyme FAD. In general, the glucose concentration is relative to electrochemical oxidation of the product H2O2 or electrochemical reduction of O2 at the working electrode [44]. The electrons that are transferred are recognized and collected by the counter electrode; thus, the number of glucose molecules present is directly proportional to electron flow [42]. Table 1 lists the advantages and disadvantages of all generations of glucose biosensors.
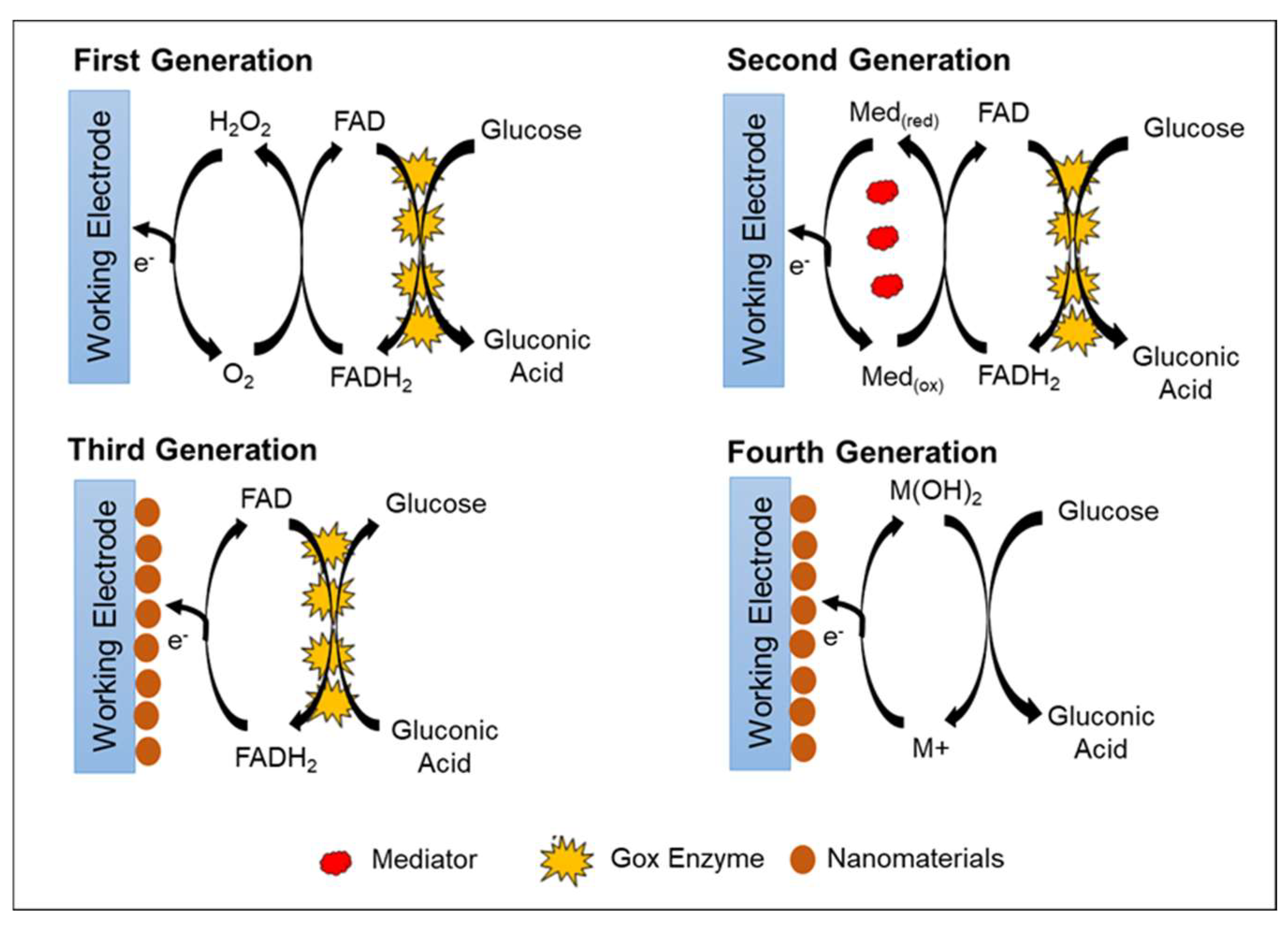
Figure 2. Schematic representation of four generations of glucose biosensors.
The advantages of the first-generation glucose biosensor are its simple design and miniaturization of the biosensor [45][46]. However, the first-generation glucose biosensor has limitations in terms of high operation potential needed for the amperometric measurement of H2O2. This high operation potential may interfere with other electroactive molecules (such as ascorbic acid and uric acids) and some drugs (e.g., acetaminophen) [42]. Another disadvantage is that oxygen deficiency may occur due to the limited oxygen solubility in biological fluids, which causes fluctuations in oxygen tension [47]. The oxygen deficiency then affects the sensor response, narrowing the linearity of the glucose concentration detection ranges.
A variety of techniques have been developed to address the limitations of the first-generation enzymatic glucose biosensor, which are interference from electroactive molecules and oxygen deficiency. Nafion, polyurethane, polycarbonate, or acetate layers were added on the surface of electrode as a selective or protective membrane to minimize the interference toward the electrode and provide mechanical stability to GOx enzyme against denaturalization [48][49]. Electrodes were further modified by co-deposition with metallized materials such as ruthenium and rhodium to lower the operating potentials to approximately 0–0.2 V, which is optimal for preventing electroactivity interference [47][50]. Another approach is to employ oxygen-rich carbon paste enzyme electrodes, which have become an internal source of oxygen due to high oxygen solubility [47].
The second-generation enzymatic glucose biosensor is based on artificial redox mediators in replacing the oxygen-dependent electrode. Mediators are tiny, low-molecular-weight, soluble redox components that act as artificial electron transfer agents. The mediators facilitate electron transport from the FAD active redox center of the enzyme to the working electrode surface [45]. This feature decreases the operational potential of the biosensors at moderate redox potentials, allowing them to avoid the oxidation of other interfering species [51]. Various types of electron mediators that are effective for GOx include ferrocene derivatives, ferricyanide, quinone compounds, conducting polymer salt tetrathiafulvalene-tetracyanoquinodimethane (TTF-TCNQ), transition metal complexes, and phenothiazine [52][53].
During glucose conversion, the electrons produced are collected by the mediator, and the mediator will be reduced to M(red). The mediator releases electrons and transfers the electrons to the electrode at the applied oxidation potential of the mediator. The reduction of the mediator helps facilitate the re-oxidation of the reduced form of GOx (FADH2) to GOx (FAD). Further oxidation of the mediator at the electrode surface regenerates M(ox) and two electrons. Thus, the glucose concentration level is proportional to the number of electrons transferred to the counter electrode. With the help of the mediator, measurement of the glucose concentration becomes independent of oxygen partial pressure and can be conducted at a lower potential to minimize interference from electroactive species [54][55].
The weakness of using natural or artificial mediators in glucose biosensor applications is the difficulty to maintain the presence of the mediator near the electrode and enzyme surface [56]. Mediators are small and highly diffusive, so they require additional and complicated methods to secure them near the electrode [57]. Although mediators can react rapidly with the enzyme compared with oxygen, there is also a possibility of dissolved oxygen competing with the mediator, thereby reducing the efficiency of the system and causing a build-up of H2O2. Another possibility is the reaction between mediator and interference species in the blood, which reduces the accuracy and efficiency of the analytical system [42].
The common oxidation potential of GOx active site (FAD) is around −0.45 V or −0.34 V versus Ag/AgCl [58]. Thus, a suitable mediator applied should have redox potential that is more positive than FAD [59]. Among all electron mediators, ferrocene and its derivatives are commonly applied in the fabrication of electrochemical glucose biosensors. Ferrocene and its derivatives are of interest due to their properties of a wide range of redox potentials, pH independence, rapid electron transfer rate, and high stability in both conditions (oxidized and reduced forms) [45][60].
The problems with mediators, such as poor electron transport, mediator leakage, and poor stability, can be overcome by incorporating polymers and their derivative to the mediator. Polymers improve the mediator biocompatibility, stability, and electrical conductivity, and provide a large surface area. Dendrimers, conducting polymers (polypyrrole and polythiophene), carbon nanotubes (CNTs), chitosan, polyelectrolyte, and polyethylenimine are commonly used [61][62][63]. Jiang et al. [63] reported the use of a ferrocene-modified polyelectrolyte film-coated electrode for amperometric glucose biosensors. The ferrocene group present on the polyelectrolyte skeleton structure acts as a mediator to shuttle electron transfer between FAD-active redox center of GOx enzyme to the working electrode. The modified electrode shows good linearity for glucose detection in the range of 0.2–5 mM.
In third-generation enzymatic glucose biosensors, direct electron transfer between the enzyme and electrode is introduced without the need for natural or synthetic mediators. The FAD-active redox center of the enzyme is covalently or electrochemically linked to the working electrode by nanomaterials. Nanomaterials act as a matrix to enable GOx to be immobilized directly in proximity and facilitate direct electron transfer. Thus, the obtained electrochemical signal is correlated with the glucose concentration [64].
In recent years, efforts to achieve direct electron transfer using various types and sizes of nanomaterials and nanocomposite have been extensively explored due to their excellent physical, chemical, and electronic properties [32][33][65]. Different nanomaterials display various main functions in improving glucose biosensor performance based on their unique properties. However, the basic functions of nanomaterials in biosensors are aiding biomolecule immobilization and labelling, catalysis of electrochemical reactions, increasing the electron transfer rate, and acting as the reactant [32]. The most commonly used nanomaterials are metal [66][67][68], carbon-based [69][70], and metal oxide [71][72][73].
During biosensor fabrication, nanomaterial-modified electrodes have a great potential to adsorb biomolecules and serve as an immobilization support for biomolecules. The direct adsorption of biomolecules onto bulk materials frequently results in denaturation and loss of bioactivity, whereas nanoparticles preserve biomolecule bioactivity [74]. Nanomaterials provide a microenvironment similar to the redox protein in the native system, thereby allowing more freedom for biomolecules to immobilize [75]. Several nanomaterials carry charges via functionalization, providing an electrostatic surface to attach the biomolecules with different charges [74]. The incorporation of suitable surface functional groups on nanoparticles can produce a strong binding of biomolecules with nanoparticles. The high-conductivity properties of nanoparticles allow them to function as a signal-generating probe and a signal amplifier [43]. The third generation of enzymatic glucose biosensors has numerous advantages, including high selectivity and sensitivity toward glucose rather than interfering species such as ascorbic acid and uric acid, a rapid response time, and a low operating potential [76]. Some limitations of the third-generation enzymatic glucose biosensors are enzyme leaching and a good conductivity of nanomaterials to enhance direct electron transfer between the deeply buried FAD-active redox center of the enzyme and the working electrode.
Finally, the fourth generation of glucose biosensors, also known as non-enzymatic glucose biosensors, employs direct electron transfer through electro-oxidation of glucose to gluconic acid at the nanomaterial matrix with strong electrocatalytic activity [77]. In the non-enzymatic glucose biosensor, atoms from nanomaterials act as electrocatalyst in the glucose reaction [78]. Recently, many studies focused on the non-enzymatic glucose biosensor, which employs various types of nanomaterials and nanocomposite materials in the modification of the electrode. However, several issues limit the application of fourth-generation glucose biosensors for commercial use in monitoring patients with diabetes, such as poor selectivity and the requirement for alkaline condition during analysis. Indeed, with a broader understanding of the mechanisms of catalytic properties of nanomaterials, the 3D enzyme mimicking glucose biosensor can be developed.
Table 1. The advantages and disadvantages of all generations of glucose biosensor.
| Types of Glucose Sensor | Advantages | Disadvantages | Reference |
|---|---|---|---|
| First Generation (Enzymatic) |
|
|
[79] |
| Second Generation (Enzymatic) |
|
|
[63] |
| Third Generation (Enzymatic) |
|
|
[76] |
| Fourth Generation (Non-Enzymatic) |
|
|
[35] |
References
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866.
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196.
- Güemes, M.; Rahman, S.A.; Hussain, K. What is a normal blood glucose? Arch. Dis. Child. 2016, 101, 569.
- National Institute for Health and Clinical Excellence (NICE). Type 2 Diabetes: Prevention in People at High Risk (NICE Public Health Guideline 38); National Institute for Health and Clinical Excellence, Ed.; National Institute for Health and Care Excellence (NICE): London, UK, 2012; pp. 1–42.
- American Diabetes, A. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69.
- Clark, L.J.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29.
- Baghayeri, M.; Veisi, H.; Ghanei-Motlagh, M. Amperometric glucose biosensor based on immobilization of glucose oxidase on a magnetic glassy carbon electrode modified with a novel magnetic nanocomposite. Sens. Actuator B Chem. 2017, 249, 321–330.
- Khun, K.; Ibupoto, Z.H.; Lu, J.; AlSalhi, M.S.; Atif, M.; Ansari, A.A.; Willander, M. Potentiometric glucose sensor based on the glucose oxidase immobilized iron ferrite magnetic particle/chitosan composite modified gold coated glass electrode. Sens. Actuator B Chem. 2012, 173, 698–703.
- Nouira, W.; Maaref, A.; Elaissari, H.; Vocanson, F.; Siadat, M.; Jaffrezic-Renault, N. Comparative study of conductometric glucose biosensor based on gold and on magnetic nanoparticles. Mater. Sci. Eng. C 2013, 33, 298–303.
- Xu, S.; Qi, H.; Zhou, S.; Zhang, X.; Zhang, C. Mediatorless amperometric bienzyme glucose biosensor based on horseradish peroxidase and glucose oxidase cross-linked to multiwall carbon nanotubes. Microchim. Acta 2014, 181, 535–541.
- Cheng, Y.; Gong, X.; Yang, J.; Zheng, G.; Zheng, Y.; Li, Y.; Xu, Y.; Nie, G.; Xie, X.; Chen, M.; et al. A touch-actuated glucose sensor fully integrated with microneedle array and reverse iontophoresis for diabetes monitoring. Biosens. Bioelectron. 2022, 203, 114026.
- Chen, H.; Mei, Z.; Qi, K.; Wang, Y.; Chen, R. A wearable enzyme-free glucose sensor based on nickel nanoparticles decorated laser-induced graphene. J. Electroanal. Chem. 2022, 920, 116585.
- Liu, N.; Xiang, X.; Sun, M.; Li, P.; Qin, H.; Liu, H.; Zhou, Y.; Wang, L.; Wu, L.; Zhu, J. Flexible hydrogel non-enzymatic QCM sensor for continuous glucose monitoring. Biosens. Bioelectron. X 2022, 10, 100110.
- Scholten, K.; Meng, E. A review of implantable biosensors for closed-loop glucose control and other drug delivery applications. Int. J. Pharm. 2018, 544, 319–334.
- Fedalto, L.; de Oliveira, P.R.; Agustini, D.; Kalinke, C.; Banks, C.E.; Bergamini, M.F.; Marcolino-Junior, L.H. Novel and highly stable strategy for the development of microfluidic enzymatic assays based on the immobilization of horseradish peroxidase (HRP) into cotton threads. Talanta 2023, 252, 123889.
- Lee, J.; Ji, J.; Hyun, K.; Lee, H.; Kwon, Y. Flexible, disposable, and portable self-powered glucose biosensors visible to the naked eye. Sens. Actuator B Chem. 2022, 372, 132647.
- Yang, J.; Chen, H.; Zhu, C.; Huang, Z.; Ou, R.; Gao, S.; Yang, Z. A miniature CuO nanoarray sensor for noninvasive detection of trace salivary glucose. Anal. Biochem. 2022, 656, 114857.
- Liu, Y.; Zhong, L.; Zhang, S.; Wang, J.; Liu, Z. An ultrasensitive and wearable photoelectrochemical sensor for unbiased and accurate monitoring of sweat glucose. Sens. Actuator B Chem. 2022, 354, 131204.
- Eslami, R.; Azizi, N.; Ghaffarian, S.R.; Mehrvar, M.; Zarrin, H. Highly sensitive and selective non-enzymatic measurement of glucose using arraying of two separate sweat sensors at physiological pH. Electrochim. Acta 2022, 404, 139749.
- Zhang, X.; Zhao, J.; Wang, C.; Zhu, L.; Pan, X.; Liu, Y.; Li, J.; Guo, X.; Chen, D. Measurement of sucrose in beverages using a blood glucose meter with cascade-catalysis enzyme particle. Food Chem. 2023, 398, 133951.
- Wang, H.; Zhu, W.; Xu, T.; Zhang, Y.; Tian, Y.; Liu, X.; Wang, J.; Ma, M. An integrated nanoflower-like MoS2@CuCo2O4 heterostructure for boosting electrochemical glucose sensing in beverage. Food Chem. 2022, 396, 133630.
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126.
- Goud, K.Y.; Reddy, K.K.; Khorshed, A.; Kumar, V.S.; Mishra, R.K.; Oraby, M.; Ibrahim, A.H.; Kim, H.; Gobi, K.V. Electrochemical diagnostics of infectious viral diseases: Trends and challenges. Biosens. Bioelectron. 2021, 180, 113112.
- Mohamad Nor, N.; Ramli, N.H.; Poobalan, H.; Qi Tan, K.; Abdul Razak, K. Recent Advancement in Disposable Electrode Modified with Nanomaterials for Electrochemical Heavy Metal Sensors. Crit. Rev. Anal. Chem. 2021, 1–36.
- Umapathi, R.; Ghoreishian, S.M.; Rani, G.M.; Cho, Y.; Huh, Y.S. Review—Emerging Trends in the Development of Electrochemical Devices for the On-Site Detection of Food Contaminants. ECS Sens. Plus 2022, 1, 044601.
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord. Chem. Rev. 2022, 453, 214305.
- Grieshaber, D.; MacKenzie, R.; Voros, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400.
- Shruthi, G.; Amitha, C.; Mathew, B.B. Biosensors: A Modern Day Achievement. J. Instrum. Tech. 2014, 2, 26–39.
- Witkowska Nery, E.; Kundys, M.; Jeleń, P.S.; Jönsson-Niedziółka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282.
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501.
- Peng, H.-P.; Liang, R.-P.; Zhang, L.; Qiu, J.-D. Facile preparation of novel core–shell enzyme–Au–polydopamine–Fe3O4 magnetic bionanoparticles for glucosesensor. Biosens. Bioelectron. 2013, 42, 293–299.
- Batool, R.; Rhouati, A.; Nawaz, M.H.; Hayat, A.; Marty, J.L. A Review of the Construction of Nano-Hybrids for Electrochemical Biosensing of Glucose. Biosensors 2019, 9, 46.
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165.
- Wang, S.-S.; Qiu, W.-J.; Wang, T.-P.; Lee, C.-L. Tuning structures of Pt shells on Pd nanocubes as neutral glucose oxidation catalysts and sensors. Appl. Surf. Sci. 2022, 605, 154670.
- Naikoo, G.A.; Salim, H.; Hassan, I.U.; Awan, T.; Arshad, F.; Pedram, M.Z.; Ahmed, W.; Qurashi, A. Recent Advances in Non-Enzymatic Glucose Sensors Based on Metal and Metal Oxide Nanostructures for Diabetes Management—A Review. Front. Chem. 2021, 9, 748957.
- Alexander, S.; Baraneedharan, P.; Balasubrahmanyan, S.; Ramaprabhu, S. Highly sensitive and selective non enzymatic electrochemical glucose sensors based on Graphene Oxide-Molecular Imprinted Polymer. Mater. Sci. Eng. C 2017, 78, 124–129.
- Dhara, K.; Thiagarajan, R.; Nair, B.G.; Thekkedath, G.S.B. Highly sensitive and wide-range nonenzymatic disposable glucose sensor based on a screen printed carbon electrode modified with reduced graphene oxide and Pd-CuO nanoparticles. Microchim. Acta 2015, 182, 2183–2192.
- He, C.; Wang, J.; Gao, N.; He, H.; Zou, K.; Ma, M.; Zhou, Y.; Cai, Z.; Chang, G.; He, Y. A gold electrode modified with a gold-graphene oxide nanocomposite for non-enzymatic sensing of glucose at near-neutral pH values. Microchim. Acta 2019, 186, 722.
- De Lima, L.F.; de Freitas, A.d.S.M.; Ferreira, A.L.; Maciel, C.C.; Ferreira, M.; de Araujo, W.R. Enzymeless glucose sensor based on disposable Ecoflex®/graphite thermoplastic composite substrate modified with Sens. Actuators Rep. 2022, 4, 100102.
- Zhang, J.; Guan, P.; Li, Y.; Li, W.; Guo, Q. Polyaniline/Cerium Oxide Hybrid Modified Carbon Paste Electrode for Non-Enzymatic Glucose Detection. Bull. Korean Chem. Soc. 2016, 37, 985–986.
- Sun, F.; Wang, X.; You, Z.; Xia, H.; Wang, S.; Jia, C.; Zhou, Y.; Zhang, J. Sandwich structure confined gold as highly sensitive and stable electrochemical non-enzymatic glucose sensor with low oxidation potential. J. Mater. Sci. Technol. 2022, 123, 113–122.
- Yoo, E.-H.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576.
- Taguchi, M.; Ptitsyn, A.; McLamore, E.S.; Claussen, J.C. Nanomaterial-mediated Biosensors for Monitoring Glucose. J. Diabetes Sci. Technol. 2014, 8, 403–411.
- Vaddiraju, S.; Burgess, D.J.; Tomazos, I.; Jain, F.C.; Papadimitrakopoulos, F. Technologies for Continuous Glucose Monitoring: Current Problems and Future Promises. J. Diabetes Sci. Technol. 2010, 4, 1540–1562.
- Harper, A.; Anderson, M.R. Electrochemical Glucose Sensors—Developments Using Electrostatic Assembly and Carbon Nanotubes for Biosensor Construction. Sensors 2010, 10, 8248.
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.H.; Ahn, S.J.; Lee, J.J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors 2010, 10, 4855–4886.
- Wang, J.; Lu, F. Oxygen-Rich Oxidase Enzyme Electrodes for Operation in Oxygen-Free Solutions. J. Am. Chem. Soc. 1998, 120, 1048–1050.
- Kulkarni, T.; Slaughter, G. Application of Semipermeable Membranes in Glucose Biosensing. Membranes 2016, 6, 55.
- Murphy, L.J. Reduction of Interference Response at a Hydrogen Peroxide Detecting Electrode Using Electropolymerized Films of Substituted Naphthalenes. Anal. Chem. 1998, 70, 2928–2935.
- Yang, S.; Atanasov, P.; Wilkins, E. A glucose biosensor based on an oxygen electrode: In-vitro performances in model buffer solution and in blood plasma. Biomed. Instrum. Technol. 1996, 30, 55–61.
- Wang, J.; Liu, J.; Chen, L.; Lu, F. Highly Selective Membrane-Free, Mediator-Free Glucose Biosensor. Anal. Chem. 1994, 66, 3600–3603.
- Nagarale, R.K.; Lee, J.M.; Shin, W. Electrochemical properties of ferrocene modified polysiloxane/chitosan nanocomposite and its application to glucose sensor. Electrochim. Acta 2009, 54, 6508–6514.
- Dzyadevich, S.V.; Arkhipova, V.N.; Soldatkin, A.P.; El’skaya, A.V.; Shul’ga, A.A. Glucose conductometric biosensor with potassium hexacyanoferrate(III) as an oxidizing agent. Anal. Chim. Acta 1998, 374, 11–18.
- Chaubey, A.; Malhotra, B.D. Mediated biosensors. Biosens. Bioelectron. 2002, 17, 441–456.
- Saleem, M.; Yu, H.; Wang, L.; Zain ul, A.; Khalid, H.; Akram, M.; Abbasi, N.M.; Huang, J. Review on synthesis of ferrocene-based redox polymers and derivatives and their application in glucose sensing. Anal. Chim. Acta 2015, 876, 9–25.
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301.
- Dominguez-Benetton, X.; Srikanth, S.; Satyawali, Y.; Vanbroekhoven, K.; Pant, D. Enzymatic Electrosynthesis: An Overview on the Progress in Enzyme-Electrodes for the Production of Electricity, Fuels and Chemicals. J. Microb. Biochem. Technol. 2013, S6, 1–20.
- Godet, C.; Boujtita, M.; El Murr, N. Direct electron transfer involving a large protein: Glucose oxidase. New J. Chem. 1999, 23, 795–797.
- Conghaile, P.; Kamireddy, S.; MacAodha, D.; Kavanagh, P.; Leech, D. Mediated glucose enzyme electrodes by cross-linking films of osmium redox complexes and glucose oxidase on electrodes. Anal. Bioanal. Chem. 2013, 405, 3807–3812.
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505.
- Åženel, M.; Nergiz, C.; Çevik, E. Novel reagentless glucose biosensor based on ferrocene cored asymmetric PAMAM dendrimers. Sens. Actuator B. Chem. 2013, 176, 299–306.
- Åženel, M. Construction of reagentless glucose biosensor based on ferrocene conjugated polypyrrole. Synth. Met. 2011, 161, 1861–1868.
- Jiang, Z.; Shangguan, Y.; Zheng, Q. Ferrocene-Modified Polyelectrolyte Film-Coated Electrode and Its Application in Glucose Detection. Polymers 2019, 11, 551.
- Wang, Z.; Liu, S.; Wu, P.; Cai, C. Detection of Glucose Based on Direct Electron Transfer Reaction of Glucose Oxidase Immobilized on Highly Ordered Polyaniline Nanotubes. Anal. Chem. 2009, 81, 1638–1645.
- Chen, J.; Zheng, X.; Li, Y.; Zheng, H.; Liu, Y.; Suye, S.-i. A Glucose Biosensor Based on Direct Electron Transfer of Glucose Oxidase on PEDOT Modified Microelectrode. J. Electrochem. Soc. 2020, 167, 067502.
- Sapountzi, E.; Braiek, M.; Vocanson, F.; Chateaux, J.-F.O.; Jaffrezic-Renault, N.; Lagarde, F. Gold nanoparticles assembly on electrospun poly(vinyl alcohol)/poly(ethyleneimine)/glucose oxidase nanofibers for ultrasensitive electrochemical glucose biosensing. Sens. Actuator B. Chem. 2017, 238, 392–401.
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Donnelly, R.F.; De Wael, K. Wearable hollow microneedle sensing patches for the transdermal electrochemical monitoring of glucose. Talanta 2022, 249, 123695.
- Dudkaitė, V.; Bagdžiūnas, G. Functionalization of Glucose Oxidase in Organic Solvent: Towards Direct Electrical Communication across Enzyme-Electrode Interface. Biosensors 2022, 12, 335.
- Shukla, S.K.; Deshpande, S.R.; Shukla, S.K.; Tiwari, A. Fabrication of a tunable glucose biosensor based on zinc oxide/chitosan-graft-poly(vinyl alcohol) core-shell nanocomposite. Talanta 2012, 99, 283–287.
- Sadak, O. One-pot scalable synthesis of rGO/AuNPs nanocomposite and its application in enzymatic glucose biosensor. Nanocomposites 2021, 7, 44–52.
- Kafi, A.K.M.; Bin Kasri, A.; Jose, R. Glucose Biosensor Based on Glucose Oxidase-Horseradish Peroxidase/Multiporous Tin Oxide (SnO2) Modified Electrode. J. Nanosci. Nanotechnol. 2021, 21, 3059–3064.
- Myndrul, V.; Coy, E.; Babayevska, N.; Zahorodna, V.; Balitskyi, V.; Baginskiy, I.; Gogotsi, O.; Bechelany, M.; Giardi, M.T.; Iatsunskyi, I. MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor. Biosens. Bioelectron. 2022, 207, 114141.
- Zhou, F.; Jing, W.; Liu, S.; Mao, Q.; Xu, Y.; Han, F.; Wei, Z.; Jiang, Z. Electrodeposition of gold nanoparticles on ZnO nanorods for improved performance of enzymatic glucose sensors. Mater. Sci. Semicond. 2020, 105, 104708.
- Liébana, S.; Drago, G.A. Bioconjugation and stabilisation of biomolecules in biosensors. Essays Biochem. 2016, 60, 59–68.
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021.
- Bollella, P.; Gorton, L.; Ludwig, R.; Antiochia, R. A Third Generation Glucose Biosensor Based on Cellobiose Dehydrogenase Immobilized on a Glassy Carbon Electrode Decorated with Electrodeposited Gold Nanoparticles: Characterization and Application in Human Saliva. Sensors 2017, 17, 1912.
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 2013, 3, 3487–3502.
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118.
- Putzbach, W.; Ronkainen, N. Immobilization Techniques in the Fabrication of Nanomaterial-Based Electrochemical Biosensors: A Review. Sensors 2013, 13, 4811.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
13.1K
Revisions:
2 times
(View History)
Update Date:
14 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





