Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lee Fah Yap | -- | 2074 | 2022-12-13 08:59:37 | | | |
| 2 | Camila Xu | -40 word(s) | 2034 | 2022-12-15 09:58:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yap, L.F.; Wong, A.K.C.; Paterson, I.C.; Young, L.S. Epstein-Barr Virus Lytic Genes in Carcinogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/38687 (accessed on 07 February 2026).
Yap LF, Wong AKC, Paterson IC, Young LS. Epstein-Barr Virus Lytic Genes in Carcinogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/38687. Accessed February 07, 2026.
Yap, Lee Fah, Anna Kang Chee Wong, Ian C. Paterson, Lawrence S. Young. "Epstein-Barr Virus Lytic Genes in Carcinogenesis" Encyclopedia, https://encyclopedia.pub/entry/38687 (accessed February 07, 2026).
Yap, L.F., Wong, A.K.C., Paterson, I.C., & Young, L.S. (2022, December 13). Epstein-Barr Virus Lytic Genes in Carcinogenesis. In Encyclopedia. https://encyclopedia.pub/entry/38687
Yap, Lee Fah, et al. "Epstein-Barr Virus Lytic Genes in Carcinogenesis." Encyclopedia. Web. 13 December, 2022.
Copy Citation
Epstein-Barr virus (EBV) was the first human tumor virus to be discovered and is a causative agent for several cancer types of epithelial and lymphoid origin. EBV has two life cycles comprised of latent and lytic phases. The lytic cycle is when new virions are produced, whereas the latent cycle is a state of persistent infection without productive viral replication. It has been recognized that latent infection is the predominant mode of infection in EBV-associated cancers and the expression of a restricted set of latent genes drives disease development.
EBV
lytic genes
oncogenesis
gene expression
1. Introduction
EBV is the most prevalent virus infection in humans with approximately 95% of the world’s population sustaining an asymptomatic life-long persistent infection. The discovery that EBV is the causative agent of infectious mononucleosis (IM) represented a breakthrough in our understanding of primary EBV infection in healthy individuals [1]. A wealth of data demonstrates that EBV is transmitted through saliva with subsequent infection of naïve B cells in the naso/oropharynx. EBV induces proliferation of these B cells into activated B blasts that escape primary T-cell responses and undergo a germinal centre reaction driven through a series of viral latency programs. This culminates in EBV residing in the resting memory B cell pool, thereby establishing a persistent latent infection [2][3]. The EBV lytic cycle is switched on when EBV-infected memory B cells differentiate into plasma cells or when the virus enters differentiating epithelial cells, and this results in the production of new virions for transmission to other hosts [4].
Disruption of this intimate host-viral interaction can lead to malignant transformation. Unlike the pathogenic process in EBV-induced lymphomagenesis where the virus appears to be the initiator, EBV contributes to the development of epithelial tumors, namely nasopharyngeal carcinoma (NPC) and EBV-associated gastric carcinoma (EBVaGC), as a consequence of the aberrant establishment of virus latency in epithelial cells with existing pre-malignant changes that disable the normal differentiation process [5].
The EBV genome comprises approximately 172 kb and encodes around 100 open reading frames that are generally divided into two categories, latent and lytic genes. A limited subset of viral gene products, the so-called latent proteins, consists of six nuclear antigens (EBNAs 1, 2, 3A, 3B, 3C, and -LP) and three latent membrane proteins (LMPs 1, 2A, and 2B). Over the years, these latent proteins have been regarded as key molecules in the pathogenesis of EBV-associated cancers by regulating various critical cellular processes and pathways [5]. The contribution of these latent proteins to the oncogenic process remains the subject of intense study. By contrast, although there are more than 80 EBV lytic genes, for the majority of these genes far less attention has been paid to their possible roles in oncogenesis. Nonetheless, it is now becoming increasingly clear that the EBV lytic phase also plays an important role in EBV-driven carcinogenesis. In particular, an early lytic gene, BARF1, is now recognized as being consistently expressed in NPC and EBVaGC, highlighting the need to be more open-minded about the possible contribution of EBV genes outside of those traditionally associated with latent infection. This also stresses the need to obtain more comprehensive EBV gene expression profiles in EBV-associated cancers. The advent of next generation sequencing technology has allowed the discovery and interrogation of exogenous pathogens associated with various types of cancers. RNA sequencing (RNAseq) captures genetic and transcriptional profiles of cancer cells, which can be leveraged to examine not only the host genome but also pathogen genomes infecting host cells. In recent years, an increasing number of research groups have used RNAseq analysis to determine the transcriptomic profiles of EBV-associated cancers [6][7][8][9][10][11][12][13][14][15][16][17][18][19]. Although the pattern of EBV gene expression varies between malignancies, these studies consistently demonstrated that a number of EBV lytic genes are more widely expressed than previously recognized, and the expression levels of some of these genes are similar to those of latent genes.
2. EBV Lytic Cycle
As with many viruses that can establish a persistent infection, the switch from a latent to lytic EBV infection leads to virus replication with the production of new viral particles, and eventually the lysis of productively infected cells. The EBV lytic cycle can be activated in virus-infected B cells and epithelial cells by diverse stimuli in vitro, including phorbol ester and histone deacetylase inhibitors (HDACs) [20][21]. While the precise in vivo conditions responsible for this process remain to be explored, it is intimately related to the differentiation of both B cells and epithelial cells. The lytic cycle is characterized by the expression of a large number of viral proteins and is divided into three temporal and functional stages: immediate early, early, and late [22]. Zta (encoded by BZLF1) and Rta (encoded by BRLF1) are the immediate early transcription factors in charge of activating the cascade of EBV lytic gene expression. Early genes mostly encode proteins associated with virus DNA replication, and late gene products mainly support the formation of viral particles (Figure 1).
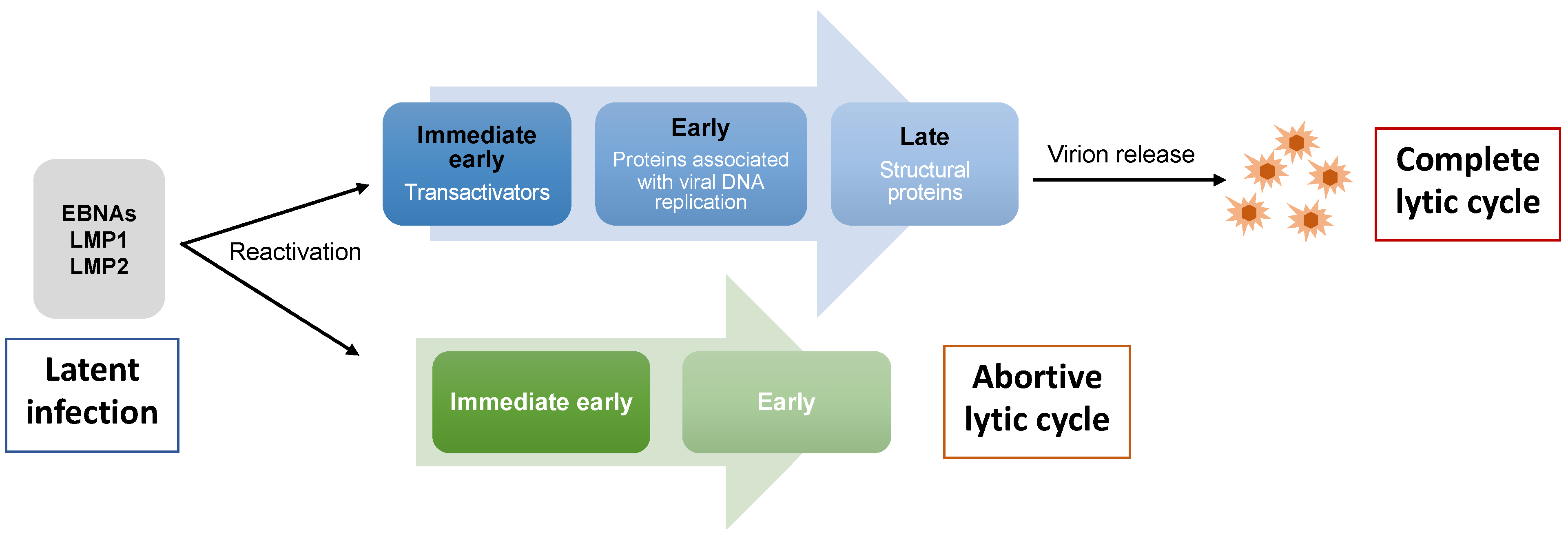
Figure 1. EBV lytic cycle. During latent infection, a restricted number of EBV latent genes are expressed in infected cells. Upon reactivation, cells that undergo complete lytic cycle express the immediate early genes (BZLF1, BRLF1), followed by early genes required for viral genome replication. Late genes that encode mainly viral structural proteins are then expressed and this is followed by the production of new viral particles. However, some cells that are reactivated undergo abortive lytic cycle where they express BZLF1 together with one or more early genes in the absence of late genes, and thus do not produce mature virions.
Zta and Rta synergistically stimulate the expression of multiple early lytic genes, including those that encode the components of the core replication machinery: BALF5 (the DNA polymerase), BALF2 (the single-stranded DNA-binding protein homolog), BMRF1 (the DNA polymerase processivity factor), BSLF1 (the primase homolog), BBLF4 (the helicase homolog) and BBLF2/3 (a potential homolog of the third component of the helicase-primase complex) [23]. In addition to its function as a transactivator, Zta binds to oriLyt (lytic origin of DNA replication) to initiate EBV DNA replication [24].
Traditionally, it has been thought that the latent and lytic cycles are two mutually exclusive mechanisms that contribute to lifelong infection by EBV. The expression of a restricted set of latent genes is compatible with persistent infection because it allows the virus to escape from host immunity. By contrast, the expression of lytic genes during viral replication is primarily found in the epithelium of oropharynx and salivary glands and it is thought to promote the spread of new viral particles [25][26]. It also likely that full lytic infection can occur periodically in B cells, but this has not been demonstrated in vivo. There is a significant immune response, both humoral and cell-mediated, to EBV lytic antigens in all individuals who are infected with EBV. This demonstrates that lytic infection is a continuous feature of virus infection, providing a constant stimulus to the immune system.
A possible tumorigenic role for lytic genes has emerged since the concept of “abortive lytic cycle” was proposed [27]. Zta preferentially binds and transactivates methylated promoters [28]. While a complete lytic cycle (i.e., production of progeny virus) immediately post-infection cannot be achieved while the viral genome is not methylated, Zta is still able to induce the expression of several early genes. The expression of BZLF1 together with one or more early genes in the absence of lytic genes primarily encoding late structural proteins is termed the abortive lytic cycle (Figure 1).
3. Expression of EBV Lytic Genes in Tumors
The co-existence of latency and abortive lytic cycles has been documented in EBV-associated malignancies, including NPC, EBVaGC, and BL [29][30][31]. These studies consistently reported the presence of BZLF1/BRLF1 and early genes, while late genes were either detected at low frequency or low levels. Unexpectedly, despite evidence for an incomplete lytic cycle, the expression of some late lytic genes has been detected in tumor samples by RNAseq analyses [6][7][8][9][10][11][12][13][14][15][16][17][18][19]. Some late genes have been classified as “leaky” because they are expressed at low levels early in the lytic phase and further increased following EBV DNA replication [32]. However, this does not explain the presence of some “true late genes” in tumor samples whose expression is usually dependent upon lytic DNA replication. Whether EBV acquires a yet-to-be-identified strategy to express late genes that could contribute to the development of disease remains to be determined.
Although there have been reports demonstrating the detection of lytic proteins in a variety of EBV tumors, comprehensive characterization of EBV transcriptomic profiles has only been made possible with the advent of RNAseq analysis. Due to technical and/or clinical challenges, most previous studies have used bulk tumor tissues. Thus, it remains to be determined whether such virus lytic RNA expression was derived from EBV-positive tumor cells or surrounding EBV-positive B cells that happen to be undergoing lytic cycle. Nonetheless, given that several independent research groups reported similar observations across different cancer types, it is now clear that EBV-associated tumors often express a wide-range of lytic genes that could play pivotal roles in EBV oncogenesis. This notion is reinforced by single-cell transcriptomic analysis showing co-existence of EBV latent and lytic genes in tumor cells isolated from primary NPC tissues [17]. The EBV lytic genes that have consistently been detected in biopsies from different tumor types using RNAseq are listed in Table 1. Of these, a number have also been detected at the protein level in tissues or cell lines, or antibodies against them detected in patient specimens (Table 2). As the tumorigenic roles of the two immediate early genes BZLF1 and BRLF1 have been discussed and reviewed previously [33][34], researchers focused their attention on early and late genes with known mechanisms that are relevant to EBV oncogenesis.
Table 1. Lytic genes that are detected in primary tissues of EBV-associated cancers by RNAseq.
| Gene Name | Kinetics | Lytic Function | Cancer Types | References |
|---|---|---|---|---|
| BZLF1 | IE | Transactivator | GC, NPC, COAD, BL, DLBCL, PTCL | [6][7][8][9][10] |
| BRLF1 | IE | Transactivator | GC, NPC, DLBCL | [7][8][10][11][12] |
| BORF2 | E | Ribonucleotide reductase large subunit | BL, ENKTCL | [13][14] |
| BSLF1 | E | Primase | GC, DLBCL | [7][15] |
| BSLF2/BMLF1 | E | mRNA export factor ICP27 homolog | PTCL, DLBCL, AITL | [6][15][16] |
| BALF1 | E | vBcl-2 | GC, NPC | [8][11][15][17] |
| BALF2 | E | Single-stranded DNA-binding protein | GC, NPC, COAD, BL, DLBCL, ENKTCL | [7][8][9][11][14][15][17][18] |
| BALF3 | E | Terminase large subunit | GC, NPC, BL, DLBCL, AITL, ENKTCL | [11][12][14][15][16][17][18] |
| BHLF1 | E | Involved in viral DNA synthesis | AITL, BL, DLBCL, | [14][15][16] |
| BHRF1 | E | vBcl-2 | GC, NPC, BL, DLBCL | [7][9][10][12][14][15] |
| BMRF1 | E | DNA polymerase processivity factor | GC, COAD, BL, DLBCL, ENKTCL | [7][9][13][14][15] |
| BALF5 | E | DNA polymerase catalytic subunit | GC, NPC, BL, DLBCL, ENKTCL | [7][8][11][12][14][15][18] |
| BARF1 | E | Soluble decoy for CSF-1 | GC, ENKTCL | [8][10][11][13] |
| BBLF4 | E | Helicase | NPC, GC | [7][12] |
| BILF1 | E | gp64, vGPCR | GC, NPC, BL, DLBCL, ENKTCL, | [9][11][12][15][17][18] |
| BNLF2a | E | Inhibtor of TAP | GC, NPC, BL, DLBCL, PTCL, AITL, ENKTCL | [6][9][11][13][15][16][17] |
| BNLF2b | E | Not reported | GC, NPC, DLBCL, PTCL, ENKTCL | [6][11][13][17][18] |
| LF3 | E | Involves in viral DNA synthesis | GC, NPC, BL, AITL | [11][14][16][17] |
| BMRF2 | E/L | Membrane proteins | BL, DLBCL, ENKTCL | [13][14][15] |
| BCRF1 | L | vIL-10 | GC, NPC, AITL, BL, | [7][9][10][12][16] |
| BALF4 | L | Envelope glycoprotein B | GC, NPC, BL, DLBCL, ENKTCL | [7][8][9][11][12][14][15][17][18] |
| BKRF2 | L | gL, gp25 | BL, DLBCL | [14][15] |
| BLLF1 | L | gp350/220 | GC | [7][10] |
| BNRF1 | L | Major tegument protein | GC, NPC, BL, AITL, ENKTCL | [8][11][12][13][14][16][17][18] |
| BCLF1 | L | Major Capsid Protein | NPC, GC | [7][12] |
| LF1 | Unknown | Not reported | GC, NPC, DLBCL | [8][11][12][15][17][19] |
| LF2 | Unknown | Protein that binds Rta | GC, NPC, BL, DLBCL | [8][9][11][12][14][15][17] |
GC gastric cancer, NPC nasopharyngeal carcinoma, COAD colon adenocarcinoma, BL Burkitt lymphoma, DLBCL diffuse large B cell lymphoma, AITL angio-immunoblastic T cell lymphoma, PTCL peripheral T cell lymphoma, ENKTCL extra nodal natural killer T cell lymphoma.
Table 2. Detection of proteins or antibodies against EBV lytic genes that are expressed in primary EBV-associated cancers by RNAseq.
| Gene Name | Samples | Assays | References |
|---|---|---|---|
| BZLF1 | Biopsy, FNA, cell line | IHC, ICC, WB | [31][35][36][37] |
| BRLF1 | Plasma, cell line | ELISA, WB, IF | [37][38][39][40] |
| BORF2 | Plasma | ELISA | [38] |
| BSLF1 | Cell line | WB | [41] |
| BSLF2/BMLF1 | Plasma, cell line | Protein array, WB | [38][42][43] |
| BALF2 | Biopsy, plasma | IHC, ELISA | [38][44] |
| BHLF1 | Cell line | IF | [45] |
| BHRF1 | Biopsy, plasma | IHC, WB, protein array | [38][46][47] |
| BMRF1 | Biopsy, serum, saliva, cell line | IHC, ELISA, WB | [37][48][49][50] |
| BALF5 | Plasma, cell line | Protein array, WB | [38][51] |
| BARF1 | Biopsy | WB | [52] |
| BBLF4 | Cell line | WB | [41] |
| BNLF2a | Cell line | WB, IF | [53][54] |
| LF3 | FNA, biopsy | ICC, IHC | [31][36] |
| BMRF2 | Cell line | WB, IF | [55] |
| BCRF1 | Allograft, serum | IHC, ELISA | [56][57] |
| BALF4 | Plasma, serum | Protein array, ELISA | [38][58] |
| BKRF2 | Cell line | IP | [59] |
| BLLF1 | Serum, cell line | IF, WB | [60][61][62] |
| BNRF1 | Cell line | WB | [63] |
| LF2 | Plasma | ELISA | [38] |
FNA fine needle aspiration, IHC immunohistochemistry, ICC immunocytochemistry, WB western blotting, ELISA enzyme-linked immunosorbent assay, IF immunofluorescence, IP immunoprecipitation.
References
- Henle, G.; Henle, W.; Diehl, V. Relation of Burkitt’s tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc. Natl. Acad. Sci. USA 1968, 59, 94–101.
- Babcock, G.J.; Decker, L.L.; Volk, M.; Thorley-Lawson, D.A. EBV persistence in memory B cells in vivo. Immunity 1998, 9, 395–404.
- Thorley-Lawson, D.A.; Gross, A. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 2004, 350, 1328–1337.
- Laichalk, L.L.; Thorley-Lawson, D.A. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 2005, 79, 1296–1307.
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein–Barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer 2016, 16, 789–802.
- Nakhoul, H.; Lin, Z.; Wang, X.; Roberts, C.; Dong, Y.; Flemington, E. High-throughput sequence analysis of peripheral T-Cell lymphomas indicates subtype-specific viral gene expression patterns and immune cell microenvironments. Msphere 2019, 4, e00248-19.
- Song, H.; Lim, Y.; Im, H.; Bae, J.M.; Kang, G.H.; Ahn, J.; Baek, D.; Kim, T.-Y.; Yoon, S.-S.; Koh, Y. Interpretation of EBV infection in pan-cancer genome considering viral life cycle: LiEB (Life cycle of Epstein-Barr virus). Sci. Rep. 2019, 9, 3465.
- Zhang, R.; Strong, M.J.; Baddoo, M.; Lin, Z.; Wang, Y.-P.; Flemington, E.K.; Liu, Y.-Z. Interaction of Epstein-Barr virus genes with human gastric carcinoma transcriptome. Oncotarget 2017, 8, 38399.
- Abate, F.; Ambrosio, M.R.; Mundo, L.; Laginestra, M.A.; Fuligni, F.; Rossi, M.; Zairis, S.; Gazaneo, S.; De Falco, G.; Lazzi, S. Distinct viral and mutational spectrum of endemic Burkitt lymphoma. PLoS Pathog. 2015, 11, e1005158.
- Tang, W.; Morgan, D.R.; Meyers, M.O.; Dominguez, R.L.; Martinez, E.; Kakudo, K.; Kuan, P.F.; Banet, N.; Muallem, H.; Woodward, K. Epstein-barr virus infected gastric adenocarcinoma expresses latent and lytic viral transcripts and has a distinct human gene expression profile. Infect. Agent. Cancer 2012, 7, 21.
- Borozan, I.; Zapatka, M.; Frappier, L.; Ferretti, V. Analysis of Epstein-Barr virus genomes and expression profiles in gastric adenocarcinoma. J. Virol. 2018, 92, e01239-17.
- Hu, L.; Lin, Z.; Wu, Y.; Dong, J.; Zhao, B.; Cheng, Y.; Huang, P.; Xu, L.; Xia, T.; Xiong, D. Comprehensive profiling of EBV gene expression in nasopharyngeal carcinoma through paired-end transcriptome sequencing. Front. Med. 2016, 10, 61–75.
- Peng, R.-J.; Han, B.-W.; Cai, Q.-Q.; Zuo, X.-Y.; Xia, T.; Chen, J.-R.; Feng, L.-N.; Lim, J.Q.; Chen, S.-W.; Zeng, M.-S. Genomic and transcriptomic landscapes of Epstein-Barr virus in extranodal natural killer T-cell lymphoma. Leukemia 2019, 33, 1451–1462.
- Kaymaz, Y.; Oduor, C.I.; Yu, H.; Otieno, J.A.; Ong’echa, J.M.; Moormann, A.M.; Bailey, J.A. Comprehensive transcriptome and mutational profiling of endemic Burkitt lymphoma reveals EBV type–specific differences. Mol. Cancer Res. 2017, 15, 563–576.
- Strong, M.J.; O’Grady, T.; Lin, Z.; Xu, G.; Baddoo, M.; Parsons, C.; Zhang, K.; Taylor, C.M.; Flemington, E.K. Epstein-Barr virus and human herpesvirus 6 detection in a non-Hodgkin’s diffuse large B-cell lymphoma cohort by using RNA sequencing. J. Virol. 2013, 87, 13059–13062.
- Bayda, N.; Tilloy, V.; Chaunavel, A.; Bahri, R.; Halabi, M.A.; Feuillard, J.; Jaccard, A.; Ranger-Rogez, S. Comprehensive Epstein-Barr virus transcriptome by RNA-sequencing in angioimmunoblastic T cell lymphoma (AITL) and other lymphomas. Cancers 2021, 13, 610.
- Jin, S.; Li, R.; Chen, M.-Y.; Yu, C.; Tang, L.-Q.; Liu, Y.-M.; Li, J.-P.; Liu, Y.-N.; Luo, Y.-L.; Zhao, Y. Single-cell transcriptomic analysis defines the interplay between tumor cells, viral infection, and the microenvironment in nasopharyngeal carcinoma. Cell Res. 2020, 30, 950–965.
- Xiong, J.; Cui, B.-W.; Wang, N.; Dai, Y.-T.; Zhang, H.; Wang, C.-F.; Zhong, H.-J.; Cheng, S.; Ou-Yang, B.-S.; Hu, Y. Genomic and transcriptomic characterization of natural killer T cell lymphoma. Cancer Cell 2020, 37, 403–419.e406.
- Murata, T. Encyclopedia of EBV-encoded lytic genes: An update. Hum. Herpesviruses 2018, 395–412.
- Hausen, H.Z.; O’NEILL, F.J.; Freese, U.K.; HECKER, E. Persisting oncogenic herpesvirus induced by the tumour promoter TPA. Nature 1978, 272, 373–375.
- Kallin, B.; Luka, J.; Klein, G. Immunochemical characterization of Epstein-Barr virus-associated early and late antigens in n-butyrate-treated P3HR-1 cells. J. Virol. 1979, 32, 710–716.
- Honess, R.W.; Roizman, B. Regulation of herpesvirus macromolecular synthesis I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 1974, 14, 8–19.
- Fixman, E.D.; Hayward, G.; Hayward, S. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J. Virol. 1992, 66, 5030–5039.
- Scepers, A.; Pich, D.; Hammerschmidt, W. Activation oforiLyt, the Lytic Origin of DNA Replication of Epstein–Barr Virus, by BZLF1. Virology 1996, 220, 367–376.
- Greenspan, J.S.; Greenspan, D.; Lennette, E.T.; Abrams, D.I.; Conant, M.A.; Petersen, V.; Freese, U.K. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N. Engl. J. Med. 1985, 313, 1564–1571.
- Temple, R.M.; Zhu, J.; Budgeon, L.; Christensen, N.D.; Meyers, C.; Sample, C.E. Efficient replication of Epstein–Barr virus in stratified epithelium in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 16544–16549.
- Kalla, M.; Hammerschmidt, W. Human B cells on their route to latent infection–early but transient expression of lytic genes of Epstein-Barr virus. Eur. J. Cell Biol. 2012, 91, 65–69.
- Dickerson, S.J.; Xing, Y.; Robinson, A.R.; Seaman, W.T.; Gruffat, H.; Kenney, S.C. Methylation-dependent binding of the epstein-barr virus BZLF1 protein to viral promoters. PLoS Pathog. 2009, 5, e1000356.
- Martel-Renoir, D.; Grunewald, V.; Touitou, R.; Schwaab, G.; Joab, I. Qualitative analysis of the expression of Epstein–Barr virus lytic genes in nasopharyngeal carcinoma biopsies. J. Gen. Virol. 1995, 76, 1401–1408.
- Ramayanti, O.; Juwana, H.; Verkuijlen, S.A.; Adham, M.; Pegtel, M.D.; Greijer, A.E.; Middeldorp, J.M. Epstein-Barr virus mRNA profiles and viral DNA methylation status in nasopharyngeal brushings from nasopharyngeal carcinoma patients reflect tumor origin. Int. J. Cancer 2017, 140, 149–162.
- Xue, S.a.; Labrecque, L.G.; Lu, Q.L.; Ong, S.K.; Lampert, I.A.; Kazembe, P.; Molyneux, E.; Broadhead, R.L.; Borgstein, E.; Griffin, B.E. Promiscuous expression of Epstein-Barr virus genes in Burkitt’s lymphoma from the central African country Malawi. Int. J. Cancer 2002, 99, 635–643.
- Djavadian, R.; Hayes, M.; Johannsen, E. CAGE-seq analysis of Epstein-Barr virus lytic gene transcription: 3 kinetic classes from 2 mechanisms. PLoS Pathog. 2018, 14, e1007114.
- Germini, D.; Sall, F.B.; Shmakova, A.; Wiels, J.; Dokudovskaya, S.; Drouet, E.; Vassetzky, Y. Oncogenic properties of the EBV ZEBRA protein. Cancers 2020, 12, 1479.
- Rosemarie, Q.; Sugden, B. Epstein–Barr Virus: How Its Lytic Phase Contributes to Oncogenesis. Microorganisms 2020, 8, 1824.
- Brousset, P.; Knecht, H.; Rubin, B.; Drouet, E.; Chittal, S.; Meggetto, F.; Al Saati, T.; Bachmann, E.; Denoyel, G.; Sergeant, A. Demonstration of Epstein-Barr virus replication in Reed-Sternberg cells of Hodgkin’s disease. Blood 1993, 82, 872–876.
- Xue, S.-A.; Lu, Q.-L.; Poulsom, R.; Karran, L.; Jones, M.; Griffin, B.E. Expression of two related viral early genes in Epstein-Barr virus-associated tumors. J. Virol. 2000, 74, 2793–2803.
- Adamson, A.L.; Darr, D.; Holley-Guthrie, E.; Johnson, R.A.; Mauser, A.; Swenson, J.; Kenney, S.C. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 2000, 74, 1224–1233.
- Song, L.; Song, M.; Camargo, M.C.; Van Duine, J.; Williams, S.; Chung, Y.; Kim, K.-M.; Lissowska, J.; Sivins, A.; Gao, W. Identification of anti-Epstein-Barr virus (EBV) antibody signature in EBV-associated gastric carcinoma. J. Gastric Cancer 2021, 24, 858–867.
- Zhu, Y.-H.; Wei, Y.-S.; Li, H.; Liang, W.-B.; Du, B.; Zhang, G.-Q.; Zhang, L. Construction and characterization of monoclonal antibodies specific for the R transactivator 185 of Epstein-Barr virus. J. Virol. Methods 2007, 144, 12–16.
- Bentz, G.L.; Liu, R.; Hahn, A.M.; Shackelford, J.; Pagano, J.S. Epstein–Barr virus BRLF1 inhibits transcription of IRF3 and IRF7 and suppresses induction of interferon-β. J. Virol. 2010, 402, 121–128.
- Yokoyama, N.; Fujii, K.; Hirata, M.; Tamai, K.; Kiyono, T.; Kuzushima, K.; Nishiyama, Y.; Fujita, M.; Tsurumi, T. Assembly of the Epstein–Barr virus BBLF4, BSLF1 and BBLF2/3 proteins and their interactive properties. J. Gen. Virol. 1999, 80, 2879–2887.
- Cook, I.; Shanahan, F.; Farrell, P. Epstein-Barr virus SM protein. J. Virol. 1994, 205, 217–227.
- Wong, K.-M.; Levine, A.J. Characterization of proteins encoded by the Epstein-Barr virus transactivator gene BMLF1. Virol. J. 1989, 168, 101–111.
- Kondo, S.; Okuno, Y.; Murata, T.; Dochi, H.; Wakisaka, N.; Mizokami, H.; Moriyama-Kita, M.; Kobayashi, E.; Kano, M.; Komori, T. EBV genome variations enhance clinicopathological features of nasopharyngeal carcinoma in a non-endemic region. Cancer Sci. 2022, 113, 2446.
- Lieberman, P.; Hardwick, J.; Hayward, S. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J. Virol. 1989, 63, 3040–3050.
- Liu, M.Y.; Shih, Y.Y.; Li, L.Y.; Chou, S.P.; Sheen, T.S.; Chen, C.L.; Yang, C.S.; Chen, J.Y. Expression of the Epstein-Barr virus BHRF1 gene, a homologue of Bcl-2, in nasopharyngeal carcinoma tissue. J. Med. Virol. 2000, 61, 241–250.
- Nicholls, J.; Kremmer, E.; Meseda, C.A.; Mackett, M.; Hahn, P.; Gulley, M.L.; Brink, A.; Swinnen, L.J.; Greenspan, J.; De Souza, Y. Comparative analysis of the expression of the epstein-barr virus (EBV) anti-apoptotic gene BHRF1 in nasopharyngeal carcinoma and EBV-related lymphoid diseases. J. Med. Virol. 2001, 65, 105–113.
- Cohen, M.; Vistarop, A.G.; Huaman, F.; Narbaitz, M.; Metrebian, F.; De Matteo, E.; Preciado, M.V.; Chabay, P.A. Epstein-Barr virus lytic cycle involvement in diffuse large B cell lymphoma. Hematol. Oncol. 2018, 36, 98–103.
- Nadala, E.; Tan, T.; Wong, H.; Ting, R. ELISA for the detection of serum and saliva IgA against the BMRFI gene product of Epstein-Barr virus. J. Med. Virol. 1996, 50, 93–96.
- Zhang, L.; Wu, H.; Sun, G.; Xu, X.; Sun, X.; Cao, L. Trichloromethane fraction of Incarvillea compacta induces lytic cytotoxicity and apoptosis in Epstein-Barr virus-positive gastric cancer AGS cells. BMC Complement. Altern. Med. 2016, 16, 344.
- Tsurumi, T.; Kobayashi, A.; Tamai, K.; Daikoku, T.; Kurachi, R.; Nishiyama, Y. Functional expression and characterization of the Epstein-Barr virus DNA polymerase catalytic subunit. J. Virol. 1993, 67, 4651–4658.
- Seto, E.; Yang, L.; Middeldorp, J.; Sheen, T.S.; Chen, J.Y.; Fukayama, M.; Eizuru, Y.; Ooka, T.; Takada, K. Epstein–Barr virus (EBV)-encoded BARF1 gene is expressed in nasopharyngeal carcinoma and EBV-associated gastric carcinoma tissues in the absence of lytic gene expression. J. Med. Virol. 2005, 76, 82–88.
- Horst, D.; Van Leeuwen, D.; Croft, N.P.; Garstka, M.A.; Hislop, A.D.; Kremmer, E.; Rickinson, A.B.; Wiertz, E.J.; Ressing, M.E. Specific targeting of the EBV lytic phase protein BNLF2a to the transporter associated with antigen processing results in impairment of HLA class I-restricted antigen presentation. J. Immunol. 2009, 182, 2313–2324.
- Strong, M.J.; Laskow, T.; Nakhoul, H.; Blanchard, E.; Liu, Y.; Wang, X.; Baddoo, M.; Lin, Z.; Yin, Q.; Flemington, E.K. Latent expression of the Epstein-Barr virus (EBV)-encoded major histocompatibility complex class I TAP inhibitor, BNLF2a, in EBV-positive gastric carcinomas. J. Virol. 2015, 89, 10110–10114.
- Gore, M.; Hutt-Fletcher, L.M. The BDLF2 protein of Epstein–Barr virus is a type II glycosylated envelope protein whose processing is dependent on coexpression with the BMRF2 protein. J. Virol. 2009, 383, 162–167.
- Itano, H.; Zhang, W.; Ritter, J.H.; McCarthy, T.J.; Yew, N.S.; Mohanakumar, T.; Patterson, G.A. Endobronchial transfection of naked viral interleukin-10 gene in rat lung allotransplantation. Ann. Thorac. Surg. 2001, 71, 1126–1133.
- Tanner, J.E.; Mitoma, F.D.; Rooney, C.M.; Alfieri, C. Anti-interleukin-10 antibodies in patients with chronic active Epstein-Barr virus infection. J. Infect. Dis. 1997, 176, 1454–1461.
- Hu, B.; Hong, G.; Li, Z.; Xu, J.; Zhu, Z.; Li, L. Expression of VCA (viral capsid antigen) and EBNA1 (Epstein—Barr-virus-encoded nuclear antigen 1) genes of Epstein–Barr virus in Pichia pastoris and application of the products in a screening test for patients with nasopharyngeal carcinoma. Biotechnol. Appl. Biochem. 2007, 47, 59–69.
- Yaswen, L.R.; Stephens, E.B.; Davenport, L.C.; Hutt-Fletcher, L.M. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. J. Virol. 1993, 195, 387–396.
- Nuebling, C.M.; Buck, M.; Boos, H.; Von Deimling, A.; Mueller-Lantzsch, N. Expression of Epstein-Barr virus membrane antigen gp350/220 in E. coli and in insect cells. J. Virol. 1992, 191, 443–447.
- Bertoni, G.; Kostyal, D.A.; Reisert, P.S.; Humphreys, R.E.; Sairenji, T. Synthetic peptides to identify antigenic determinants on Epstein-Barr virus gp350/220. Intervirology 1990, 31, 290–294.
- Ge, J.; Huang, Y.; Hu, X.; Zhong, J. A surface-modified baculovirus vector with improved gene delivery to B-lymphocytic cells. J. Biotechnol. 2007, 129, 367–372.
- Tsai, K.; Chan, L.; Gibeault, R.; Conn, K.; Dheekollu, J.; Domsic, J.; Marmorstein, R.; Schang, L.M.; Lieberman, P.M. Viral reprogramming of the Daxx histone H3. 3 chaperone during early Epstein-Barr virus infection. J. Virol. 2014, 88, 14350–14363.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
778
Revisions:
2 times
(View History)
Update Date:
15 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




