
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fatima-Zahra Akensous | -- | 4812 | 2022-12-07 19:23:25 | | | |
| 2 | Peter Tang | Meta information modification | 4812 | 2022-12-12 03:44:33 | | |
Video Upload Options
Date palm (Phoenix dactylifera L.) is constantly hindered due to detrimental abiotic constraints. Thus, there is a crucial need to deal with this problem. The application of biostimulants, such as the arbuscular mycorrhizal fungi (AMF), plant growth-promoting rhizobacteria (PGPR), and organic amendments hold tremendous potential to ameliorate the growth and yield of date palm significantly. The strengthening of biostimulants’ main common modes of action is exerted through five main functions: biostimulation (essentially), biofertilization, bioprotection, biological control, and the role of bio-effector. Moreover, synergistic and complementary effects manifest through biochemical and nutritional benefits, as well as molecular modulation. In this sense, available data provide suggestive findings that corroborate the beneficial roles of biostimulants, thereby positioning them as promising eco-friendly tools that work toward resilience to abiotic stresses in date palm.
1. Introduction
2. History and Distribution of Date Palm
3. Date Palm, A Pillar in the Oasis Ecosystem
4. Pests, Diseases, and Anthropogenic Constraints
5. Effects of Abiotic Stresses and Adaptive Strategies in Date Palm
|
Abiotic Stress |
Stress Level |
Growth Stage |
Cultivar/Variety |
Main Effects |
References |
|---|---|---|---|---|---|
|
Drought |
Watering cessation for 7–8 days before harvest |
Seedlings |
- |
Heat-shock proteins (HSPs), chaperone proteins, and heat stress Transcription Factors (TFs) genes’ expression Cell death elimination Enrichment of phytohormones- related, wax, secondary metabolism, fatty acids biosynthesis, and plant cell wall pathways |
[17] |
|
Drought |
70%, 100% evapotranspiration (ETc) |
10–12-year-old orchards |
“Mazafati” |
Increase in bunch weight, fruit weight, fruit starch, yielding, and water-use efficiency (WUE) Increase in soluble solids and sugar content A rise in total phenolic compounds, peroxidase (POX), polyphenol Amelioration of (PPO) activities A rise in calcium (Ca), iron (Fe), and zinc (Zn) |
[51] |
|
Drought |
6.9, 13.95, 27.5% of polyethylene glycol 6000 (PEG) |
3 month seedlings |
“Sagie” |
Enhancement of phenolic and flavonoid content Rise of Catalase (CAT), peroxidase (POX), and polyphenol oxidase (PPO) activities |
[52] |
|
Drought |
0 (control), −0.41, −0.82, −1.23, −1.63 MPa of mannitol |
4–5-year-old suckers |
“Barhee”, “Ruziz”, “Sukary” |
Decrease in leaf and root numbers, leaf, and root dry weights, and total dry weight A decline in relative water content (RWC), photosynthetic and rate of transpiration, water-use efficiency (WUE), and mesophyll conductance Increase in [CO2]i |
[53] |
|
Drought |
Irrigation reduced to 50% of the control |
2 year old seedlings |
- |
Decrease in shoot growth A decline in leaf gas exchange Decrease in intrinsic leaf water-use efficiency (WUEi) |
[54] |
|
Drought |
Gradual decline in humidity |
Plantlets |
“Sewi” |
Dehydration A decline in photosynthetic pigments Decline in surviving chances |
[55] |
|
Drought |
Irrigation reduced to 50% of the control |
2-year-old seedlings |
- |
Decrease in leaf hydration, foliar total and reduced ascorbate, chlorophyll a/b ratio, sugars, and organic acids Increase in total reduced glutathione (GSH), oxidized glutathione (GSSG), the GSSG/GSH ratio, amino acids, and 5,8,11,14- eicosatetraenoic acid |
[56] |
|
Drought |
Irrigation reduced to 50% and 25% of the control |
2-year-old plants |
- |
A rise in isoprene emission rates and a decline in soil water content (SWC) Upregulation of primary metabolism, stress response, photosynthesis, and antioxidant-related proteins Downregulation of gene expression, metabolic, and secondary metabolism-related proteins |
[25] |
|
Drought |
50, 100, 150% of evapotranspiration levels |
Trees |
“Succary” |
A decline in date palm yielding Affected fruit traits An overall decline in fruit metabolites |
[57] |
|
Drought |
50, 75, 100% of watering demand |
Trees |
“Khalas” |
Affected fruit yielding as well as quality |
[58] |
|
Salinity |
0, 240 mM NaCl |
Seedlings |
“Khalas”, “Manoma”, “Barni”, “Nashukharma”, “Hilali-Omani”, “Fard”, “Abunarenja”, “Nagal”, “Umsila”, “Zabad” |
Reduction in shoot as well as root dry weights, and leaf area Decrease in photosynthetic properties |
[59] |
|
Salinity |
50, 100, 150 mM NaCl |
2 month seedlings |
“Khalas” |
Enhancement of proline content and thiobarbituric acid reactive substances (TBARS) A rise in Catalase (CAT) as well as Superoxide Dismutase (SOD) activities Variation within cDNA start codon-targeted (cDNASCoT) marker genes’ expression |
[60] |
|
Salinity |
5, 10, 15 dS m−1 of salt water |
Trees |
“Ajwat AlMadinah”, “Naghal”, “Khnizi”, “Barhi”, “Makhtoumi”, “Farad”, “Khisab”, “Nabtat-Saif“, “Shagri”, “Abu-Maan”, “Jabri”, “Sukkari”, “Rothan” |
Excluding of Na+ Retaining of K+ Decrease in osmotic potential |
[61] |
|
Salinity |
0, 300 mM NaCl |
Seedlings |
“Khalas” |
Decline in photosynthetic capacity, stomatal conductance (gs), rate of transpiration (E), as well as internal carbon dioxide concentration [CO2]i Variation within genes expression Transcripts enrichment implicated in metabolism pathways |
[62] |
|
Salinity |
50, 300 mM NaCl |
Seedlings |
“Khalas” |
Decrease in photosynthetic capacity, stomatal conductance (gs), rate of transpiration (E), and root system traits Hypermethylated and hypomethylated DNA regions, coupled with insignificant genes expression |
[63] |
|
Salinity |
0, 240 mM NaCl |
Seedlings |
“Umsila”, “Zabad” |
Decrease in leaf area, physiological traits, and leaf water potential (LWP) Increase in leaf total soluble sugars, proline and glycine betaine |
[64] |
|
Salinity |
0, 240 mM NaCl |
Seedlings |
“Umsila”, “Zabad” |
A decline in leaf fresh and dry weights |
[65] |
|
Salinity |
0, 300 mM NaCl |
Seedlings |
“Khalas” |
Decrease in leaf area, leaf and root dry weights, K+ accumulation, and roots’ Casparian strips Enhancement of stress-related metabolites (e.g., osmolytes and antioxidant enzymes) |
[66] |
|
Salinity |
5 dS m−1, 15 dS m−1 of saline water |
Trees |
“Lulu”, “Khalas”, “Shahlah” |
Negative effect on height Decrease in tree water use (ETc) Variation in the consumed water productivity (CWP) |
[67] |
|
Salinity |
<1, 12–15, 18–20 dS m−1 of saline water |
4-year-old trees |
- |
A decline in actual water use |
[68] |
|
Salinity |
5, 10, 15 dS m−1 of salt water |
Trees |
- |
Decrease in trunk height and diameter, brunch total number, yielding of dates Increase in canopy temperature (CT) |
[69] |
|
Salinity |
4 g/L, 8 g/L, 12 g/L, 16 g/L NaCl |
Seedlings |
“Deglet Nour” |
Drop in seeds’ germination, radicle length, and Catalase (CAT) activity A rise in total protein content, superoxide dismutase (SOD), and secondary metabolites |
[60] |
|
Salinity |
5, 10, 15 dS m−1 of salt water |
Trees |
“Ajwat Al Madinah”, “Naghal”, “Barhi”, “Shagri”, “Abu Maan”, “Jabri”, “Sukkari”, “Rothan”, “Khinizi”, “Maktoumi” |
Increase in minerals, mainly K, P, and Ca |
[70] |
|
Salinity |
Irrigation levels based on crop evapotranspiration (ETc) at 50%, 100%, and 150% of saline water |
Trees |
“Succary” |
Decrease in dates yielding, fruit weight and size, total soluble solids (TTS), acidity, fruit moisture content, and total sugar and non-reducing sugar content in fruits |
[57] |
|
Salinity |
0, 240 mM NaCl |
Seedlings |
“Umsila”, “Zabad” |
Production of salinity- related metabolites |
[71] |
|
Salinity |
3.2–4.5 dS m−1 salt water (ECw) |
Trees |
- |
Increase in transpiration, soil evaporation, percolation, and salt accumulation |
[72] |
|
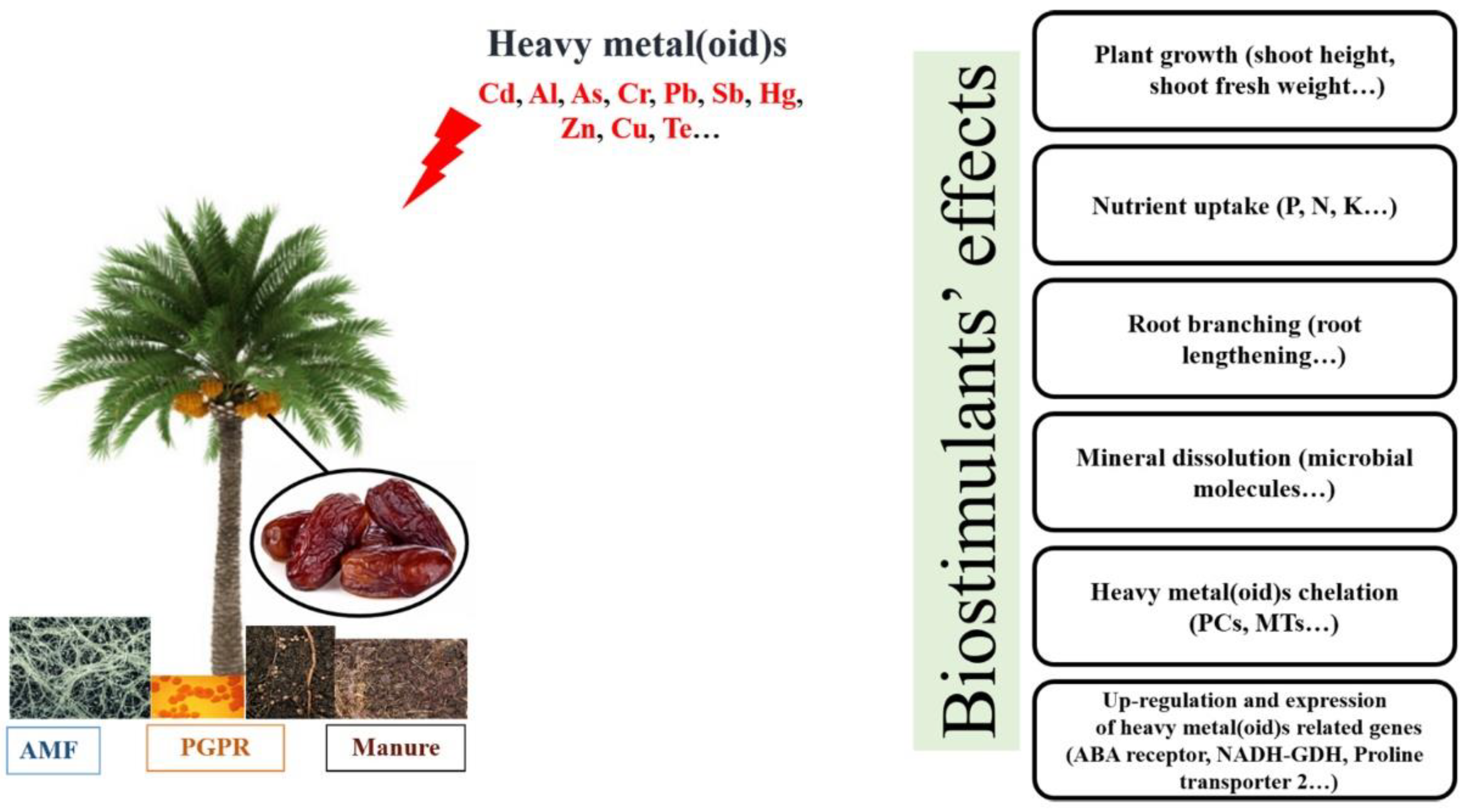
Heavy metal(oid)s |
Cadmium (Cd), chromium (Cr) |
Seedlings |
“Deglet Nour” |
Decrease in phytochelatin synthase (pcs) and metallothionein (mt) genes’ expression |
[73] |
|
Heavy metal(oid)s |
Antimony (Sb), cadmium (Cd), lead (Pb), chromium (Cr), arsenic (As), aluminum (Al) |
Date fruits |
“Sakay Mabroum”, “Kadary”, “Safawy Al-Madina”, “Eklas Al-Hassa”, “Barny Al-Madina”, “Rashadya Al-qaseem”, “Sakay Normal” |
As along with Pb surpassing the maximal allowable levels (MAL) |
[74] |
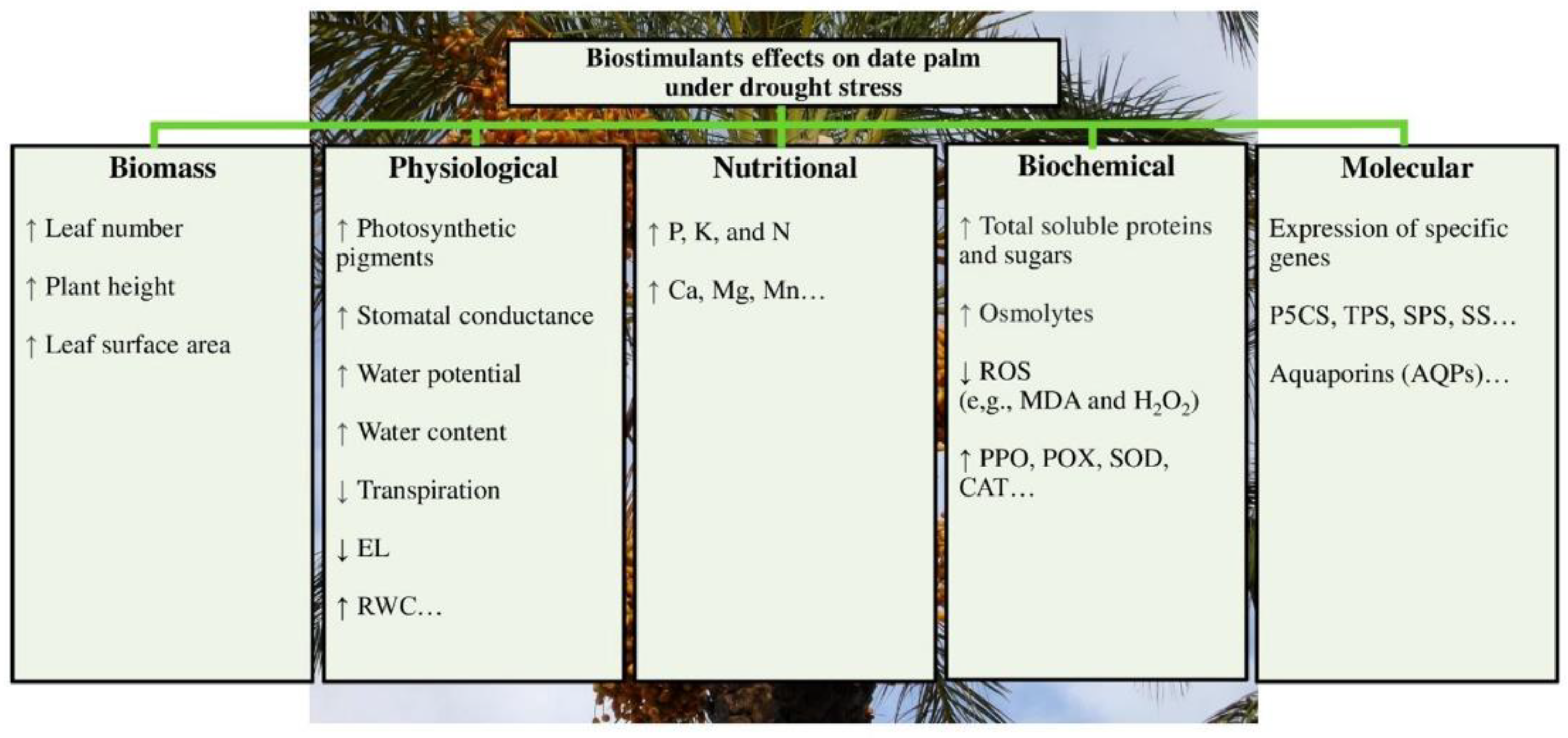
6. Biostimulants Attenuate Abiotic Stresses’ Effects in Date Palm
|
Abiotic Stress |
Level of Stress |
Biostimulants |
Main Effects |
References |
||
|---|---|---|---|---|---|---|
|
AMF |
PGPR |
Organic Amendment |
||||
|
Drought |
75, 25% FC |
Rhizoglomus irregulare Aoufous consortium |
PGPR consortium |
Grass-based compost Green waste-based compost |
Enhancement of growth traits and physiological parameters Improvement of N and P content Increase in sugar and protein content Decrease in MDA and H2O2 Decrease in soil pH and boosting of electrical conductivity (EC), organic matter (OM), and total organic carbon (TOC), |
[30] |
|
Drought |
100, 75, 50, 25% FC |
A complex of 28 different species |
Bacillus S48 |
- |
Improvement of RWC Enhancement of proline content Decrease in SOD, CAT, POX, and glutathione S-transferase (GST) Increase in soil EC |
[78] |
|
Drought |
Water regimes: 32 L/h for well-watered (WW); 16 L/h for drought stress (DS) |
Aoufous consortium |
PGPR consortium |
Organic waste-based compost |
Improvement of plant biomass Amelioration of plant-water relations Enhancement of P uptake A rise in total soluble sugar and protein content Decrease in MDA as well as H2O2 Improvement of soil traits, such as OM, P, and glomalin content |
[79] |
|
Salinity |
0, 50, 100, 200 mM NaCl |
- |
Endophytic bacteria |
- |
Ferric ion (Fe3+) chelation, K+ solubilization, phosphate ion (PO43−) and zinc ion (Zn2+), and ammonia (NH3) production 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase together with IAA production capacity |
[80] |
|
Salinity |
0, 240 mM NaCl |
Aoufous consortium |
- |
- |
Improvement of growth and physiological traits Enhancement of water potential Amelioration of P, K as well as Ca content Decrease in MDA and H2O2 and rise in SOD, CAT, POX as well as APX activities |
[81] |
|
Salinity |
0, 240 mM NaCl |
Aoufous consortium |
- |
Green waste-based compost |
Amelioration of physiological parameters Improvement of P, potassium ion (K+), and calcium ion (Ca2+) content Enhancement of proline Reduction in the effect of lipid peroxidation and H2O2 |
[82] |
|
Salinity |
Up to 7.6 dS m−1 NaCl |
Identification of Albahypha drummondii, Dominikia disticha, Funneliformis coronatus, Rhizoglomus irregular |
- |
- |
Positive correlation of soil salinity and intensity of mycorrhization Negative correlation of soil salinity and easily extractable glomalin |
[83] |
|
Salinity |
0, 120, 240 mM NaCl |
Aoufous consortium Rhizophagus irregularis |
PGPR consortium |
Green waste-based compost |
Enhancement of growth traits and antioxidant defensive machinery |
[84] |
|
Salinity |
0, 10, 20 g·L−1 NaCl |
Autochthonous AMF Exogenous AMF |
- |
- |
Negatively impacted growth as well as physiological properties |
[85] |
|
Heavy metal(oid)s |
- |
- |
- |
Organic manure |
Enhancement of heavy metal(oid)s content in date palm fruits |
[86] |
|
Heavy metal(oid)s |
Pb(NO3)2 200 mg/L |
Glomus spp. |
Rhizobium leguminosarum |
- |
Improvement of root length, root fresh weight, shoot height, shoot fresh weight as well as the germination index Enhancement of seedling length, root basal diameter, and dry biomass |
[87] |
|
Heavy metal(oid)s |
- |
- |
Exiguobacterium sp. |
- |
Identification of proteins/ enzymes involved in reducing heavy metal(oid)s contamination |
[88] |
7. AMF–PGPR Co-Inoculation and/or Organic Amendments
-
Strengthened in-common effects
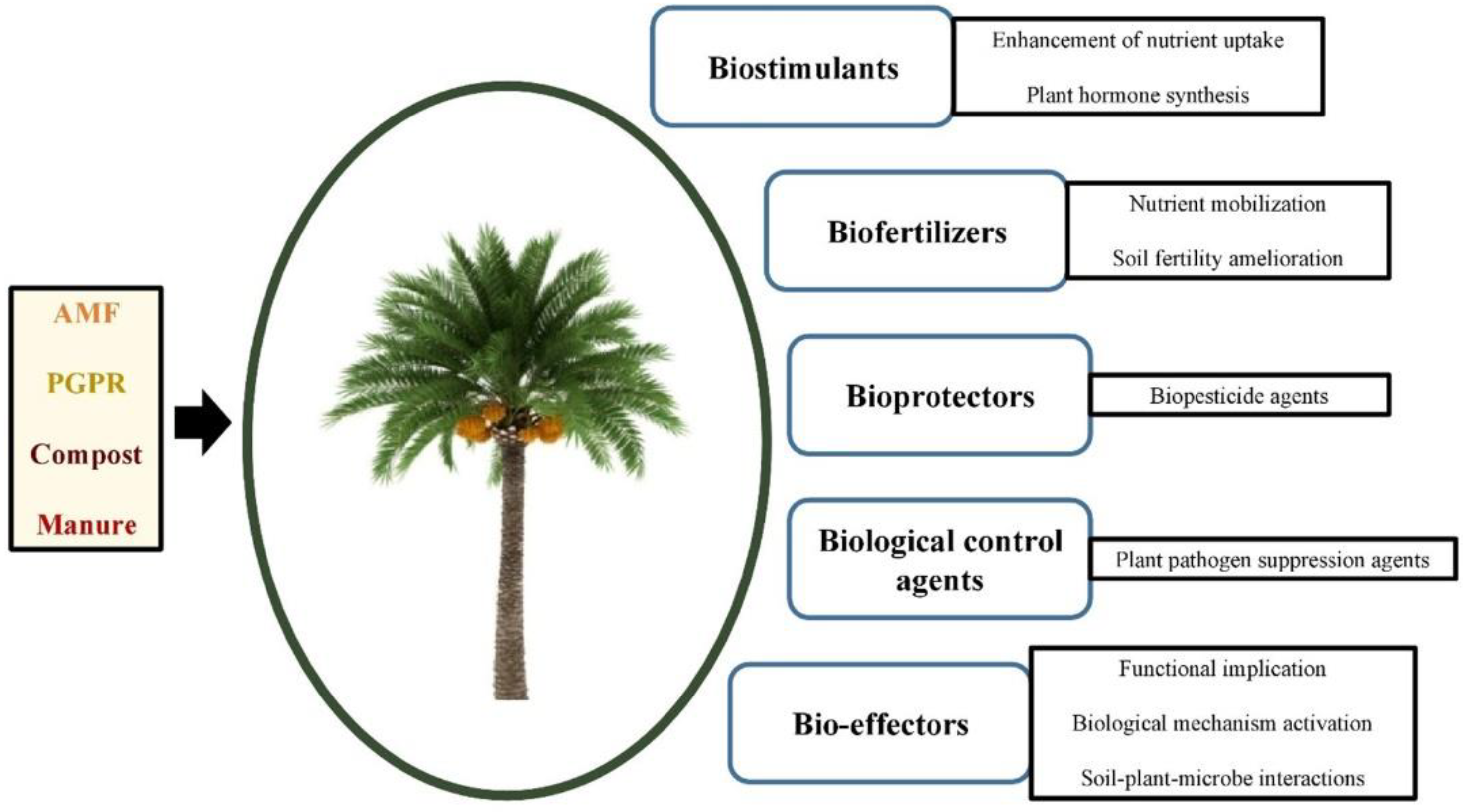
-
Synergistic and complementary effects
8. Biostimulants and Plant Hormones: Initiating and Suppressing Effects
8.1. AMF–Plant Hormones
8.2. PGPR–Plant Hormones
References
- Al-Ghussain, L.; Global Warming: Review on Driving Forces and Mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21, https://doi.org/10.1002/ep.13041.
- Papalexiou, S.M.; Montanari, A.; Global and Regional Increase of Precipitation Extremes Under GlobalWarming. Water Resour. Res. 2019, 55, 4901–4914, https://doi.org/10.1029/2018WR024067.
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N.; Climate Change, Food Security and Mycotoxins: DoWe Know Enough?. Fungal Biol. Rev. 2017, 31, 143–154, https://doi.org/10.1016/j.fbr.2017.04.002.
- Drobek, M.; Fra˛c, M.; Cybulska, J.; Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335, https://doi.org/10.3390/agronomy9060335.
- Minhas, P.S.; Rane, J.; Pasala, R.K. (Eds.). Abiotic Stress Management for Resilient Agriculture; Springer: Singapore, 2017; pp. ISBN 978-981-10-5743-4.
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K.; Traversing the Links between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12, https://doi.org/10.3389/fpls.2018.00012.
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al.et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, PH and Heavy Metals. Molecules 2020, 25, 5359, https://doi.org/10.3390/molecules25225359.
- Mabhaudhi, T.; Mpandeli, S.; Nhamo, L.; Chimonyo, V.G.P.; Nhemachena, C.; Senzanje, A.; Naidoo, D.; Modi, A.T.; Prospects for Improving Irrigated Agriculture in Southern Africa: Linking Water, Energy and Food. Water 2018, 10, 1881, https://doi.org/10.3390/w10121881.
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Soil Salinity, a Serious Environmental Issue and Plant Responses: A Metabolomics Perspective. Metabolites 2021, 11, 724, https://doi.org/10.3390/metabo11110724.
- Ouhaddou, R.; Ben-Laouane, R.; Lahlali, R.; Anli,M.; Ikan, C.; Boutasknit, A.; Slimani, A.; Oufdou, K.; Baslam,M.; Ait Barka, E.; et al.et al. Application of Indigenous Rhizospheric Microorganisms and Local Compost as Enhancers of Lettuce Growth, Development, and Salt Stress Tolerance. Microorganisms 2022, 10, 1625, https://doi.org/10.3390/microorganisms10081625.
- Shahid, S.A.; Zaman, M.; Heng, L.. Introduction to Soil Salinity, Sodicity and Diagnostics Techniques; Springer: Cham: Switzerland, 2018; pp. ISBN 9783319961897.
- Liu, L.; Li, W.; Song, W.; Guo, M.; Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci. Total Environ. 2018, 633, 206–219, https://doi.org/10.1016/j.scitotenv.2018.03.161.
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.Y.; Ahammed, G.J.; Qi, Z.Y.; Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 2017, 8, 1492, 10.3389/fpls.2017.01492.
- Mia, M.A.T.; Mosaib, M.G.; Khalil, M.I.; Islam, M.A.; Gan, S.H.; Potentials and Safety of Date Palm Fruit against Diabetes: A Critical Review. Foods 2020, 9, 1557, https://doi.org/10.3390/foods9111557.
- Shehzad, M.; Rasheed, H.; Naqvi, S.A.; Al-Khayri, J.M.; Lorenzo, J.M.; Alaghbari, M.A.; Manzoor, M.F.; Aadil, R.M.; Therapeutic Potential of Date Palm against Human Infertility: A Review. Metabolites 2021, 11, 408, https://doi.org/10.3390/metabo11060408.
- Al-Alawi, R.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y.; Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front. Plant Sci. 2017, 8, 845, https://doi.org/10.3389/fpls.2017.00845.
- Safronov, O.; Kreuzwieser, J.; Haberer, G.; Alyousif, M.S.; Schulze, W.; Al-Harbi, N.; Arab, L.; Ache, P.; Stempfl, T.; Kruse, J.; et al.et al. Detecting Early Signs of Heat and Drought Stress in Phoenix dactylifera (Date Palm). PLoS ONE 2017, 12, e0177883, https://doi.org/10.1371/journal.pone.0177883.
- Benaffari,W.; Boutasknit, A.; Anli, M.; Ait-El-mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ahmed, H.B.; Mitsui, T.; Baslam, M.; Meddich, A.; et al. The Native Arbuscular Mycorrhizal Fungi and Vermicompost-Based Organic Amendments Enhance Soil Fertility, Growth Performance, and the Drought Stress Tolerance of Quinoa. Plants 2022, 11, 393, https://doi.org/10.3390/plants11030393.
- Hasanuzzaman, M.; Hakeem, K.R.; Nahar, K.; Alharby, H.F.. Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Cham: Switzerland, 2019; pp. Volume 7, pp. 1–490.
- Jogawat, A.. Osmolytes and Their Role in Abiotic Stress Tolerance in Plants; In Molecular Plant Abiotic Stress; Wiley: New York: NY, USA, 2019; pp. 91–104, ISBN 9781119463665.
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V.; Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681, https://doi.org/10.3390/antiox9080681.
- Sarkar, T.; Thankappan, R.; Mishra, G.P.; Nawade, B.D.; Advances in the Development and Use of DREB for Improved Abiotic Stress Tolerance in Transgenic Crop Plants. Physiol. Mol. Biol. Plants 2019, 25, 1323–1334, https://doi.org/10.1007/s12298-019-00711-2.
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E.; In Silico Contact Pressure of Metal-on- Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241, https://doi.org/10.3390/met12081241.
- Mahmud, S.; Paul, G.K.; Afroze, M.; Islam, S.; Gupt, S.B.R.; Razu, M.H.; Biswas, S.; Zaman, S.; Uddin, M.S.; Khan, M.; et al.et al. Efficacy of Phytochemicals Derived from Avicennia Officinalis for the Management of COVID-19: A Combined In Silico and Biochemical Study. Molecules 2021, 26, 2210, https://doi.org/10.3390/molecules26082210.
- Ghirardo, A.; Nosenko, T.; Kreuzwieser, J.; Winkler, J.B.; Kruse, J.; Albert, A.; Schnitzler, J.P.; Protein Expression Plasticity Contributes to Heat and Drought Tolerance of Date Palm. Oecologia 2021, 197, 903–919, 10.1007/s00442-021-04907-w.
- Al-Harrasi, I.; Jana, G.A.; Patankar, H.V.; Al-Yahyai, R.; Rajappa, S.; Kumar, P.P.; Yaish, M.W.; A Novel Tonoplast Na+/H+ Antiporter Gene from Date Palm (PdNHX6) Confers Enhanced Salt Tolerance Response in Arabidopsis. Plant Cell Rep. 2020, 39, 1079–1093, https://doi.org/10.1007/s00299-020-02549-5.
- Rekik, I.; Chaâbene, Z.; Kriaa,W.; Rorat, A.; Franck, V.; Hafedh, M.; Elleuch, A.; Transcriptome Assembly and Abiotic Related Gene Expression Analysis of Date Palm Reveal Candidate Genes Involved in Response to Cadmium Stress. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2019, 225, 108569, https://doi.org/10.1016/j.cbpc.2019.108569.
- Jana, G.A.; Yaish, M.W.; Genome-Wide Identification and Functional Characterization of Glutathione Peroxidase Genes in Date Palm (Phoenix dactylifera L.) under Stress Conditions. Plant Gene 2020, 23, 100237, https://doi.org/10.1016/j.plgene.2020.100237.
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A.; The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5, https://doi.org/10.1186/s40538-017-0089-5.
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al.et al. Biofertilizers as Strategies to Improve Photosynthetic Apparatus, Growth, and Drought Stress Tolerance in the Date Palm. Front. Plant Sci. 2020, 11, 516818, https://doi.org/10.3389/fpls.2020.516818.
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R.; Insight Into the Role of PGPR in Sustainable Agriculture and Environment. Front. Sustain. Food Syst. 2021, 5, 183, 2021.
- Lahbouki, S.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Ait-El-Mokhtar, M.; El Gabardi, S.; Douira, A.; Wahbi, S.; Outzourhit, A.; et al.et al. Arbuscular Mycorrhizal Fungi and/or Organic Amendment Enhance the Tolerance of Prickly Pear (Opuntia Ficus-indica) under Drought Stress. J. Arid Environ. 2022, 199, 104703, https://doi.org/10.1016/j.jaridenv.2021.104703.
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K.; Roles of Arbuscular Mycorrhizal Fungi on Soil Fertility: Contribution in the Improvement of Physical, Chemical, and Biological Properties of the Soil. Front. Fungal Biol. 2022, 3, 723892, https://doi.org/10.3389/ffunb.2022.723892.
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R.; Insight Into the Role of PGPR in Sustainable Agriculture and Environment. Front. Sustain. Food Syst. 2021, 5, 183, https://doi.org/10.3389/fsufs.2021.667150.
- Slimani, A.; Raklami, A.; Oufdou, K.; Meddich, A.; Isolation and Characterization of PGPR and Their Potenzial for Drought Alleviation in Barley Plants. Gesunde Pflanz. 2022, -, 1–15, https://doi.org/10.1007/s10343-022-00709-z.
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I.; Composting Parameters and Compost Quality: A Literature Review. Org. Agric. 2018, 8, 141–158, https://doi.org/10.1007/s13165-017-0180-z.
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D.; Seed Germination Test for Toxicity Evaluation of Compost: Its Roles, Problems and Prospects. Waste Manag. 2018, 71, 109–114, https://doi.org/10.1016/j.wasman.2017.09.023.
- Whalen, J.K.; Thomas, B.W.; Sharifi, M.; Novel Practices and Smart Technologies to Maximize the Nitrogen Fertilizer Value of Manure for Crop Production in Cold Humid Temperate Regions. Adv. Agron. 2019, 153, 1–85, https://doi.org/10.1016/bs.agron.2018.09.002.
- Chao, C.C.T.; Krueger, R.R.; The Date Palm (Phoenix dactylifera L.): Overview of Biology, Uses, and Cultivation. HortScience 2007, 42, 1077–1082, https://doi.org/10.21273/HORTSCI.42.5.1077.
- Gros-Balthazard, M.; Hazzouri, K.; Flowers, J.; Genomic Insights into Date Palm Origins. Genes 2018, 9, 502, https://doi.org/10.3390/genes9100502.
- Sallon, S.; Solowey, E.; Cohen, Y.; Korchinsky, R.; Egli, M.; Woodhatch, I.; Simchoni, O.; Kislev, M.; Germination, Genetics, and Growth of an Ancient Date Seed. Science 2008, 320, 1464, DOI: 10.1126/science.1153600.
- El-Juhany, L.I.; Degradation of Date Palm Trees and Date Production in Arab Countries: Causes and Potential Rehabilitation. Aust. J. Basic Appl. Sci. 2010, 4, 3998–4010, https://doi.org/10.1016/j.radphyschem.2006.10.004.
- Sghaier-Hammami, B.; Baazaoui, N.; Drira, R.; Drira, N.; Jorrín-Novo, J.V.; Proteomic Insights of Date Palm Embryogenesis and Responses to Environmental Stress. In The Date Palm Genome; Springer: Cham, Switzerland 2021, 2, 85–99, ISBN 978-3-030-73746-7.
- Hussain, M.I.; Farooq, M.; Syed, Q.A.; Nutritional and Biological Characteristics of the Date Palm Fruit (Phoenix dactylifera L.)—A Review. Food Biosci. 2020, 34, 100509, https://doi.org/10.1016/j.fbio.2019.100509.
- Mihi, A.; Tarai, N.; Chenchouni, H.; Can Palm Date Plantations and Oasification Be Used as a Proxy to Fight Sustainably against Desertification and Sand Encroachment in Hot Drylands?. Ecol. Indic. 2019, 105, 365–375, https://doi.org/10.1016/j.ecolind.2017.11.027.
- Raho, O.; Boutasknit, A.; Anli, M.; Ben-Laouane, R.; Rahou, Y.A.; Ouhaddou, R.; Duponnois, R.; Douira, A.; Modafar, C.; El Meddich, A.; et al. Impact of Native Biostimulants/Biofertilizers and Their Synergistic Interactions On the Agro-Physiological and Biochemical Responses of Date Palm Seedlings. Gesunde Pflanz. 2022, 74, 1053–1069, https://doi.org/10.1007/s10343-022-00668-5.
- Al-Dosary, N.M.N.; Al-Dobai, S.; Faleiro, J.R.; Review on the Management of Red PalmWeevil Rhynchophorus ferrugineus Olivier in Date Palm Phoenix dactylifera L.. Emir. J. Food Agric. 2016, 28, 34–44, doi: 10.9755/ejfa.2015-10-897.
- Ibrahim, K.M.; The Role of Date Palm Tree in Improvement of the Environment. Acta Hortic. 2010, 882, 777–778, 10.17660/ActaHortic.2010.882.87.
- Al-Hammadi, M.S.; Al-Shariqi, R.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M.; Molecular Identification of Fungal Pathogens Associated with Date Palm Root Diseases in the United Arab Emirates. J. Plant Pathol. 2019, 101, 141–147, https://doi.org/10.1007/s42161-018-0089-8.
- Saijo, Y.; Loo, E.P.; Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytol. 2020, 225, 87–104, https://doi.org/10.1111/nph.15989.
- Alikhani-Koupaei, M.; Fatahi, R.; Zamani, Z.; Salimi, S.; Effects of Deficit Irrigation on Some Physiological Traits, Production and Fruit Quality of ‘Mazafati’ Date Palm and the Fruit Wilting and Dropping Disorder. Agric. Water Manag. 2018, 209, 219–227, https://doi.org/10.1016/j.agwat.2018.07.024.
- Sakran, M.I.; El Rabey, H.A.; Almulaiky, Y.Q.; Al-Duais, M.A.; Elbakry, M.; Faridi, U.; The Antioxidant Enzymatic Activity of Date Palm Seedlings under Abiotic Drought Stress. Indian J. Pharm. Educ. Res. 2018, 52, 442–448, doi:10.5530/ijper.52.3.51.
- Al-Khateeb, S.A.; Al-Khateeb, A.A.; El-Beltagi, H.S.; Sattar, M.N.; Genotypic Variation for Drought Tolerance in Three Date Palm (Phoenix dactylifera L.) Cultivars. Fresenius Environ. Bull. 2019, 28, 4671–4683.
- Kruse, J.; Adams, M.; Winkler, B.; Ghirardo, A.; Alfarraj, S.; Kreuzwieser, J.; Hedrich, R.; Schnitzler, J.P.; Rennenberg, H.; Optimization of Photosynthesis and Stomatal Conductance in the Date Palm Phoenix dactylifera during Acclimation to Heat and Drought. New Phytol. 2019, 223, 1973–1988, https://doi.org/10.1111/nph.15923.
- Zein El Din, A.F.M.; Ibrahim, M.F.M.; Farag, R.; Abd El-Gawad, H.G.; El-Banhawy, A.; Alaraidh, I.A.; Rashad, Y.M.; Lashin, I.; Abou El-Yazied, A.; Elkelish, A.; et al.et al. Influence of Polyethylene Glycol on Leaf Anatomy, Stomatal Behavior,Water Loss, and Some Physiological Traits of Date Palm Plantlets Grown In Vitro and Ex Vitro. Plants 2020, 9, 1440, https://doi.org/10.3390/plants9111440.
- Du, B.; Kruse, J.; Winkler, J.B.; Alfarraj, S.; Albasher, G.; Schnitzler, J.P.; Ache, P.; Hedrich, R.; Rennenberg, H.; Metabolic Responses of Date Palm (Phoenix dactylifera L.) Leaves to Drought Differ in Summer andWinter Climate. Tree Physiol. 2021, 41, 1685–1700, https://doi.org/10.1093/treephys/tpab027.
- Mattar, M.A.; Soliman, S.S.; Al-Obeed, R.S.; Effects of Various Quantities of Three Irrigation Water Types on Yield and Fruit Quality of ‘Succary’ Date Palm. Agronomy 2021, 11, 796, https://doi.org/10.3390/agronomy11040796.
- Alnaim, M.A.; Mohamed, M.S.; Mohammed, M.; Munir, M.; Effects of Automated Irrigation Systems and Water Regimes on Soil Properties, Water Productivity, Yield and Fruit Quality of Date Palm. Agriculture 2022, 12, 343, https://doi.org/10.3390/agriculture12030343.
- Al Kharusi, L.; Assaha, D.; Al-Yahyai, R.; Yaish, M.; Screening of Date Palm (Phoenix dactylifera L.) Cultivars for Salinity Tolerance. Forests 2017, 8, 136, https://doi.org/10.3390/f8040136.
- Bouhouch, S.; Eshelli, M.; Slama, H.B.; Bouket, A.C.; Oszako, T.; Okorski, A.; Rateb, M.E.; Belbahri, L.; Morphological, Biochemical, and Metabolomic Strategies of the Date Palm (Phoenix dactylifera L., Cv. Deglet Nour) Roots Response to Salt Stress. Agronomy 2021, 11, 2389, https://doi.org/10.3390/agronomy11122389.
- Dghaim, R.; Hammami, Z.; Al Ghali, R.; Smail, L.; Haroun, D.; Dghaim, R.; Hammami, Z.; Al Ghali, R.; Smail, L.; Haroun, D. The Mineral Composition of Date Palm Fruits (Phoenix dactylifera L.) under Low to High Salinity Irrigation. Molecules 2021, 26, 7361, https://doi.org/10.3390/molecules26237361.
- Yaish, M.W.; Patankar, H.V.; Assaha, D.V.M.; Zheng, Y.; Al-Yahyai, R.; Sunkar, R.; Genome-Wide Expression Profiling in Leaves and Roots of Date Palm (Phoenix dactylifera L.) Exposed to Salinity. BMC Genom. 2017, 18, 246, https://doi.org/10.1186/s12864-017-3633-6.
- Al-Harrasi, I.; Al-Yahyai, R.; Yaish, M.W.; Differential DNA Methylation and Transcription Profiles in Date Palm Roots Exposed to Salinity. PLoS ONE 2018, 13, e0191492, https://doi.org/10.1371/journal.pone.0191492.
- Al Kharusi, L.; Sunkar, R.; Al-Yahyai, R.; Yaish, M.W.; ComparativeWater Relations of Two Contrasting Date Palm Genotypes under Salinity. Int. J. Agron. 2019, 2019, 4262013, https://doi.org/10.1155/2019/4262013.
- Al Kharusi, L.; Al Yahyai, R.; Yaish, M.W.; Antioxidant Response to Salinity in Salt-Tolerant and Salt-Susceptible Cultivars of Date Palm. Agriculture 2019, 9, 8, https://doi.org/10.3390/agriculture9010008.
- Jana, G.A.; Al Kharusi, L.; Sunkar, R.; Al-Yahyai, R.; Yaish, M.W.; Metabolomic Analysis of Date Palm Seedlings Exposed to Salinity and Silicon Treatments. Plant Signal. Behav. 2019, 14, 1663112, https://doi.org/10.1080/15592324.2019.1663112.
- Al-Muaini, A.; Green, S.; Dakheel, A.; Abdullah, A.H.; Sallam, O.; Abou Dahr, W.A.; Dixon, S.; Kemp, P.; Clothier, B.; Water Requirements for Irrigation with Saline Groundwater of Three Date-Palm Cultivars with Different Salt-Tolerances in the Hyper- Arid United Arab Emirates. Agric. Water Manag. 2019, 222, 213–220, https://doi.org/10.1016/j.agwat.2019.05.022.
- Abdelhadi, A.W.; Salih, A.A.; Sultan, K.; Alsafi, A.; Tashtoush, F.; Actual Water Use of Young Date Palm Trees As Affected By Aminolevulinic Acid Application and Different Irrigation Water Salinities. Irrig. Drain. 2020, 69, 427–440, https://doi.org/10.1002/ird.2414.
- Serret, M.D.; Al-Dakheel, A.J.; Yousfi, S.; Fernáandez-Gallego, J.A.; Elouafi, I.A.; Araus, J.L.; Vegetation Indices Derived from Digital Images and Stable Carbon and Nitrogen Isotope Signatures as Indicators of Date Palm Performance under Salinity. Agric. Water Manag. 2020, 230, 105949, https://doi.org/10.1016/j.agwat.2019.105949.
- Dghaim, R.; Hammami, Z.; Al Ghali, R.; Smail, L.; Haroun, D.; The Mineral Composition of Date Palm Fruits (Phoenix dactylifera L.) under Low to High Salinity Irrigation. Molecules 2021, 26, 7361, https://doi.org/10.3390/molecules26237361.
- Al Kharusi, L.; Jana, G.A.; Patankar, H.V.; Yaish, M.W.; Comparative Metabolic Profiling of Two Contrasting Date Palm Genotypes Under Salinity. Plant Mol. Biol. Rep. 2021, 39, 351–363, https://doi.org/10.1007/s11105-020-01255-6.
- Zemni, N.; Slama, F.; Bouksila, F.; Bouhlila, R.; Simulating and Monitoring Water Flow, Salinity Distribution and Yield Production under Buried Diffuser Irrigation for Date Palm Tree in Saharan Jemna Oasis (North Africa). Agric. Ecosyst. Environ. 2022, 325, 107772, https://doi.org/10.1016/j.agee.2021.107772.
- Chaâbene, Z.; Rorat, A.; Rekik Hakim, I.; Bernard, F.; Douglas, G.C.; Elleuch, A.; Vandenbulcke, F.; Mejdoub, H.; Insight into the Expression Variation of Metal-Responsive Genes in the Seedling of Date Palm (Phoenix dactylifera). Chemosphere 2018, 197, 123–134, https://doi.org/10.1016/j.chemosphere.2017.12.146.
- Salama, K.F.; Randhawa, M.A.; Al Mulla, A.A.; Labib, O.A.; Heavy Metals in Some Date Palm Fruit Cultivars in Saudi Arabia and Their Health Risk Assessment. Int. J. Food Prop. 2019, 22, 1684–1692, https://doi.org/10.1080/10942912.2019.1671453.
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F.; Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 505, https://doi.org/10.3390/metabo10120505.
- Rouphael, Y.; Colla, G.; Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40, https://doi.org/10.3389/fpls.2020.00040.
- Shareef, H.J.; Al-Tememi, I.H.; Abdi, G.; Foliar Nutrition of Date Palm: Advances and Applications. A Review. Folia Oecologica 2021, 48, 82–99, https://doi.org/10.2478/foecol-2021-0010.
- Harkousse, O.; Slimani, A.; Jadrane, I.; Aitboulahsen, M.; Mazri, M.A.; Zouahri, A.; Ouahmane, L.; Koussa, T.; Najib, M.; Feddy, A.; et al. Role of Local Biofertilizer in Enhancing the Oxidative Stress Defence Systems of Date Palm Seedling (Phoenix dactylifera) against Abiotic Stress. Appl. Environ. Soil Sci. 2021, 2021, 6628544, https://doi.org/10.1155/2021/6628544.
- Akensous, F.-Z.; Anli, M.; Boutasknit, A.; Ben-Laouane, R.; Ait-Rahou, Y.; Ahmed, H.B.; Nasri, N.; Hafidi, M.; Meddich, A.; Boosting Date Palm (Phoenix dactylifera L.) Growth under Drought Stress: Effects of Innovative Biostimulants. Gesunde Pflanz. 2022, 74, 961–982, https://doi.org/10.1007/s10343-022-00651-0.
- Glick, B.; Glick, B.; Glick, B.; Eid, A.; Salem, S.S.; Glick, B.R.; Isolation and Characterization of Endophytic Plant Growth- Promoting Bacteria from Date Palm Tree (Phoenix dactylifera L.) and Their Potential Role in Salinity Tolerance. Antonie Van Leeuwenhoek 2015, 107, 1519–1532, https://doi.org/10.1007/s10482-015-0445-z.
- Ait-El-Mokhtar, M.; Laouane, R.B.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A.; Use of Mycorrhizal Fungi in Improving Tolerance of the Date Palm (Phoenix dactylifera L.) Seedlings to Salt Stress. Sci. Hortic. 2019, 253, 429–438, https://doi.org/10.1016/j.scienta.2019.04.066.
- Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A.; Alleviation of Detrimental Effects of Salt Stress on Date Palm (Phoenix dactylifera L.) by the Application of Arbuscular Mycorrhizal Fungi and/or Compost. Front. Sustain. Food Syst. 2020, 4, 131, https://doi.org/10.3389/fsufs.2020.00131.
- Chebaane, A.; Symanczik, S.; Oehl, F.; Azri, R.; Gargouri, M.; Mäder, P.; Mliki, A.; Fki, L.; Arbuscular Mycorrhizal Fungi Associated with Phoenix dactylifera L. Grown in Tunisian Sahara Oases of Different Salinity Levels. Symbiosis 2020, 81, 173–186, https://doi.org/10.1007/s13199-020-00692-x.
- Toubali, S.; Tahiri, A.; Anli, M.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Oufdou, K.; Ait-Rahou, Y.; Ben-Ahmed, H.; et al.et al. Physiological and Biochemical Behaviors of Date Palm Vitroplants Treated with Microbial Consortia and Compost in Response to Salt Stress. Appl. Sci. 2020, 10, 8665, https://doi.org/10.3390/app10238665.
- Outamamat, E.; Bourhia, M.; Dounas, H.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Al Feddy, M.N.; Mnasri, B.; Ouahmane, L.; et al. Application of Native or Exotic Arbuscular Mycorrhizal Fungi Complexes and Monospecific Isolates from Saline Semi-Arid Mediterranean Ecosystems Improved Phoenix dactylifera’s Growth and Mitigated Salt Stress Negative Effects. Plants 2021, 10, 2501, https://doi.org/10.3390/plants10112501.
- Elsadig, E.H.; Aljuburi, H.J.; Elamin, A.H.B.; Gafar, M.O.; Impact of Organic Manure and Combination of N P K S, on Yield, Fruit Quality and Fruit Mineral Content of Khenazi Date Palm (Phoenix dactylifera L.) Cultivar. J. Sci. Agric. 2017, 1, 335, doi: 10.25081/jsa.2017.v1.848.
- Ghadbane, M.; Medjekal, S.; Benderradji, L.; Belhadj, H.; Daoud, H.; Assessment of Arbuscular Mycorrhizal Fungi Status and Rhizobium on Date Palm (Phoenix dactylifera L.) Cultivated in a Pb Contaminated Soil.. Springer: Cham, Switzerland 2021, -, 703–707, https://doi.org/10.1007/978-3-030-51210-1_111.
- Sugumar, T.; Srinivasan, P.; Muthukumar, B.; Natarajan, E.; Whole Genome Sequencing and Genome Annotation of PGPR ‘Exiguobacterium Sp. TNDT2’ Isolated from Dates Palm Tree Rhizospheric Soil. Indian J. Agric. Res. 2021, 1, 1–7, DOI: 10.18805/IJARe.A-5665.
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S.; Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370, https://doi.org/10.3390/d12100370.
- Hafez, M.; Abdallah, A.M.; Mohamed, A.E.; Rashad, M.; Influence of Environmental-Friendly Bio-Organic Ameliorants on Abiotic Stress to Sustainable Agriculture in Arid Regions: A Long Term Greenhouse Study in Northwestern Egypt. J. King Saud Univ.-Sci. 2022, 34, 102212, https://doi.org/10.1016/j.jksus.2022.102212.
- Bonini, P.; Rouphael, Y.; Miras-Moreno, B.; Lee, B.; Cardarelli, M.; Erice, G.; Cirino, V.; Lucini, L.; Colla, G.; Microbial-Based Biostimulant Enhances Sweet Pepper Performance by Metabolic Reprogramming of Phytohormone Profile and Secondary Metabolism. Front. Plant Sci. 2020, 11, 567388, https://doi.org/10.3389/fpls.2020.567388.
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P.; Biofertilizers: A Potential Approach for Sustainable Agriculture Development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335, https://doi.org/10.1007/s11356-016-8104-0.
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L.; Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Their Synergistic Interactions to Counteract the Negative Effects of Saline Soil on Agriculture: Key Macromolecules and Mechanisms. Microorganisms 2021, 9, 1491, https://doi.org/10.3390/microorganisms9071491.
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A.; Crosstalk amongst Phytohormones from Planta and PGPR under Biotic and Abiotic Stresses. Plant Growth Regul. 2020, 90, 189–203, https://doi.org/10.1007/s10725-020-00571-x.
- Masters-Clark, E.; Shone, E.; Paradelo, M.; Hirsch, P.R.; Clark, I.M.; Otten,W.; Brennan, F.; Mauchline, T.H.; Development of a Defined Compost System for the Study of Plant-Microbe Interactions. Sci. Rep. 2020, 10, 7521, https://doi.org/10.1038/s41598-020-64249-0.
- Bulgari, R.; Franzoni, G.; Ferrante, A.; Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306, https://doi.org/10.3390/agronomy9060306.
- Lephatsi, M.M.; Meyer, V.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Plant Responses to Abiotic Stresses and Rhizobacterial Biostimulants: Metabolomics and Epigenetics Perspectives. Metabolites 2021, 11, 457, https://doi.org/10.3390/metabo11070457.
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R.; of Reactive Oxygen Species and Hormone Signaling during Abiotic Stress. Plant J. 2021, 105, 459–476, https://doi.org/10.1111/tpj.15010.
- Bhoi, A.; Yadu, B.; Chandra, J.; Keshavkant, S.; Contribution of Strigolactone in Plant Physiology, Hormonal Interaction and Abiotic Stresses. Planta 2021, 254, 28, https://doi.org/10.1007/s00425-021-03678-1.
- Saeed, W.; Naseem, S.; Ali, Z.; Strigolactones Biosynthesis and Their Role in Abiotic Stress Resilience in Plants: A Critical Review. Front. Plant Sci. 2017, 8, 1487, https://doi.org/10.3389/fpls.2017.01487.
- Fernández-Lizarazo, J.C.; Moreno-Fonseca, L.P.; Mechanisms for Tolerance to Water-Deficit Stress in Plants Inoculated with Arbuscular Mycorrhizal Fungi: A Review. Agron. Colomb. 2016, 34, 179–189, https://doi.org/10.15446/agron.colomb.v34n2.55569.
- Bakshi, P.; Handa, N.; Gautam, V.; Kaur, P.; Sareen, S.; Mir, B.A.; Bhardwaj, R.; Role and Regulation of Plant Hormones as a Signal Molecule in Response to Abiotic Stresses. Plant Signal. Mol. Role Regul. Stress. Environ. 2019, -, 303–317, https://doi.org/10.1016/B978-0-12-816451-8.00018-6.
- Rehman, A.; Azhar, M.T.; Hinze, L.; Qayyum, A.; Li, H.; Peng, Z.; Qin, G.; Jia, Y.; Pan, Z.; He, S.; et al.et al. Insight into Abscisic Acid Perception and Signaling to Increase Plant Tolerance to Abiotic Stress. J. Plant Interact. 2021, 16, 222–237, https://doi.org/10.1080/17429145.2021.1925759.
- Li, T.; Sun, Y.; Ruan, Y.; Xu, L.; Hu, Y.; Hao, Z.; Zhang, X.; Li, H.; Wang, Y.; Yang, L.; et al.et al. Potential Role of D-Myo-Inositol- 3-Phosphate Synthase and 14-3-3 Genes in the Crosstalk between Zea mays and Rhizophagus intraradices under Drought Stress. Mycorrhiza 2016, 26, 879–893, https://doi.org/10.1007/s00572-016-0723-2.
- Tsukanova, K.A.; Chebotar, V.; Meyer, J.J.M.; Bibikova, T.N.; Effect of Plant Growth-Promoting Rhizobacteria on Plant Hormone Homeostasis. S. African J. Bot. 2017, 113, 91–102, https://doi.org/10.1016/j.sajb.2017.07.007.
- Sami, F.; Faizan, M.; Faraz, A.; Siddiqui, H.; Yusuf, M.; Hayat, S.; Nitric Oxide-Mediated Integrative Alterations in Plant Metabolism to Confer Abiotic Stress Tolerance, NO Crosstalk with Phytohormones and NO-Mediated Post Translational Modifications in Modulating Diverse Plant Stress. Nitric Oxide-Biol. Chem. 2018, 73, 22–38, https://doi.org/10.1016/j.niox.2017.12.005.
- ˙Ipek, M.; Mutluay, E.. Enhancing the Physiological and Molecular Responses of Horticultural Plants to Drought Stress through Plant Growth–Promoting Rhizobacterias; Elsevier: Amsterdam: The Netherlands, 2022; pp. 185–199.
- Korver, R.A.; Koevoets, I.T.; Testerink, C.; Out of Shape During Stress: A Key Role for Auxin. Trends Plant Sci. 2018, 23, 783–793, DOI:https://doi.org/10.1016/j.tplants.2018.05.01.
- He, J.; Xin, P.; Ma, X.; Chu, J.; Wang, G.; Gibberellin Metabolism in Flowering Plants: An Update and Perspectives. Front. Plant Sci. 2020, 11, 5–10, https://doi.org/10.3389/fpls.2020.00532.
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T.; Cytokinin Action in Response to Abiotic and Biotic Stresses in Plants. Plant. Cell Environ. 2019, 42, 998–1018, https://doi.org/10.1111/pce.13494.
- Prakash, V.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Corpas, F.J.; Nitric Oxide (NO) and Salicylic Acid (SA): A Framework for Their Relationship in Plant Development under Abiotic Stress. Plant Biol. 2021, 23, 39–49, https://doi.org/10.1111/plb.13246.
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B.; Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568, https://doi.org/10.3390/ijms22168568.
- Bleecker, A.B.; Kende, H.; Ethylene: A Gaseous Signal Molecule in Plants.. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18, https://doi.org/10.1146/annurev.cellbio.16.1.1.
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Prasad, S.M.; Singh, V.P.; A Brief Appraisal of Ethylene Signaling under Abiotic Stress in Plants. Plant Signal. Behav. 2020, 15, 1782051, https://doi.org/10.1080/15592324.2020.1782051.




