
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Koushik Halder | -- | 2262 | 2022-12-08 20:40:52 | | | |
| 2 | Vivi Li | Meta information modification | 2262 | 2022-12-09 04:15:37 | | |
Video Upload Options
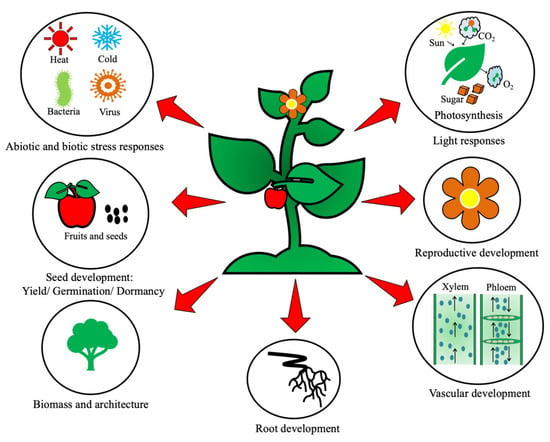
Brassinosteroid hormones (BRs) multitask to smoothly regulate a broad spectrum of vital physiological processes in plants, such as cell division, cell expansion, differentiation, seed germination, xylem differentiation, reproductive development and light responses (photomorphogenesis and skotomorphogenesis). Their importance is inferred when visible abnormalities arise in plant phenotypes due to suboptimal or supraoptimal hormone levels. This group of steroidal hormones are major growth regulators, having pleiotropic effects and conferring abiotic stress resistance to plants. Numerous abiotic stresses are the cause of significant loss in agricultural yield globally. However, plants are well equipped with efficient stress combat machinery. Scavenging reactive oxygen species (ROS) is a unique mechanism to combat the deleterious effects of abiotic stresses.
1. Introduction
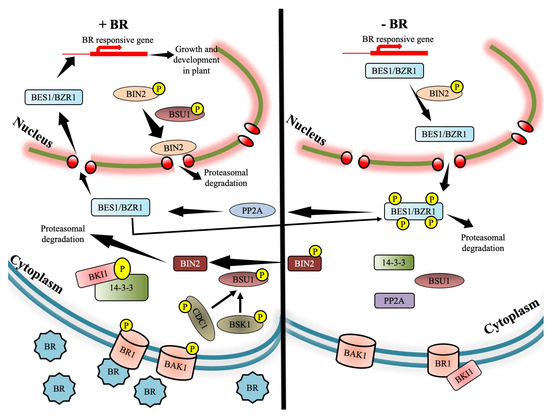
2. Brassinosteroid Signaling Cascade
2.1. Jump-Start of BRI1 Receptor Kinase by Brassinosteroid
2.2. Interaction of BRI1 with Receptor Complex Associates
2.3. Joining the Dots to Complete the BR Signaling Cascade
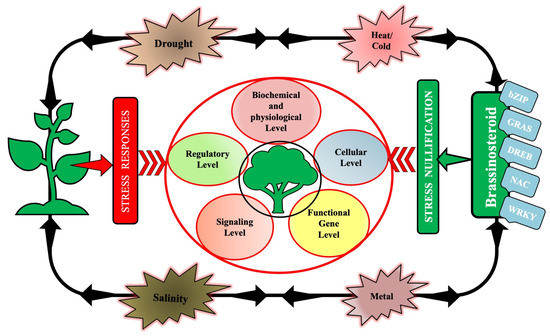
3. Signaling and Regulation by BRs (Endogenous and Exogenous) in Plants under Abiotic Stress
References
- Hasanuzzaman, M. Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 1–859. ISBN 9789811521560.
- Saddiq, M.S.; Afzal, I.; Iqbal, S.; Hafeez, M.B.; Raza, A. Low Leaf Sodium Content Improves the Grain Yield and Physiological Performance of Wheat Genotypes in Saline-Sodic Soil. Pesqui. Agropecu. Trop. 2021, 51.
- Zahra, N.; Mahmood, S.; Raza, Z.A. Salinity Stress on Various Physiological and Biochemical Attributes of Two Distinct Maize (Zea mays L.) Genotypes. J. Plant Nutr. 2018, 41, 1368–1380.
- Zahra, N.; Wahid, A.; Shaukat, K.; Rasheed, T. Role of Seed Priming and Foliar Spray of Calcium in Improving Flag Leaf Growth, Grain Filling and Yield Characteristics in Wheat (Triticum aestivum)—A Field Appraisal. Int. J. Agric. Biol. 2020, 24, 1591–1600.
- Wei, Z.; Li, J. Brassinosteroids Regulate Root Growth, Development, and Symbiosis. Mol. Plant 2016, 9, 86–100.
- Nazir, F.; Hussain, A.; Fariduddin, Q. Interactive Role of Epibrassinolide and Hydrogen Peroxide in Regulating Stomatal Physiology, Root Morphology, Photosynthetic and Growth Traits in Solanum lycopersicum L. under Nickel Stress. Environ. Exp. Bot. 2019, 162, 479–495.
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids Make Plant Life Easier under Abiotic Stresses Mainly by Modulating Major Components of Antioxidant Defense System. Front. Environ. Sci. 2015, 2, 67.
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Khan, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and Physiological Responses in Plant Growth and Abiotic Stresses. Plant Stress 2021, 2, 100029.
- Mubarik, M.S.; Khan, S.H.; Sajjad, M.; Raza, A.; Hafeez, M.B.; Yasmeen, T.; Rizwan, M.; Ali, S.; Ali, S.; Arif, M.S. A Manipulative Interplay between Positive and Negative Regulators of Phytohormones: A Way Forward for Improving Drought Tolerance in Plants. Physiol. Plant 2021, 172, 1269–1290.
- Planas-Riverola, A.; Gupta, A.; Betegoń-Putze, I.; Bosch, N.; Ibañes, M.; Cano-Delgado, A.I. Brassinosteroid Signaling in Plant Development and Adaptation to Stress. Development 2019, 146, dev151894.
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and, Physiological Functions in Plants under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061.
- Zhang, C.; Bai, M.; Chong, K. Brassinosteroid-Mediated Regulation of Agronomic Traits in Rice. Plant Cell Rep. 2014, 33, 683–696.
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined Effects of Brassinosteroid and Kinetin Mitigates Salinity Stress in Tomato through the Modulation of Antioxidant and Osmolyte Metabolism. Plant Physiol. Biochem. 2020, 147, 31–42.
- Ahammed, G.J.; Li, X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Enhanced Photosynthetic Capacity and Antioxidant Potential Mediate Brassinosteriod-Induced Phenanthrene Stress Tolerance in Tomato. Environ. Pollut. 2015, 201, 58–66.
- Divi, U.K.; Rahman, T.; Krishna, P. Gene Expression and Functional Analyses in Brassinosteroid-Mediated Stress Tolerance. Plant Biotechnol. J. 2016, 14, 419–432.
- Tang, W.; Deng, Z.; Wang, Z.Y. Proteomics shed light on the brassinosteroid signaling mechanisms. Curr. Opin. Plant Biol. 2010, 13, 27–33.
- Johnson, K.L.; Ingram, G.C. Sending the Right Signals: Regulating Receptor Kinase Activity. Curr. Opin. Plant Biol. 2005, 8, 648–656.
- Vert, G.; Nemhauser, J.L.; Geldner, N.; Hong, F.; Chory, J. Molecular Mechanisms of Steroid Hormone Signaling in Plants. Annu. Rev. Cell Dev. Biol. 2005, 21, 177–201.
- Tang, W.; Tae-Wuk, K.; Oses-Prieto, J.A.; Yu, S.; Deng, Z.; Zhu, S.; Wang, R.; Burlingame, A.L.; Wang, Z.-Y. BSKs Mediate Signal Transduction from the Receptor Kinase BRI1 in Arabidopsis. Science 2008, 321, 557–560.
- Kim, T.W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid Signal Transduction from Cell-Surface Receptor Kinases to Nuclear Transcription Factors. Nat. Cell Biol. 2009, 11, 1254–1260.
- Karlova, R.; Boeren, S.; Van Dongen, W.; Kwaaitaal, M.; Aker, J.; Vervoort, J.; De Vries, S. Identification of in Vitro Phosphorylation Sites in the Arabidopsis Thaliana Somatic Embryogenesis Receptor-like Kinases. Proteomics 2009, 9, 368–379.
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Huber, S.C.; Clouse, S.D. Sequential Transphosphorylation of the BRI1/BAK1 Receptor Kinase Complex Impacts Early Events in Brassinosteroid Signaling. Dev. Cell 2008, 15, 220–235.
- Wang, X.; Goshe, M.B.; Soderblom, E.J.; Phinney, B.S.; Kuchar, A.; Li, J.; Asami, T.; Yoshida, S.; Huber, S.C.; Clouse, S.D.; et al. Identification and Functional Analysis of in Vivo Phosphorylation Sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 Receptor Kinase. Plant Cell 2005, 17, 1685–1703.
- Kinoshita, T.; Caño-Delgado, A.; Seto, H.; Hiranuma, S.; Fujioka, S.; Yoshida, S.; Chory, J. Binding of Brassinosteroids to the Extracellular Domain of Plant Receptor Kinase BRI1. Nature 2005, 433, 167–171.
- Wang, X.; Li, X.; Meisenhelder, J.; Hunter, T.; Yoshida, S.; Asami, T.; Chory, J. Autoregulation and Homodimerization Are Involved in the Activation of the Plant Steroid Receptor BRI1. Dev. Cell 2005, 8, 855–865.
- Wang, X.; Chory, J. Brassinoteroids Regulate Dissociation of BKI1, a Negative Regulator of BRI1 Signaling, from the Plasma Membrane. Science 2006, 313, 1118–1122.
- Wang, Z.-Y.; Seto, H.; Fujioka, S.; Yoshida, S.; Chory, J. BRI1 Is a Critical Component of a Plasma-Membrane Receptor for Plants Steroids. Nature 2001, 410, 380–383.
- Nam, K.H.; Li, J. BRI1/BAK1, a Receptor Kinase Pair Mediating Brassinosteroid Signaling. Cell 2002, 110, 203–212.
- Li, J.; Wen, J.; Lease, K.A.; Doke, J.T.; Tax, F.E.; Walker, J.C. BAK1, an Arabidopsis LRR Receptor-like Protein Kinase, Interacts with BRI1 and Modulates Brassinosteroid Signaling. Cell 2002, 110, 213–222.
- He, J.X.; Gendron, J.M.; Yang, Y.; Li, J.; Wang, Z.Y. The GSK3-like Kinase BIN2 Phosphorylates and Destabilizes BZR1, a Positive Regulator of the Brassinosteroid Signaling Pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 10185–10190.
- Yin, Y.; Wang, Z.Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 Accumulates in the Nucleus in Response to Brassinosteroids to Regulate Gene Expression and Promote Stem Elongation. Cell 2002, 109, 181–191.
- Vert, G.; Chory, J. Downstream Nuclear Events in Brassinosteroid Signalling. Nature 2006, 441, 96–100.
- Gampala, S.S.; Kim, T.W.; He, J.X.; Tang, W.; Deng, Z.; Bai, M.Y.; Guan, S.; Lalonde, S.; Sun, Y.; Gendron, J.M.; et al. An Essential Role for 14-3-3 Proteins in Brassinosteroid Signal Transduction in Arabidopsis. Dev. Cell 2007, 13, 177–189.
- Ryu, H.; Kim, K.; Cho, H.; Park, J.; Choe, S.; Hwang, I. Nucleocytoplasmic Shuttling of BZR1 Mediated by Phosphorylation Is Essential in Arabidopsis Brassinosteroid Signaling. Plant Cell 2007, 19, 2749–2762.
- Mora-García, S.; Vert, G.; Yin, Y.; Caño-Delgado, A.; Cheong, H.; Chory, J. Nuclear Protein Phosphatases with Kelch-Repeat Domains Modulate the Response to Brassinosteroids in Arabidopsis. Genes Dev. 2004, 18, 448–460.
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The Physiological and Molecular Mechanism of Brassinosteroid in Response to Stress: A Review. Biol. Res. 2018, 51, 1–15.
- Nolan, T.; Chen, J.; Yin, Y. Cross-Talk of Brassinosteroid Signaling in Controlling Growth and Stress Responses. Biochem. J. 2017, 474, 2461–2661.
- Fàbregas, N.; Lozano-Elena, F.; Blasco-Escámez, D.; Tohge, T.; Martínez-Andújar, C.; Albacete, A.; Osorio, S.; Bustamante, M.; Riechmann, J.L.; Nomura, T.; et al. Overexpression of the Vascular Brassinosteroid Receptor BRL3 Confers Drought Resistance without Penalizing Plant Growth. Nat. Commun. 2018, 9, 1–13.
- Zou, L.J.; Deng, X.G.; Zhang, L.E.; Zhu, T.; Tan, W.R.; Muhammad, A.; Zhu, L.J.; Zhang, C.; Zhang, D.W.; Lin, H.H. Nitric Oxide as a Signaling Molecule in Brassinosteroid-Mediated Virus Resistance to Cucumber Mosaic Virus in Arabidopsis thaliana. Physiol. Plant 2018, 163, 196–210.
- Ye, H.; Liu, S.; Tang, B.; Chen, J.; Xie, Z.; Nolan, T.M.; Jiang, H.; Guo, H.; Lin, H.Y.; Li, L.; et al. RD26 Mediates Crosstalk between Drought and Brassinosteroid Signalling Pathways. Nat. Commun. 2017, 8, 14573.
- Sharma, I.; Kaur, N.; Pati, P.K. Brassinosteroids: A Promising Option in Deciphering Remedial Strategies for Abiotic Stress Tolerance in Rice. Front. Plant Sci. 2017, 8, 2151.
- Wang, Y.; Cao, J.J.; Wang, K.X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Zhoua, J. BZR1 Mediates Brassinosteroid-Induced Autophagy and Nitrogen Starvation in Tomato. Plant Physiol. 2019, 179, 671–685.
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in Plant Tolerance to Abiotic Stress. J. Plant Growth Regul. 2020, 39, 1451–1464.
- Amraee, L.; Rahmani, F.; Abdollahi Mandoulakani, B. 24-Epibrassinolide Alters DNA Cytosine Methylation of Linum Usitatissimum L. under Salinity Stress. Plant Physiol. Biochem. 2019, 139, 478–484.
- Sharma, A.; Thakur, S.; Kumar, V.; Kanwar, M.K.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R.; Alam, P.; Ahmad, P. Pre-Sowing Seed Treatment with 24-Epibrassinolide Ameliorates Pesticide Stress in Brassica juncea L. through the Modulation of Stress Markers. Front. Plant Sci. 2016, 7, 1569.
- Sharma, A.; Yuan, H.; Kumar, V.; Ramakrishnan, M.; Kohli, S.K.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Zheng, B. Castasterone Attenuates Insecticide Induced Phytotoxicity in Mustard. Ecotoxicol. Environ. Saf. 2019, 179, 50–61.
- Yin, W.; Dong, N.; Niu, M.; Zhang, X.; Li, L.; Liu, J.; Liu, B.; Tong, H. Brassinosteroid-Regulated Plant Growth and Development and Gene Expression in Soybean. Crop J. 2019, 7, 411–418.
- Yue, J.; You, Y.; Zhang, L.; Fu, Z.; Wang, J.; Zhang, J.; Guy, R.D. Exogenous 24-Epibrassinolide Alleviates Effects of Salt Stress on Chloroplasts and Photosynthesis in Robinia Pseudoacacia L. Seedlings. J. Plant Growth Regul. 2019, 38, 669–682.
- Nolan, T.M.; Vukasinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 298–318.




