Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José S. Câmara | -- | 4829 | 2022-12-08 18:50:30 | | | |
| 2 | Dean Liu | -7 word(s) | 4822 | 2022-12-09 09:38:58 | | | | |
| 3 | Dean Liu | Meta information modification | 4822 | 2022-12-12 08:43:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Câmara, J.S.; Locatelli, M.; Pereira, J.A.M.; Oliveira, H.; Arlorio, M.; Fernandes, I.; Perestrelo, R.; Freitas, V.; Bordiga, M. Significance of Anthocyanins. Encyclopedia. Available online: https://encyclopedia.pub/entry/38362 (accessed on 08 February 2026).
Câmara JS, Locatelli M, Pereira JAM, Oliveira H, Arlorio M, Fernandes I, et al. Significance of Anthocyanins. Encyclopedia. Available at: https://encyclopedia.pub/entry/38362. Accessed February 08, 2026.
Câmara, José S., Monica Locatelli, Jorge A. M. Pereira, Hélder Oliveira, Marco Arlorio, Iva Fernandes, Rosa Perestrelo, Victor Freitas, Matteo Bordiga. "Significance of Anthocyanins" Encyclopedia, https://encyclopedia.pub/entry/38362 (accessed February 08, 2026).
Câmara, J.S., Locatelli, M., Pereira, J.A.M., Oliveira, H., Arlorio, M., Fernandes, I., Perestrelo, R., Freitas, V., & Bordiga, M. (2022, December 08). Significance of Anthocyanins. In Encyclopedia. https://encyclopedia.pub/entry/38362
Câmara, José S., et al. "Significance of Anthocyanins." Encyclopedia. Web. 08 December, 2022.
Copy Citation
Anthocyanins are widespread and biologically active water-soluble phenolic pigments responsible for a wide range of vivid colours, from red (acidic conditions) to purplish blue (basic conditions), present in fruits, vegetables, and coloured grains.
anthocyanins (ACNs)
occurrence
health benefits
1. Anthocyanins Classification, Chemical Properties and Biosynthesis
The benefits of consuming medicinal herbs, fruits, and vegetables on a regular basis for human health have gained widespread acceptance in recent years. This is mostly due to their composition, which is rich in several non-nutrient bioactive chemicals, such as phenolics, which have long been known for their ability to influence various processes and pathways in the human body, such as regulating glucose levels and increasing anti-inflammatory, antioxidant, anticancer, anti-mutagenic, and neuroprotective properties [1]. Among the phenolic compounds, one of the polyphenols class’s most extensively studied members is the anthocyanins (ACNs), representing an interesting class of water-soluble compounds (pigments). One reason for this is that they offer a viable alternative to the most used synthetic food dyes, which can produce hazardous consequences for human health, and are thus becoming increasingly significant to the food business and consumers. The flavonoid pathway is the one responsible for the synthesis of anthocyanins. Many fruits, vegetables, and edible flowers, as well as their derivatives such as juices, red wines, and tea, exhibit intense red, orange, violet, and blue colours [2]. They are the primary sources of these colours. In addition, they have drawn a lot of interest because of their nutritional value, pharmacokinetic profile, pharmacological mechanisms, and health-promoting qualities [3].
These molecules are useful substances that can boost antioxidant defences, reduce the effects of chronic inflammation, free radicals, and mutation risk, as well as slow or even stop the onset and progression of many chronic non-communicable and degenerative diseases. Anthocyanins are natural colorants that are less hazardous than synthetic ones and have a variety of technological potential applications in a variety of industrial domains, including the food and textile industries. The goal of this comprehensive review was to evaluate and clarify these applications. Researchers also discussed some of the most recent research on the health advantages of anthocyanins, with a particular emphasis on the prevention of the most common oxidative stress-related human disorders, such as cancer, diabetes, and cardiovascular and neurological diseases.
2. Significance of ACNs, from Plants to Human Health Benefits
Plants are organisms not provided with motility organs, and for this reason they are highly exposed to external conditions, namely unfavourable edaphoclimatic parameters such as drought, high-temperature fluctuations, and radiation exposure, salinity, soil nutrients deficiency, among others. To cope with these challenges, plants produce a huge diversity of secondary metabolites that allowed their survival and colonization of the most disperse ecosystems on the planet. But these metabolites also confer protection and advantages to other organisms that include plants in their diets, namely herbivores and mammals (Figure 1).
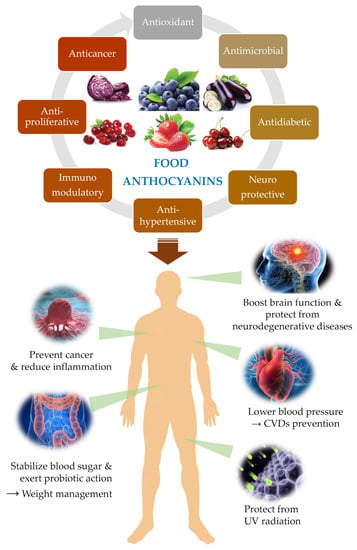
Figure 1. Overview of major human protective effects associated to ACNs consumption reported in the literature.
Overall, the benefits associated with ACNs consumption for the promotion and protection of human health span the most diverse functions and effects in our body as synthetised in Figure 1.
Effectively, there is an overwhelming number of plant secondary metabolites that confer protection to humans, and ACNs are among these bioactive compounds. Researchers will unveil some of the latest studies about the health benefits of ACNs, mainly focusing on the protection against the most prevalent human diseases, namely cardiovascular and neurodegenerative diseases, cancer, and diabetes. The contribution of ACNs to visual health is also very relevant and will be briefly explored (Figure 1).
2.1. Cardiovascular Diseases
Cardiovascular diseases (CVDs) are a set of disorders that affect the heart and blood vessels and have earned increasing awareness due to high prevalence and mortality worldwide [4][5][6]. It has been expected that the ratio of CVD-related deaths to worldwide deaths would rise from 28% in 1990 to 31.5% in 2020 [4]. Hypertension, dyslipidemia, atherosclerosis, oxidative stress, inflammation, and enteric dysbacteriosis are the major conditions linked to CVDs [6]. Moreover, the development of CVDs is connected to the production of free radicals from several sources, namely the mitochondrial electron transport chain, angiotensin II-mediated NADPH oxidase system, xanthin oxidase, and inducible nitric oxidase synthase [7]. In this sense, many synthetic drugs have been applied to the treatment of CVDs, but some side effects, namely gastrointestinal reaction, hyperkalemia, and arrhythmias, were also identified [6]. On the other hand, key findings from epidemiological and clinical studies revealed an inverse relationship between CVDs and the intake of some antioxidant natural products (e.g., fruits, vegetables, teas, cereals, nuts) [5][6]. Moreover, research reported that consumption of ACN-rich foods (e.g., berries, nuts) [8][9][10], ACN extracts [4][11][12], and purified ACNs as supplements [13][14][15] resulted in beneficial functions in the prevention of CVDs, such as decreasing of low-density lipoprotein-cholesterol (LDL-C), triglycerides, blood pressure, inflammatory and oxidative stress biomarkers, as well as increasing high-density lipoprotein-cholesterol (HDL-C) and improving vascular endothelial functions. Nevertheless, the effective dose of ACN necessary to promote beneficial functions has not been well established.
Regarding the consumption of anthocyanin-rich foods (e.g., berries, nuts), Askari et al. [10] investigated the association between nuts and legume consumption with Framingham risk score and CVD risk factors in older adult men. The results suggest that older adult men with higher consumption of these food products have higher serum levels of HDL-C and are less likely to have high LDL-C serum levels compared with those with lower consumption. However, no association was observed between nuts and legumes with Framingham’s risk score and other CVD risk factors. On the other hand, Nurfaradilla et al. [16] evaluate the effect of Hibiscus sabdariffa extract coadministration with the frequently prescribed angiotensin-converting enzyme (ACE) inhibitor captopril on blood pressure and biomarkers of the renin-angiotensin-aldosterone system. The data obtained suggest that Hibiscus sabdariffa aqueous extract has potential in the prevention of CVDs, since the plasma renin level, serum angiotensin-converting enzyme (ACE) activity, and plasma angiotensin II level were also significantly reduced. Del Bo’ and collaborators [12] examined the ability of an ACN-rich extract from blueberry, single ACNs (e.g., cyanidin, delphinidin, and malvidin-3-glucoside), and related metabolites (e.g., protocatechuic, gallic and syringic acid) to reduce the inflammation-driven adhesion of monocytes (THP-1) on endothelial cell and secretion of cell adhesion molecules E-selectin and vascular cell adhesion molecule (VCAM-1). The anthocyanin-rich extract and malvidin-3-glucoside (Mv3glu) had an effect at all the concentrations analysed, whereas Cy3glu decreased the adhesion by about 41.8% only at the high concentrations (10 μg/mL).
Previous in vivo studies have demonstrated that ACN supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with CVDs. In this sense, Zhang, and collaborators [17] performed a randomized controlled trial to evaluate the dose-response effect of ACNs supplementation on oxidative and inflammatory response in individuals with dyslipidaemia. The results demonstrated that the subjects who received 320 mg/day ACNs for 12 weeks showed further improvement in reducing serum IL-6 and tumour necrosis factor α (TNF-α), serum malonaldehyde (MDA), urine 8-iso-prostaglandin F2α (urine 8-iso-PGF2α), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) than those who received 80 mg/day and 40 mg/day ACNs. On the other hand, Aboonabi et al. [18] sought to uncover whether ACN supplementation would ameliorate cardiometabolic abnormalities commonly associated with metabolic syndrome patients and inhibit the activation of platelet surface markers. The data obtained demonstrated that four weeks of anthocyanin supplementation significantly reduced cardiometabolic risk factors comprising the average serum fasting blood glucose (by 13.3%, p < 0.05) and lipid profiles by a significant reduction in triglyceride (by 24.9%, p < 0.05) and LDL- cholesterol (by 33.1%, p < 0.05) in the metabolic syndrome patients. Moreover, in females, the anthocyanin supplementation also decreased high sensitivity C-reactive protein level (by 28%, p < 0.05). Li et al. [14] demonstrated that cyanidin reduced lipopolysaccharide-induced mitochondrial reactive oxygen species (ROS) production. Moreover, cyanidin showed to be a more potent reductant of cytochrome C than ascorbate at the same concentration [13]. These authors proposed that cyanidin and specific flavonols combined with cytochrome C at low concentrations (few µM) were able to inhibit the pro-apoptotic cardiolipin-induced peroxidase activity of cytochrome C. In sum, the intake of berries as nutraceuticals or functional foods could be suggested for the prevention and control of CVDs.
2.2. Neurodegenerative Diseases
Neurodegenerative diseases including Parkinson’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis are symptomatically characterized by the impairment of cognitive or motor functions [19]. Dietary ACNs can enhance cognitive performance, exert neuroprotective effects against neurodegenerative disorders, and minimize cognitive decline, suggesting their potential application for the prevention of several neurological diseases (e.g., Alzheimer’s, Parkinson’s disease). Notwithstanding, the underlying mechanisms of their action are not completely recognized. The synaptic plasticity, neurogenesis, passage across the blood-brain barrier, modulation of cell signalling pathways and expression of genes involved in neuroinflammation, are some of the proposed mechanisms [20][21]. The ability of ACNs to change cell signalling pathways and expression of several different genes engaged in the regulation of numerous processes including inflammatory responses, redox balance, cell migration, and metabolism has been earlier demonstrated [21][22]. In this sense, Milenkovic, and collaborators [21] evaluated the effects of 12-week ACN-rich bilberry extract supplementation (0.02%) on global gene expression in the hippocampus of ApoE−/− mice with the purpose of understanding the molecular mechanisms underlying anthocyanin neuroprotective effects. The results suggested that ACNs-rich bilberry extract altered the overall gene expression pattern in the hippocampus of ApoE−/− mice. In addition, the bioinformatic analyses proposed that the identified 1698 differentially expressed genes could be involved in numerous cellular and molecular processes, comprising inflammation, neuronal function, metabolic processes, and signal transduction, which are involved in cognitive function and neurodegenerative disorders (e.g., Alzheimer’s, Parkinson’s disease). Furthermore, Vauzour et al. [23] elucidated the underlying mechanisms of actions associated with anthocyanin-rich extract, specifically neuronal signalling, and synaptic function/integrity. The results suggest that the consumption of anthocyanin-rich extract improves learning capabilities in aged rats, counteracting spatial memory loss in aged brains, through the modulation of several cell signalling events implicated predominantly in synaptic plasticity, apoptosis, and cytoskeleton remodelling. These behavioural variations are complemented by a regional rise of brain-derived neurotrophic factor mRNA expression within the hippocampus, highlighting a possible mechanism of action attributable to the memory enhancements detected in the aged animals [23].
ACN is well documented to have a protective effect on nerve tissue by crossing the blood-brain barrier. Amyloid-β toxicity and the modified amyloid precursor protein that process the potential aggregation of glycation products are key pathogenic features of Alzheimer’s disease [24]. Wen et al. [25] investigated the synergistic neuroprotective effect of metformin and cyanidin 3-O-galactoside (Cy3gal) by behavioural and histopathological assays and metabolite analysis in SAMP8 mice. The behaviour experiments showed that the SAMP8 mice treated with metformin and Cy3gal showed improved spatial learning and memory compared with the SAMP8 model group, which suggests their positive effect on postponing the progression of Alzheimer’s disease. Moreover, El-Shiekh et al. [26] demonstrated that Hibiscus—ACNs enriched extract prevents memory impairment, and this could be attributed to the amelioration of streptozotocin -induced neuroinflammation and amyloidogenesis. Moreover, according to these authors, Hibiscus represents a promising safe agent that can be repurposed for Alzheimer’s disease through exerting anti-inflammatory, anti-acetylcholinesterase, antioxidant, and anti-amyloidogenic activities. The effects of Cy3glu on M1/M2 polarization and the mechanism to regulate anti-inflammation and phagocytosis, both in vitro and in vivo, were investigated by Sanjay and collaborators [27]. The results demonstrate the effects of Cy3glu in shifting the M1/M2 polarization of microglia via activation of PPARγ and the TREM2-mediated enhancement of Aβ phagocytosis in Aβ42-treated HMC3 cells. Cy3glu not only has anti-inflammatory properties but also promotes eliminating accumulated β-amyloid by enhancing phagocytosis.
Parkinson’s disease is a result of the progressive loss of dopaminergic neurons in the substantia nigra [24]. Zaim et al. [28] investigated the potential neuroprotective effect of black carrots-ACNs enriched extract on human SH-SY5Y cells treated with 1-methyl-4-phenylpyridinium (MPP+) to induce Parkinson’s disease-associated cell death and cytotoxicity. The results suggest the first evidence that black carrots-ACNs enriched extract significantly protected SH-SY5Y cells from MPP+ induced neurotoxicity by inhibiting ROS mediated oxidative stress and apoptosis. Filaferro et al. [29] disclosed the antioxidant and neuroprotective activity of an ACNs-rich extract from Sweet Cherry (Prunus avium L.) using in vitro and in vivo models. The extract was demonstrated to be effective at a cellular level in reducing the cytotoxicity, intracellular reactive oxygen species (ROS) production in the cell lines tested (SH-SY5Y, BV2), after exposure to the neurotoxin Drosophila melanogaster rotenone. Moreover, the extract improved the resistance of the nematode Caenorhabditis elegans against thermally induced oxidative stress, mainly in young animals, suggesting it to be suitable for protection against oxidative stress.
Regarding to isolated ACNs, a study conducted by Chen and collaborators [30] and hippocampal metabolome alterations in aging rats demonstrated that petunidin-3,5-O-diglucosides (Pn3G5G) isolated from Lycium ruthenicum Murr. fruit enhanced biological pathways and processes, particularly biosynthesis of amino acids, ABC transporters, cysteine and methionine metabolism, protein digestion and absorption, biosynthesis of cofactors in the hippocampus of aging rats. In sum, Pn3G5G likely improves antioxidant and anti-inflammatory defences as well as keeps the normal function of the hippocampus by affecting these metabolic pathways, in that way alleviating cognitive impairment in aging rats. Furthermore, the disaggregation reaction of single amyloid β (Aβ) fibrils by delphinidin-3-galactoside (D3gal), cyanidine-3-galactoside (Cy3gal), and malvidin-3-galactoside (Mv3gal) was studied by total-internal-reflection-fluorescence microscopy with a quartz-crystal microbalance (TIRFM-QCM). The results demonstrated that D3gal promotes high disassembly ability; it completely dissolves Aβ amyloid fibrils, whereas Cy3gal interacts with Aβ amyloid fibrils, but it fails to show the disassembly activity on the fibrils. On the other hand, Mv3gal cannot even bind the fibrils. According to the authors, a possible explanation for these results is the number of hydroxyl groups in six-membered ring B, since Cy3gal and D3gal have two and three hydroxyl groups, and they interact with Aβ amyloid fibrils, whereas Mv3Ggal has only one hydroxyl group and fails to interact with the fibrils. Therefore, it was possible to conclude that a diet rich in ACNs can prevent neurodegenerative diseases.
2.3. Anticancer Effect
Oxidative damage to the cells and inflammation are two hallmarks triggering the development and progression of different forms of cancer. Therefore, the ingestion of compounds able to attenuate or disrupt these harmful processes may confer resilience to our organism against cancer if such molecules were able to reach the cells where the deleterious events are occurring. In this aspect, one of the protective roles of ACNs certainly lies in their antioxidant potential. For this reason, ACNs have been reported to exert therapeutic and preventive effects in almost every type of cancer, broadly by causing cytotoxic effects and inducing DNA damage and consequent cell cycle arrest [24][31]. Researchers will, however, focus the discussion on the most recent reports in this field and the forms of cancer, with stronger evidence of protective effects elicited by ACNs, namely prostate cancer (PC), colon cancer (CC), namely lung cancer (LC), breast cancer (BC), and melanoma.
Prostate malignancy is one of the most prevalent and lethal cancers affecting men worldwide. For this reason, the potential chemopreventive effect of ACNs in this form of cancer has been widely studied through in vitro approaches using different prostate cancer (PC) cell lines, as well as in vivo, using animal models, mostly rats and mice. Regarding the in vitro evidence, extracts from different berries, such as Vaccinium myrtillus [32], Lycium ruthenicum Murray (black fruit wolfberry) [33], or sweet cherries [34], have been recently shown to increase the apoptotic rate and decrease cell viability of PC cell lines. But such an effect was also found using extracts from other foods, such as red cabbage juice [35] or the Ready to Serve (RTS) beverage prepared from Ixora coccinea fruits [36]. The cytotoxicity against human PC cells of the Ixora coccinea fruits is very relevant because this evergreen shrub is extensively used in Indian traditional medicine and so constitutes another cheap source of bioactive ACNs against PC in a huge population with limited access to proper health care services. Concerning the protective effects of ACNs against PC, involving in vivo models, recently Lamas et al. [37] observed a dose-dependent control of inflammation and oxidative-stress in the FVB mice fed with an extract of the Brazilian berry Myrciaria jaboticaba. A similar result was found by Kim et al. [38] that reported that the ACNs-rich extract of Aronia melanocarpa (black chokeberry) can attenuate the development of testosterone-induced prostatic hyperplasia in Wistar rats. Moreover, the authors have shown that such an effect was dependent on the abundance of the major ACNs present in the extract, Cy3glu and Cy3xyl Purple rice constitutes a promising functional food due to their rich bioactive composition, namely in ACNs. For this reason, the work of Yeewa et al. [39] is very relevant as the authors reported that the hexane insoluble fraction of this coloured rice retards carcinogenesis and castration-resistant cancer growth of the prostate and such effect occurred through the suppression of androgen receptor-mediated cell proliferation and metabolism.
Epidemiological evidence points to a protective effect of diets rich in fruits, vegetables, and whole grains against CC [40]. Therefore, ACNs, being abundant in most fruits and vegetables and responsible for their colour, will certainly contribute to mitigating CC development. This assumption has been largely supported by studies involving ACNs-rich extracts from fruits and vegetable foods as well as pure ACNs solutions. Such studies were performed in CC cell lines, animal models, and clinical trials and evidence points that ACNs are largely responsible for such chemoprevention effects (reviewed in [41]). Moreover, such effects have been assayed in diverse types of CC cell lines, such as Caco-2, DLD1-1, HT-29, HCT-116, SW480, or SW620, therefore covering a wide range of types of cells associated with CC development and progression. Beyond the protective effects reported against PC [38], ACNs from Aronia melanocarpa (black chokeberry) extracts were also reported to be effective against CC cells lines, inducing apoptosis in Caco-2 cells through the Wnt/beta-catenin signalling pathway [42]. A similar effect was observed by Gill et al. [43], which assayed three different species of chokeberries (purple, red and black, the last one being the Aronia melanocarpa). According to the data obtained, Aronia melanocarpa was the most effective against CC. Moreover, such enhanced effect in comparison with the purple and red chokeberry species assayed, correlated with the darker colour, higher overall phenolic content, antioxidant capacity, and presence of caffeic and chlorogenic acids in the black chokeberry. Regarding this observation, it is relevant to highlight the work from Mazewski et al. [44] that assayed eleven extracts from different fruits sharing unconventional and strong colours, namely red and purple grapes, purple sweet potato, purple carrot, black and purple beans, black lentil, black peanut, sorghum, black rice, and blue wheat. Overall, the authors report several protective effects against CC that include cell cycle arrest, apoptosis stimulation, and inhibition of anti-apoptotic proteins and tyrosine kinase. The attribution of these protective effects to the ACNs fractions of the different natural extracts is supported by the studies performed with pure ACNs such as Cy3glu and Dp, which evidence similar effects against CC development and progression [45][46][47][48]. The use of animal models provides additional evidence of the protective effects that ACN-rich extracts elicit against CC. In this regard, Fragoso et al. [49] have shown that the lyophilized açaí pulp (Euterpe oleracea Mart) attenuates colitis-associated colon carcinogenesis. Furthermore, the authors studied the main ACN from this fruit, cyanidin 3-rutinoside, and observed that it affects the motility of RKO CC cells [49]. Additional research recently reported involving CC rat models fed with ACNs-rich extracts points to important anti-inflammatory effects [50][51], providing additional evidence of the importance of inflammation in cancer development and progression.
One of the limitations pointed to the bioactive potential of ACNs is limited availability to protect cells as most polyphenols, including ACNs, are methylated in the gut and liver, therefore hindering their absorption. In this context, Grimes et al. [52] studied the effect of entacapone with ACNs. Entacapone is an inhibitor of the enzyme catechol-O-methyltransferase (COMT) which methylates polyphenols and so its use in combination with ACNs promoted a synergetic effect in the growth inhibition of CC and BC cells.
LC is one of the most prevalent and deadly worldwide and this certainly fosters Zhang et al. [53] to study the association between dietary ACNs and LC risk. Their epidemiologic study involving the dietary habits of about 10,000 Americans points to a positive correlation between dietary ACNs consumption and LC risk in this population. Different mechanisms should be involved in the protection offered by dietary ACNs against LC. Lu et al. [54], for instance, showed that the ACNs extracted from the fruits of Vitis coignetiae Pulliat, widely used in Korean folk medicine to treat inflammatory diseases and cancers, inhibit the expression of several transcription and growth factors that promote proliferation, angiogenesis, invasion, and migration of non-small cell LC (NSCLC) cells. Similar results were obtained when LC model cells were assayed with Cy3glu and Dp solutions, unveiling the downregulation of specific pathways [55] and transcription factors [56].
Melanoma is the deadliest form of skin cancer and its incidence significantly increased in the last decades. For this reason, research for chemopreventive agents against melanoma is very active and ACNs have great potential in this field. Wang et al. [57] recently studied the effect of ACNs-rich extracts obtained from blueberries on B16-F10 melanoma cells. The authors observed a dose-dependent inhibition of the B16-F10 cell’s viability and proliferation, as well as a cell cycle arrest at the G0/G1 phase and induced early apoptosis. Rugina et al. [58] also reported that the aqueous elderberry extracts exhibited a dose-dependent effect against melanoma cells by promoting cell integrity and apoptosis and inhibiting cell proliferation. The authors showed that these extracts are rich in several ACNs, namely C3-O-sambubioside, C3-O-sambubioside-5- glucoside, C3,5-digluc, cyhexoside-pentoside, and Cy3glu. In turn, Liu et al. [59] challenged mice and human melanoma cells with Cy3glu and reported this ACN inhibits tumorigenesis both in vitro and in vivo via the oestrogen receptor beta.
BC is certainly one of the forms of cancer that more impact has in our societies and so the obtention of a natural chemopreventive agent able to invert its incidence and mortality would be a remarkable achievement. Overall, BC treatment targets the inhibition of the mechanisms that regulate oestrogen activity. This can be attained either by antagonists of the oestrogen receptor (ER) or by the inhibition of oestrogen synthesis. In this context, preclinical studies in BC/CC rodent models point to a chemopreventive effect ACNs [60]. Recently, Han et al. [61] provided evidence that Dp suppresses BC by acting in the tumour suppressor microRNA-34a in cell lines and tumour tissues, but the results in human studies are contradictory. The EU-funded project ATHENA conducted during the last 10 years shows that dietary ACNs may protect against the damage caused by radiotherapy treatment for BC [62]. Following this result, Bracone et al. [63] studied if ACN supplementation could mitigate the skin toxicity caused by radiotherapy in BC patients. Upon the study involving 193 BC patients, the authors concluded that despite ACN supplementation being well tolerated and safe, it did not prevent RT-induced local skin toxicity. In another recent epidemiologic study involving over 1500 BC patients and 1500 controls in China, it was reported that intake of flavonoids, anthocyanidins, proanthocyanidins, flavanones, flavones, flavonols, and isoflavones was associated with a lower overall BC risk [64]. Overall, thi recent data about the role of ACNs in BC is not conclusive and further studies are required.
2.4. Diabetes Mellitus
The excessive accumulation of adipose tissue in the human body, known as obesity, is the strongest risk factor for developing diabetes, namely, type 2 diabetes mellitus (T2DM), which is responsible for 90% of all diabetic patients [65]. The term diabesity represents this crosstalk between obesity and diabetes sharing inflammatory processes as a hallmark. These deleterious processes can be prevented by natural antioxidants, such as ACNs. Previous epidemiological evidence points out that dietary ACN consumption correlates with the reduction of type 2 diabetes mellitus and improvement of glucose metabolism (reviewed in [66]). Moreover, such protection seems to be dose-dependent, demonstrating the protective potential of chronic dietary ACN intake [67]. Different mechanisms seem to be involved in such protection. In diabetes, there is an up-regulation of NFκB and ACNs inhibit this signalling pathway, leading to a reduction in oxidative stress and inflammation [68]. ACNs were also associated with the downregulation of the glucose transporters GLUT2 in human intestinal Caco-2 cells [69][70]. This result is certainly related to the observation of diminished intestinal glucose absorption in patients and better postprandial glycemia in healthy populations [71][72]. In turn, Tani et al. [73] treated diabetic human aortic endothelial cells with Dp-3-rutinoside-rich blackcurrant extract and observed an increase in the secretion of glucagon-like peptide-1 which improved glucose tolerance. Other works involving mouse models provide evidence that the consumption of ACNs-rich extracts, such as those obtained from raspberry [74] and black currant [68], prevents diet-induced obesity by mitigating oxidative stress and modulating hepatic lipid metabolism. Also using high-fat diet-induced obese mice, Wu et al. [75] reported that ACNs in black rice, soybean, and purple corn increase faecal butyric acid, an anti-inflammatory molecule with regenerative properties in the intestines, and prevent liver inflammation. In a recent pilot study, Azzini et al. [76] recruited 11 overweight or obese women which received 500 mL/day of a commercial red orange juice rich in ACNs for 12 weeks. The authors report that the intervention resulted in a decrease in total cholesterol and LDL cholesterol, but no significant effects on obesity, insulin resistance, or inflammatory status. Further studies are therefore necessary to clarify the role of dietary ACNs in the prevention and management of diabetes.
2.5. Visual Health
The awareness of the protective effects ACNs elicit on visual health is not new. During World War II, for instance, bilberry jam was administered to British pilots to improve their night sight [77]. In early 2000, Kajimoto et al. [78] performed an interventional study with primary school students with pseudomyopia and showed that ACNs from bilberry (Vaccinium myrtillus L.) promoted recovery of visual acuity. This and many other protective effects attributed to ACNs for vision and eye health are eventually related to their ability to absorb light in the UV (280–400 nm) and blue light region (360–500 nm). Effectively, ACNs are the only class of polyphenols able to do that, which could protect human retinal cells against light-induced damage (reviewed in [79][80]) or more broadly against the excessive production of ROS and consequent oxidative damage to surrounding cells. Evidence points to crosstalk between this oxidative damage and inflammation which in turn affects the integrity of the blood-retinal barrier (BRB) and induce pathological neovascularization [79][81]. Overall, oxidative damage to the cells is a hallmark triggering the onset and progression of different ophthalmological diseases, such as glaucoma, age-related macular degeneration, and different retinal degenerative diseases (RTDs) [79][80][82]. Glaucoma, for instance, is one of the leading causes of irreversible blindness, often triggered by an increase in intraocular pressure. This eventually damages the optic nerve and retinal ganglion cells, ultimately leading to visual field dysfunction. While several phenolics, such as baicalein, forskolin, marijuana, ginsenoside, resveratrol, and hesperidin, were shown to reduce intraocular pressure, ACNs elicit neuroprotective effects on retinal ganglion cells. Such an effect seems to involve different mechanisms, especially antioxidant, anti-inflammatory, and anti-apoptosis mechanisms (reviewed in [80][83]). Age-related macular degeneration (AMD) is another major cause of blindness. It is the most prevalent cause of the condition in elderly populations, and there is no effective treatment for its dry form. For this reason, the protective effect of the fruit extract of Aronia melanocarpa on rat retina is so relevant. Using a NaIO3-induced dry AMD model, Xing et al. [84] showed that these ACNs-rich extracts protect the rat retina, mitigating the effect of oxidative damage and upregulating at least five crystallin proteins which protect the retina ganglion cells.
To elicit the protective effects, ACN would need to pass thorough the blood-aqueous barrier and BRB, and this ability has been shown in rats and rabbits [85]. Moreover, ACNs were reported to accumulate in ocular tissues as their total concentration in these tissues was found to be higher than that measured in plasma upon ACNs intravenous or intraperitoneal administration [83]. The route to explain this result seems to involve facilitated transport via glucose transporters, namely the constitutive isoform GLUT1 [86].
The protective effects observed in different studies using ACNs-rich extracts prompted research using purified ACNs to try to ascribe to ACNs the reported effects. Cy3glu as one of the most abundant ACNs was shown to mitigate the photooxidation-induced apoptosis and angiogenesis in the retina [87], possibly through the activation of Nrf2/HO-1 pathway and NFκB suppression [88]. In turn, the same suppression of NFκB activation together with cox-2 expression seems to be the mechanism involved in the protection of the lens epithelial cells to cope with apoptosis induced by high glucose levels, therefore preventing the development of cataracts [89]. Similarly, another important ACN, malvidin promotes SOD and catalase expression in high glucose-induced human retinal capillary endothelial cells. Such activation protects these cells not only from oxidative stress-induced damage but also involves anti-inflammatory mechanisms through the inhibition of ICAM-1 and NFκB [90]. These selected examples are clear evidence of the protective effects ACNs have on the visual health and for this reason, a growing number of applications and products are incorporating these bioactive compounds in health care products targeting researchers' vision.
References
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325.
- Francis, F.J.; Markakis, P.C. Food colorants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314.
- Kurek, M.; Hlupić, L.; Ščetar, M.; Bosiljkov, T.; Galić, K. Comparison of Two pH Responsive Color Changing Bio-Based Films Containing Wasted Fruit Pomace as a Source of Colorants. J. Food Sci. 2019, 84, 2490–2498.
- Wang, Z.; Pang, W.; He, C.; Li, Y.; Jiang, Y.; Guo, C. Blueberry Anthocyanin-Enriched Extracts Attenuate Fine Particulate Matter (PM2.5)-Induced Cardiovascular Dysfunction. J. Agric. Food. Chem. 2017, 65, 87–94.
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food. Chem. 2019, 67, 1771–1783.
- Zhou, D.D.; Luo, M.; Shang, A.; Mao, Q.Q.; Li, B.Y.; Gan, R.Y.; Li, H.B. Antioxidant Food Components for the Prevention and Treatment of Cardiovascular Diseases: Effects, Mechanisms, and Clinical Studies. Oxid. Med. Cell Longev. 2021, 2021, 6627355.
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health benefits of cyanidin-3-glucoside as a potent modulator of Nrf2-mediated oxidative stress. Inflammopharmacology 2021, 29, 907–923.
- Larsson, S.C.; Drca, N.; Bjorck, M.; Back, M.; Wolk, A. Nut consumption and incidence of seven cardiovascular diseases. Heart 2018, 104, 1615–1620.
- Bell, L.; Williams, C.M. A pilot dose-response study of the acute effects of haskap berry extract (Lonicera caerulea L.) on cognition, mood, and blood pressure in older adults. Eur. J. Nutr. 2019, 58, 3325–3334.
- Askari, M.; Daneshzad, E.; Jafari, A.; Bellissimo, N.; Azadbakht, L. Association of nut and legume consumption with Framingham 10 year risk of general cardiovascular disease in older adult men: A cross-sectional study. Clin. Nutr. ESPEN 2021, 42, 373–380.
- Igwe, E.O.; Charlton, K.E.; Roodenrys, S.; Kent, K.; Fanning, K.; Netzel, M.E. Anthocyanin-rich plum juice reduces ambulatory blood pressure but not acute cognitive function in younger and older adults: A pilot crossover dose-timing study. Nutr. Res. 2017, 47, 28–43.
- Del Bo, C.; Marino, M.; Riso, P.; Moller, P.; Porrini, M. Anthocyanins and metabolites resolve TNF-alpha-mediated production of E-selectin and adhesion of monocytes to endothelial cells. Chem. Biol. Interact. 2019, 300, 49–55.
- Lagoa, R.; Samhan-Arias, A.K.; Gutierrez-Merino, C. Correlation between the potency of flavonoids for cytochrome c reduction and inhibition of cardiolipin-induced peroxidase activity. BioFactors 2017, 43, 451–468.
- Li, F.; Lang, F.; Wang, Y.; Zhai, C.; Zhang, C.; Zhang, L.; Hao, E. Cyanidin ameliorates endotoxin-induced myocardial toxicity by modulating inflammation and oxidative stress through mitochondria and other factors. Food Chem. Toxicol. 2018, 120, 104–111.
- Horie, K.; Nanashima, N.; Maeda, H. Phytoestrogenic Effects of Blackcurrant Anthocyanins Increased Endothelial Nitric Oxide Synthase (eNOS) Expression in Human Endothelial Cells and Ovariectomized Rats. Molecules 2019, 24, 1259.
- Nurfaradilla, S.A.; Saputri, F.C.; Harahap, Y. Effects of Hibiscus Sabdariffa Calyces Aqueous Extract on the Antihypertensive Potency of Captopril in the Two-Kidney-One-Clip Rat Hypertension Model. Evid. Based Complement. Alternat. Med. 2019, 2019, 9694212.
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474.
- Aboonabi, A.; Meyer, R.R.; Gaiz, A.; Singh, I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr. Res. 2020, 76, 82–93.
- Mehdi, A.; Lamiae, B.; Samira, B.; Ramchoun, M.; Abdelouahed, K.; Tamas, F.; Hicham, B. Pomegranate (Punica granatum L.) Attenuates Neuroinflammation Involved in Neurodegenerative Diseases. Foods 2022, 11, 2570.
- Hein, S.; Whyte, A.R.; Wood, E.; Rodriguez-Mateos, A.; Williams, C.M. Systematic Review of the Effects of Blueberry on Cognitive Performance as We Age. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 984–995.
- Milenkovic, D.; Krga, I.; Dinel, A.L.; Morand, C.; Laye, S.; Castanon, N. Nutrigenomic modification induced by anthocyanin-rich bilberry extract in the hippocampus of ApoE-/- mice. J. Funct. Foods 2021, 85, 104609.
- Rodriguez-Mateos, A.; Istas, G.; Boschek, L.; Feliciano, R.P.; Mills, C.E.; Boby, C.; Gomez-Alonso, S.; Milenkovic, D.; Heiss, C. Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights From Randomized Controlled Trials, Metabolomics, and Nutrigenomics. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 967–976.
- Vauzour, D.; Rendeiro, C.; D’Amato, A.; Waffo-Teguo, P.; Richard, T.; Merillon, J.M.; Pontifex, M.G.; Connell, E.; Muller, M.; Butler, L.T.; et al. Anthocyanins Promote Learning through Modulation of Synaptic Plasticity Related Proteins in an Animal Model of Ageing. Antioxidants 2021, 10, 1235.
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J.; Shi, S. Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities. Molecules 2021, 26, 3807.
- Wen, H.; Tian, H.; Liu, C.; Zhang, X.; Peng, Y.; Yang, X.; Chen, F.; Li, J. Metformin and cyanidin 3-O-galactoside from Aronia melanocarpa synergistically alleviate cognitive impairment in SAMP8 mice. Food Funct. 2021, 12, 10994–11008.
- El-Shiekh, R.A.; Ashour, R.M.; Abd El-Haleim, E.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L.: A potent natural neuroprotective agent for the prevention of streptozotocin-induced Alzheimer’s disease in mice. Biomed. Pharmacother. 2020, 128, 110303.
- Sanjay; Shin, J.H.; Park, M.; Lee, H.J. Cyanidin-3-O-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARgamma and Abeta42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol. Neurobiol. 2022, 59, 5135–5148.
- Zaim, M.; Kara, I.; Muduroglu, A. Black carrot anthocyanins exhibit neuroprotective effects against MPP+ induced cell death and cytotoxicity via inhibition of oxidative stress mediated apoptosis. Cytotechnology 2021, 73, 827–840.
- Filaferro, M.; Codeluppi, A.; Brighenti, V.; Cimurri, F.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Bertelli, D.; Pellati, F.; Vitale, G. Disclosing the Antioxidant and Neuroprotective Activity of an Anthocyanin-Rich Extract from Sweet Cherry (Prunus avium L.) Using In Vitro and In Vivo Models. Antioxidants 2022, 11, 211.
- Chen, S.S.; Hu, N.; Wang, H.L.; Li, G.L. The major anthocyanin of Lycium ruthenicum Murr. relieves cognitive deficits, oxidative stress, neuroinflammation, and hippocampal metabolome alterations in aging rats. J. Funct. Foods 2022, 94, 105104.
- Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines 2020, 8, 336.
- Del Bubba, M.; Di Serio, C.; Renai, L.; Scordo, C.V.A.; Checchini, L.; Ungar, A.; Tarantini, F.; Bartoletti, R. Vaccinium myrtillus L. extract and its native polyphenol-recombined mixture have anti-proliferative and pro-apoptotic effects on human prostate cancer cell lines. Phytother. Res. 2021, 35, 1089–1098.
- Li, Z.L.; Mi, J.; Lu, L.; Luo, Q.; Liu, X.; Yan, Y.M.; Jin, B.; Cao, Y.L.; Zeng, X.X.; Ran, L.W. The main anthocyanin monomer of Lycium ruthenicum Murray induces apoptosis through the ROS/PTEN/PI3K/Akt/caspase 3 signaling pathway in prostate cancer DU-145 cells. Food Funct. 2021, 12, 1818–1828.
- Silva, G.R.; Vaz, C.V.; Catalao, B.; Ferreira, S.; Cardoso, H.J.; Duarte, A.P.; Socorro, S. Sweet Cherry Extract Targets the Hallmarks of Cancer in Prostate Cells: Diminished Viability, Increased Apoptosis and Suppressed Glycolytic Metabolism. Nutr. Cancer 2020, 72, 917–931.
- Drozdowska, M.; Leszczynska, T.; Koronowicz, A.; Piasna-Slupecka, E.; Dziadek, K. Comparative study of young shoots and the mature red headed cabbage as antioxidant food resources with antiproliferative effect on prostate cancer cells. RSC Adv. 2020, 10, 43021–43034.
- Shreelakshmi, S.V.; Chaitrashree, N.; Kumar, S.S.; Shetty, N.P.; Giridhar, P. Fruits of Ixora coccinea are a rich source of hytoconstituents, bioactives, exhibit antioxidant activity and cytotoxicity against human prostate carcinoma cells and development of RTS beverage. J. Food Process. Preserv. 2021, 45, e15656.
- Lamas, C.A.; Kido, L.A.; Hermes, T.A.; Nogueira-Lima, E.; Minatel, E.; Collares-Buzato, C.B.; Marostica, M.R., Jr.; Cagnon, V.H.A. Brazilian berry extract (Myrciaria jaboticaba): A promising therapy to minimize prostatic inflammation and oxidative stress. Prostate 2020, 80, 859–871.
- Kim, N.H.; Jegal, J.; Kim, Y.N.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. The Effects of Aronia melanocarpa Extract on Testosterone-Induced Benign Prostatic Hyperplasia in Rats, and Quantitative Analysis of Major Constituents Depending on Extract Conditions. Nutrients 2020, 12, 1575.
- Yeewa, R.; Naiki-Ito, A.; Naiki, T.; Kato, H.; Suzuki, S.; Chewonarin, T.; Takahashi, S. Hexane Insoluble Fraction from Purple Rice Extract Retards Carcinogenesis and Castration-Resistant Cancer Growth of Prostate Through Suppression of Androgen Receptor Mediated Cell Proliferation and Metabolism. Nutrients 2020, 12, 558.
- Birt, D.F.; Phillips, G.J. Diet, genes, and microbes: Complexities of colon cancer prevention. Toxicol. Pathol. 2014, 42, 182–188.
- Shi, N.; Chen, X.; Chen, T. Anthocyanins in Colorectal Cancer Prevention Review. Antioxidants 2021, 10, 1600.
- Wei, J.; Yu, W.; Hao, R.; Fan, J.; Gao, J. Anthocyanins from Aronia melanocarpa Induce Apoptosis in Caco-2 Cells through Wnt/beta-Catenin Signaling Pathway. Chem. Biodivers. 2020, 17, e2000654.
- Gill, N.K.; Rios, D.; Osorio-Camacena, E.; Mojica, B.E.; Kaur, B.; Soderstrom, M.A.; Gonzalez, M.; Plaat, B.; Poblete, C.; Kaur, N.; et al. Anticancer Effects of Extracts from Three Different Chokeberry Species. Nutr. Cancer 2021, 73, 1168–1174.
- Mazewski, C.; Liang, K.; Gonzalez de Mejia, E. Comparison of the effect of chemical composition of anthocyanin-rich plant extracts on colon cancer cell proliferation and their potential mechanism of action using in vitro, in silico, and biochemical assays. Food Chem. 2018, 242, 378–388.
- Baster, Z.; Li, L.; Kukkurainen, S.; Chen, J.; Pentikainen, O.; Gyorffy, B.; Hytonen, V.P.; Zhu, H.; Rajfur, Z.; Huang, C. Cyanidin-3-glucoside binds to talin and modulates colon cancer cell adhesions and 3D growth. FASEB J. 2020, 34, 2227–2237.
- Zhang, Z.; Pan, Y.; Zhao, Y.; Ren, M.; Li, Y.; Lu, G.; Wu, K.; He, S. Delphinidin modulates JAK/STAT3 and MAPKinase signaling to induce apoptosis in HCT116 cells. Environ. Toxicol. 2021, 36, 1557–1566.
- Huang, C.C.; Hung, C.H.; Hung, T.W.; Lin, Y.C.; Wang, C.J.; Kao, S.H. Dietary delphinidin inhibits human colorectal cancer metastasis associating with upregulation of miR-204-3p and suppression of the integrin/FAK axis. Sci. Rep. 2019, 9, 18954.
- Mazewski, C.; Kim, M.S.; Gonzalez de Mejia, E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560.
- Fragoso, M.F.; Romualdo, G.R.; Vanderveer, L.A.; Franco-Barraza, J.; Cukierman, E.; Clapper, M.L.; Carvalho, R.F.; Barbisan, L.F. Lyophilized acai pulp (Euterpe oleracea Mart) attenuates colitis-associated colon carcinogenesis while its main anthocyanin has the potential to affect the motility of colon cancer cells. Food Chem. Toxicol. 2018, 121, 237–245.
- Yoon, B.I.; Bae, W.J.; Choi, Y.S.; Kim, S.J.; Ha, U.S.; Hong, S.H.; Sohn, D.W.; Kim, S.W. Anti-inflammatory and Antimicrobial Effects of Anthocyanin Extracted from Black Soybean on Chronic Bacterial Prostatitis Rat Model. Chin. J. Integr. Med. 2018, 24, 621–626.
- Fernandez, J.; Garcia, L.; Monte, J.; Villar, C.J.; Lombo, F. Functional Anthocyanin-Rich Sausages Diminish Colorectal Cancer in an Animal Model and Reduce Pro-Inflammatory Bacteria in the Intestinal Microbiota. Genes 2018, 9, 133.
- Grimes, K.L.; Stuart, C.M.; McCarthy, J.J.; Kaur, B.; Cantu, E.J.; Forester, S.C. Enhancing the Cancer Cell Growth Inhibitory Effects of Table Grape Anthocyanins. J. Food Sci. 2018, 83, 2369–2374.
- Zhang, Y.; Zhu, M.; Wan, H.; Chen, L.; Luo, F. Association between Dietary Anthocyanidins and Risk of Lung Cancer. Nutrients 2022, 14, 2643.
- Lu, J.N.; Panchanathan, R.; Lee, W.S.; Kim, H.J.; Kim, D.H.; Choi, Y.H.; Kim, G.S.; Shin, S.C.; Hong, S.C. Anthocyanins from the Fruit of Vitis Coignetiae Pulliat Inhibit TNF-Augmented Cancer Proliferation, Migration, and Invasion in A549 Cells. Asian Pac. J. Cancer Prev. 2017, 18, 2919–2923.
- Chen, X.; Zhang, W.; Xu, X. Cyanidin-3-glucoside suppresses the progression of lung adenocarcinoma by downregulating TP53I3 and inhibiting PI3K/AKT/mTOR pathway. World J. Surg. Oncol. 2021, 19, 232.
- Kim, M.H.; Jeong, Y.J.; Cho, H.J.; Hoe, H.S.; Park, K.K.; Park, Y.Y.; Choi, Y.H.; Kim, C.H.; Chang, H.W.; Park, Y.J.; et al. Delphinidin inhibits angiogenesis through the suppression of HIF-1alpha and VEGF expression in A549 lung cancer cells. Oncol. Rep. 2017, 37, 777–784.
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and proapoptotic activities of anthocyanin and anthocyanidin extracts from blueberry fruits on B16-F10 melanoma cells. Food Nutr. Res. 2017, 61, 1325308.
- Rugina, D.; Hanganu, D.; Diaconeasa, Z.; Tabaran, F.; Coman, C.; Leopold, L.; Bunea, A.; Pintea, A. Antiproliferative and Apoptotic Potential of Cyanidin-Based Anthocyanins on Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 949.
- Liu, M.; Du, Y.; Li, H.; Wang, L.; Ponikwicka-Tyszko, D.; Lebiedzinska, W.; Pilaszewicz-Puza, A.; Liu, H.; Zhou, L.; Fan, H.; et al. Cyanidin-3-o-Glucoside Pharmacologically Inhibits Tumorigenesis via Estrogen Receptor beta in Melanoma Mice. Front. Oncol. 2019, 9, 1110.
- Bars-Cortina, D.; Sakhawat, A.; Pinol-Felis, C.; Motilva, M.J. Chemopreventive effects of anthocyanins on colorectal and breast cancer: A review. Semin. Cancer Biol. 2022, 81, 241–258.
- Han, B.; Peng, X.; Cheng, D.; Zhu, Y.; Du, J.; Li, J.; Yu, X. Delphinidin suppresses breast carcinogenesis through the HOTAIR/microRNA-34a axis. Cancer Sci. 2019, 110, 3089–3097.
- Cerletti, C.; De Curtis, A.; Bracone, F.; Digesu, C.; Morganti, A.G.; Iacoviello, L.; de Gaetano, G.; Donati, M.B. Dietary anthocyanins and health: Data from FLORA and ATHENA EU projects. Br. J. Clin. Pharmacol. 2017, 83, 103–106.
- Bracone, F.; De Curtis, A.; Di Castelnuovo, A.; Pilu, R.; Boccardi, M.; Cilla, S.; Macchia, G.; Deodato, F.; Costanzo, S.; Iacoviello, L.; et al. Skin toxicity following radiotherapy in patients with breast carcinoma: Is anthocyanin supplementation beneficial? Clin. Nutr. 2021, 40, 2068–2077.
- Feng, X.-L.; Ho, S.C.; Mo, X.-F.; Lin, F.-Y.; Zhang, N.-Q.; Luo, H.; Zhang, X.; Zhang, C.-X. Association between flavonoids, flavonoid subclasses intake and breast cancer risk: A case-control study in China. Eur. J. Cancer Prev. 2020, 29, 493–500.
- Shah, M.A.; Haris, M.; Faheem, H.I.; Hamid, A.; Yousaf, R.; Rasul, A.; Shah, G.M.; Khalil, A.A.K.; Wahab, A.; Khan, H.; et al. Cross-Talk between Obesity and Diabetes: Introducing Polyphenols as an Effective Phytomedicine to Combat the Dual Sword Diabesity. Curr. Pharm. Des. 2022, 28, 1523–1542.
- Sandoval-Ramirez, B.A.; Catalan, U.; Llaurado, E.; Valls, R.M.; Salamanca, P.; Rubio, L.; Yuste, S.; Sola, R. The health benefits of anthocyanins: An umbrella review of systematic reviews and meta-analyses of observational studies and controlled clinical trials. Nutr. Rev. 2022, 80, 1515–1530.
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367.
- Aboonabi, A.; Singh, I.; Rose’ Meyer, R. Cytoprotective effects of berry anthocyanins against induced oxidative stress and inflammation in primary human diabetic aortic endothelial cells. Chem. Biol. Interact. 2020, 317, 108940.
- Luna-Vital, D.; Weiss, M.; Gonzalez de Mejia, E. Anthocyanins from Purple Corn Ameliorated Tumor Necrosis Factor-alpha-Induced Inflammation and Insulin Resistance in 3T3-L1 Adipocytes via Activation of Insulin Signaling and Enhanced GLUT4 Translocation. Mol. Nutr. Food Res. 2017, 61, 1700362.
- Alzaid, F.; Cheung, H.M.; Preedy, V.R.; Sharp, P.A. Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS ONE 2013, 8, e78932.
- Yang, L.; Ling, W.; Du, Z.; Chen, Y.; Li, D.; Deng, S.; Liu, Z.; Yang, L. Effects of Anthocyanins on Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2017, 8, 684–693.
- Daneshzad, E.; Shab-Bidar, S.; Mohammadpour, Z.; Djafarian, K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1153–1165.
- Tani, T.; Nishikawa, S.; Kato, M.; Tsuda, T. Delphinidin 3-rutinoside-rich blackcurrant extract ameliorates glucose tolerance by increasing the release of glucagon-like peptide-1 secretion. Food Sci. Nutr. 2017, 5, 929–933.
- Wu, T.; Yang, L.; Guo, X.; Zhang, M.; Liu, R.; Sui, W. Raspberry anthocyanin consumption prevents diet-induced obesity by alleviating oxidative stress and modulating hepatic lipid metabolism. Food Funct. 2018, 9, 2112–2120.
- Wu, T.; Guo, X.; Zhang, M.; Yang, L.; Liu, R.; Yin, J. Anthocyanins in black rice, soybean and purple corn increase fecal butyric acid and prevent liver inflammation in high fat diet-induced obese mice. Food Funct. 2017, 8, 3178–3186.
- Azzini, E.; Venneria, E.; Ciarapica, D.; Foddai, M.S.; Intorre, F.; Zaccaria, M.; Maiani, F.; Palomba, L.; Barnaba, L.; Tubili, C.; et al. Effect of Red Orange Juice Consumption on Body Composition and Nutritional Status in Overweight/Obese Female: A Pilot Study. Oxid. Med. Cell Longev. 2017, 2017, 1672567.
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622.
- Kajimoto, O.; Sasaki, K.; Takahashi, T. Recovery effect of VMA intake on visual acuity of pseudomyopia in primary school students. J. New Rem. Clin. 2000, 49, 72–79.
- Oliveira, H.; Correia, P.; Pereira, A.R.; Araujo, P.; Mateus, N.; de Freitas, V.; Oliveira, J.; Fernandes, I. Exploring the Applications of the Photoprotective Properties of Anthocyanins in Biological Systems. Int. J. Mol. Sci. 2020, 21, 7464.
- Sim, R.H.; Sirasanagandla, S.R.; Das, S.; Teoh, S.L. Treatment of Glaucoma with Natural Products and Their Mechanism of Action: An Update. Nutrients 2022, 14, 534.
- Aires, I.D.; Boia, R.; Rodrigues-Neves, A.C.; Madeira, M.H.; Marques, C.; Ambrosio, A.F.; Santiago, A.R. Blockade of microglial adenosine A2A receptor suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells. Glia 2019, 67, 896–914.
- Santiago, A.R.; Boia, R.; Aires, I.D.; Ambrosio, A.F.; Fernandes, R. Sweet Stress: Coping With Vascular Dysfunction in Diabetic Retinopathy. Front Physiol. 2018, 9, 820.
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic Effects of Anthocyanins for Vision and Eye Health. Molecules 2019, 24, 3311.
- Xing, Y.; Liang, S.; Zhao, Y.; Yang, S.; Ni, H.; Li, H. Protection of Aronia melanocarpa Fruit Extract from Sodium-Iodate-Induced Damages in Rat Retina. Nutrients 2021, 13, 4411.
- Matsumoto, H.; Nakamura, Y.; Iida, H.; Ito, K.; Ohguro, H. Comparative assessment of distribution of blackcurrant anthocyanins in rabbit and rat ocular tissues. Exp. Eye Res. 2006, 83, 348–356.
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Bras, N.; Faria, A.; Calhau, C.; de Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport- Nanobased targeted approach. Sci. Rep. 2019, 9, 789.
- Peng, W.; Wu, Y.; Peng, Z.; Qi, W.; Liu, T.; Yang, B.; He, D.; Liu, Y.; Wang, Y. Cyanidin-3-glucoside improves the barrier function of retinal pigment epithelium cells by attenuating endoplasmic reticulum stress-induced apoptosis. Food Res. Int. 2022, 157, 111313.
- Wang, Y.; Huo, Y.; Zhao, L.; Lu, F.; Wang, O.; Yang, X.; Ji, B.; Zhou, F. Cyanidin-3-glucoside and its phenolic acid metabolites attenuate visible light-induced retinal degeneration in vivo via activation of Nrf2/HO-1 pathway and NF-kappaB suppression. Mol. Nutr. Food Res. 2016, 60, 1564–1577.
- Song, X.L.; Li, M.J.; Liu, Q.; Hu, Z.X.; Xu, Z.Y.; Li, J.H.; Zheng, W.L.; Huang, X.M.; Xiao, F.; Cui, Y.H.; et al. Cyanidin-3-O-glucoside Protects Lens Epithelial Cells against High Glucose-Induced Apoptosis and Prevents Cataract Formation via Suppressing NF-kappaB Activation and Cox-2 Expression. J. Agric. Food. Chem. 2020, 68, 8286–8294.
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxid. Med. Cell Longev. 2018, 2018, 1862462.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
3 times
(View History)
Update Date:
12 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




