1. Introduction
The term “cancer” was first used in medicine in the 1600s, which refers to cells that are developing abnormally and have the potential to infiltrate or spread to other parts of the body. Cancer is a complex and multifaceted disorder with thousands of genetic and epigenetic variations, especially in how they grow and divide. In a typical cell cycle, the cells go through the process of mitosis to reproduce themselves, leading to the cell’s normal growth. Eventually, the programmed cell-death process, known as apoptosis, causes the cells to die in order to ensure controlled growth. Once this process is disordered, the cells lose its balance and grow uncontrollably to form malignant tumours invading the surrounding tissues. The cancer cell may potentially move through the bloodstream or lymphatic system to different organs of the body and continue to spread from there. There are two types of cancer cells, namely benign and malignant. Benign cells do not spread to other parts, while malignant cells metastasize and are considered to be more destructive. There are more than 500 genes known to be connected to different forms of cancer. Cancer is one of the major causes of deaths in the world, especially for adults under 70
[1]. One of the world’s most rapidly developing therapeutic fields nowadays is oncology, and there is a need to accurately diagnose cancer at an early stage to enhance patient’s survival rate. The current research efforts are focused on cancer etiology and therapy, resulted in huge repositories of cancer-associated data which could be used for cancer prediction and early detection
[2]. The lengthy and expensive treatment methods for cancer are due to the disease’s high mortality and recurrence rate. Because of the developments in translational research and clinical trials, cancer has the highest number of clinically relevant mutations and the greatest multimodality-therapeutic options. Given the wide variety of cancers and the variety of observed manifestations, oncology may have the highest demand for customised care.
AI intends to mimic human-like cognitive abilities to address difficult healthcare challenges, including complex biological abnormalities like cancer, through algorithms and programs with appropriate data. These algorithms are designed to have a set of instructions which analyse data to take an appropriate decision or course of action
[3]. Since the human mind is only able to analyse a finite amount of data in a short amount of time, the exponential rise of AI over the past ten years is a proof that it can serve as a platform for supporting human experts to make the best possible decisions. AI-based algorithms hold great promise to pave the way to identify genetic mutations and aberrant protein interactions that can lead to cancer at a very early stage. One of the goals of current biomedical research is to ethically and safely introduce AI technology into medical settings. The greatest advancement in disease risk, diagnosis, prognosis, and therapy prediction may come from AI-based support for pathologists and doctors. The future of medical guidance will move toward speedier mapping of a new treatment for each individual through clinical applications of AI and machine learning (ML) in cancer diagnosis and treatment. Researchers may work together in real-time and share information digitally with AI to possibly treat millions of people. In this research, healthcare and AI are combined in an effort to showcase clinic technology that is changing the way people think about healthcare and to show how AI-based support enables medical practitioners to deliver accurate care. The requirement for this expertise is crucial since a technology that a medical professional is unfamiliar with has no bearing on healthcare
[4].
2. AI for Cancer Research
Since the field’s inception, experts have predicted the potential of highly tailored oncology care employing AI technologies. This promise is being realised as a result of cumulative advancements in the sciences, including the improvement of ML and deep-learning (DL) algorithms, the expansion of the breadth and variety of databases, including multiomics, and the decline in the price of massively parallelized computing power.
Fuzzy logic and neural networks are the two main methods used by AI to mimic human intelligence. In contrast to fuzzy-logic models, the results of neural-network models are very difficult to interpret and are referred to as “blackbox” models. While the data-driven AI (DAI) paradigm is guided by data, the symbolic AI (SAI) paradigm is guided by human-domain expertise. Most often employed in deterministic situations, SAI joins human-readable symbols in a relationship akin to “if-then” expressions to draw conclusions. In order to help solve situations where simple rules are sufficient, SAI explicitly incorporates human knowledge and rules into computer systems. This allows computers to reason and arrive at educated judgments. In other words, while DAI uses historical data as experience to develop mathematical equations that generate intelligent decisions, SAI focuses on reasoning with rules specified by human experts, requiring little to no learning. SAI and DDAI concepts are combined in informed AI (IAI). In order to create the target variable (i.e., data annotation) and make the models explicable, SAI takes into consideration human-domain expertise. DAI has a significant function to play in the study of cancer
[5].
It requires time and special technologies to ensure data security and privacy while making inferences from encrypted data. The Stained-Glass Transforms used by Protopia AI reduce the risk of sensitive data leakage when drawing conclusions from data, which is frequently a barrier to using the data for ML and AI. These transformations work well with many different sorts of data, including tabular, text, picture, and video. Prior to therapeutic treatments, it may anticipate prognostic markers, including patient outcome, pharmacological efficacy, and resistance, providing a very credible foundation for following therapies and providing a customised scenario
[6].
Researchers have developed tools to help with cancer identification and prognosis as a result of the availability of open-source healthcare statistics. To address problems in cancer medical treatment, DL and ML models offer dependable, quick, and efficient solutions on distributed dataset. For the analysis of distributed data, advanced-federated learning models can be deployed. Whole-blood, multi-cancer detection using deep sequencing, virtual biopsies, and NLP to infer health trajectories from medical records, and advanced clinical-decision support systems that incorporate genomics and clinomics, are some of the emerging clinically useful techniques
[7]. Oncology heavily relies on evidence-based, medicine-scoring systems for cancer-risk assessment, disease diagnosis, prognostic staging, treatment, and surveillance monitoring. These systems began as straightforward light-microscopy observations and improved to more sophisticated testing, such as gene-expression tests and next-generation sequencing of somatic and germline genomes. AI also opened doors to the synergic usage of drugs for cancer treatment.
When backed by strong AI core services and resources, AI-powered cancer research is approachable even for those without much computer knowledge. The future of digital healthcare and clinical practices is anticipated to shift toward the usage of algorithm-based AI for radiological image interpretation, EHRs, and data mining to give a more precise cancer therapy. Marketing-cancer-research companies have estimated the cost-savings from intelligent AI applications in the US healthcare sector to reach $52 billion in 2021
[8]. The AI intervention in cancer research can be more effective if proper data are available for developing the ML and DL models.
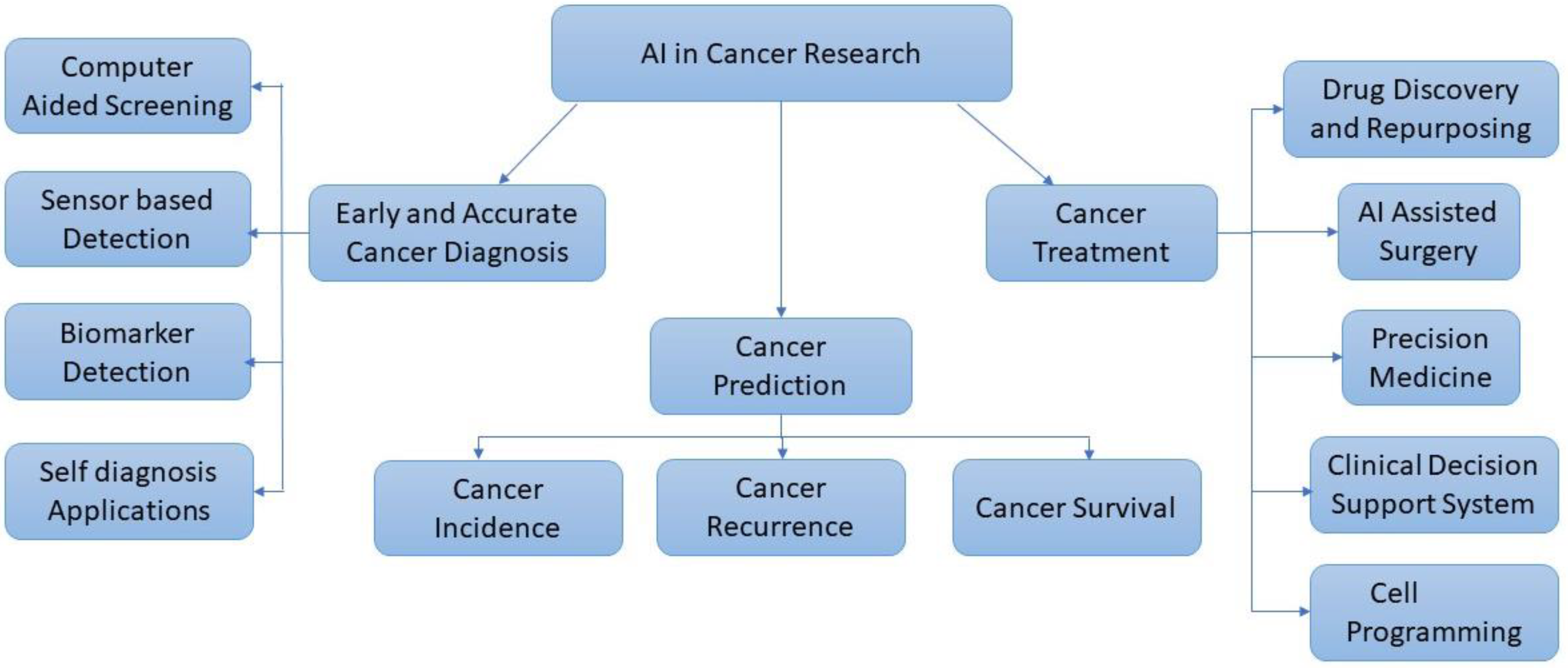
Figure 1 shows the broad approaches for cancer research using AI.
Figure 1. Approaches for cancer research using AI.
2.1. Cancer Data Repositories
Software, data, technology, and services are all part of digital health, which applies digital transformation to the healthcare industry. According to Deloitte Insights, radically interoperable data and AI promise consumer-focused and prevention-oriented healthcare. Availability of data is essential for data-driven AI research and many researchers develop frustration due to the lack of adequate data to carry out their research
[9][10].
To uncover ways to enhance cancer treatment, care, and prevention, cancer experts are continually developing new clinical trials. To assist people in locating a research study that might be suitable for them, many organisations provide online lists of open clinical trials. The following tools, lists, and searchable databases are useful for locating a cancer clinical
[11].
-
Ancora.ai.
-
Be the Match: Jason Carter Clinical Trial Search and Support Program
-
Bladder Cancer Advocacy Network
-
BreastCancerTrials.org
-
Center for Information and Study on Clinical Research Participation (CISCRP)
-
CenterWatch
-
ClinicalTrials.gov.
-
EmergingMed Clinical Trial Navigator Service
-
Lazarex Cancer Foundation
-
Melanoma Research Alliance
-
Metastatic Breast Cancer Project
-
Metastatic Prostate Cancer Project
-
National Brain Tumor Society Clinical Trial Finder
-
National Cancer Institute (NCI) Clinical Trials
-
Pancreatic Cancer Action Network Clinical Trial Finder
-
SPOHNC Clinical Trial Navigation Service
-
Targeted Agent and Profiling Utilization Registry (TAPUR) Study
-
The Leukemia & Lymphoma Society Clinical Trial Support Center
-
Us TOO Prostate Cancer Clinical Trial Finder
-
World Health Organization (WHO) International Clinical Trials Registry Platform.
2.2. Using Cloud AI Platforms
The growth of zero-code AI platforms make it possible for health specialists to use AI without having any programming experience by using APIs. The establishment of cancer-related AI relies on the trifecta of computational algorithms, databases, and computing power in an effort to make use of the inherent data richness of the subject. To achieve the level of precision that oncology strives for, each of these AI principles must be expanded beyond its existing limitations. Medical professionals across the world are exchanging information about disease diagnosis and management, drug response, and strategies for managing diseases with precision. Moreover, such data might be automatically stored to cloud and utilize its computing power for discovering insights with AI. Cloud computing is essentially the use of remote-internet-connected servers which can be used to store and process data. Google, Amazon and Microsoft are the major cloud-AI-platform providers. The AI-cloud platform allows the training of ML and DL models with a wide range of customization options. Cloud is very convenient for healthcare institutions to store massive amounts of data securely, analyse them and get useful insights. This has led to the establishment of Tumor Atlas
[10][12].
Microsoft’s significant investment in cloud computing makes sense for a subject that requires a lot of computer power to tackle challenging issues. Jasmin Fisher, a biologist from Microsoft Cambridge, U.K., developed a Bio Model Analyzer (BMA), a cloud-based application
[13] that makes it possible for biologists to simulate how cells interact and communicate with one another as well as discover the connections they form. Literome is a cloud-based method for organising genetic research that could be useful for cancer diagnosis. Due to the volume of data, oncologists would find it challenging to complete their duty alone. Hoifung Poon, a researcher at Microsoft’s Redmond, Washington lab used ML to generate sophisticated models for locating various descriptions of identical knowledge in order to produce Literome, which uses NLP capabilities that only need a modest quantity of knowledge to be available
[13].
BigQuery, a highly scalable, multi-cloud data warehouse from Google Cloud, supports the cloud-based platform that connects researchers to a large number of cancer datasets and provides the analytical and computational infrastructure to quickly evaluate that data. Medical researchers can now directly study data on the Institute of Science Biology-Cancer Genomic Cloud (ISB-CGC) platform without having to download it using Google Cloud services like Notebooks and BigQuery application programming interfaces (APIs)
[14]. The success of ISB-CGC in leveraging Google Cloud as the cornerstone of their infrastructure and data-cloud strategy has allowed the cancer-research community to receive secure, real-time access to data that is crucial to the early diagnosis of cancer.
2.3. AI for Cancer Prediction
Predictive models powered by AI are now a crucial component of cancer treatment. Predictive models can establish a person’s likelihood of getting a certain cancer by identifying the risk factors. Before the disease takes hold, AI can recognise those who are at high risk of contracting it. This enables medical professionals to keep a close eye on these patients and act quickly when necessary. DL, according to researchers at the University of Hawaii, can tell apart between mammograms from women who would later get breast cancer and those who will not. The Massachusetts Institute of Technology (MIT) researchers have developed a DL model to predict cancer risk from mammogram images. They validated the model using data from several hospitals across different continents. The algorithm correctly identified 30% of future breast cancer patients as belonging to a high-risk group. On the other hand, human doctors who used the traditional Tyrer-Cuzick methodology only flagged 18% of the cases
[4][15].
Different research teams have created random-forest-ML models to predict cancer survival and long-term cognitive outcomes
[16]. Identifying the biological mechanisms necessary for proper growth and how they affect the state of the learning tissue is crucial here. It is, therefore, exceedingly challenging for a human to measure the shift, but a machine may analyse millions of these photos from many modalities holistically to draw conclusions. In order to better personalise treatment regimens and provide better patient counselling, prediction of overall survival, recurrence risk, or other outcomes for cancer patients would be helpful.
2.4. AI for Cancer Diagnosis
The NYU Langone’s Perlmutter Cancer Center started using their AI classifier for cancer diagnosis in October 2019. This classifier can help pathologists in diagnosing cancer more accurately, reducing hospital error rates. The AI-based cancer classifiers, in general, can recognize patterns that are too subtle for the human eyes to detect. This will help physicians to perform targeted cancer therapies for patients with improved outcomes
[12]. The classifiers are normally trained using thousands of cancer samples, which could be much more than a single pathologist can see in a lifetime. Moreover, the machines will be consistent and will not be affected by inter-reader variability. Thus, AI- based systems can be used even by inexperienced radiologists to get clear diagnoses, leading to the right treatment. Researchers from the Korea Institute of Science and Technology (KIST) have developed a neuromorphic technology for cancer diagnosis which combines AI, IoT, and autonomous technologies. They used tactile-neuron devices with artificial neural network (ANN) based learning methods. When pressure is applied to a potentially cancerous site, the generating electrical spikes will increase or decrease depending on the stiffness of the object or tumor encountered.
It is envisaged that ongoing research to assist the application of AI to cancer genomes would enable multicancer early detection and tumour-site-origin determination. This may improve cancer survivors’ surveillance plans and change cancer screening, particularly for less common and rare tumours
[17]. Both scientists and physicians are becoming more and more interested in the field of radiology. The use of radiomics for diagnostics, prognostics, and treatment decision processes is becoming more and more appealing due to developments in pattern recognition, computer vision, and model building. As a potential non-invasive method of disease-heterogeneity quantification that could be used in conjunction with invasive biopsies and conventional quantitative-imaging techniques, radiomic features have the potential to quantify information about the entire tumour as well as the various textures contained within that tumour. These initiatives also make it easier for patients who are asymptomatic to get a cancer diagnosis. The creation of biosensors, which are non-invasive or implanted, may now identify the presence of cancer-related biomarkers. The three major parts of a biosensor are typically a biological sensing element, a physiochemical detector or transducer, and a signal processing system. Increased interest in the use of implanted medical devices for customised medicine has been brought about by developments in electronics and technology. The implanted biosensor can be used to monitor tumours as well
[17].
2.5. AI for Cancer Treatment
Though surgery, chemotherapy, and radiotherapy will continue to be the gold standard for cancer treatment for a very long time, concerns like how quickly a tumour is developing, if it has spread, and whether it is likely to return after treatment must be taken into consideration when treating cancer. Personalised treatment planning is increasingly important for cancer diagnosis and therapy for better outcomes. A few of the revolutionary ways that this technology may lengthen life and make more cures possible include the timing of restaging and surveillance tests, the dosages of systemic-cancer medicines and radiation, and the choice and sequencing of diagnostic and treatment measures. Automated contouring of tumour targets by DL remains a significant difficulty due to the diversity of tumour shapes, locations, and interior morphologies. Even so, automated contouring expedites the procedure and enhances the uniformity of radiation oncologists
[18].
With the help of AI, the development of treatment choices over the past 50 years has significantly extended the life expectancy of many cancer patients. This has been made feasible by imaging technologies that allow for early detection, highly personalised radiation therapy, targeted chemotherapies built on understanding of the human genome, and immunotherapies. Image-based ML methods can effectively predict high-risk cancer lesions. The radiotherapy workflow entails volumetric imaging, segmentation of the target volume and organs at risk (OAR), treatment planning, delivery of the treatment, and follow-up. Quality assurance (QA), when referring to the radiation workflow, is the final stage of confirming the treatments’ clinical acceptability and establishes the effectiveness of the total workflow
[19]. One of the most crucial components of radiation is the contours of the treatment targets and organs at risk since the effectiveness of these contours influences both the therapeutic and unfavourable effects of the therapy
[20].
According to MIT researchers, sensor technology and Internet of Things (IoT) will gather a lot of personal data that may be utilised to create individualised treatment regimens. Better diagnosis will result from it, and researchers will be able to use AI and ML to evaluate the vast amounts of data and choose the first and most effective course of action. The capacity to use individual biology rather than population biology at every stage of a patient’s medical journey boosts the effectiveness and speed of recovery when it comes to precision medicine. This entails gathering personal information from people, such as genetic information, physiological monitoring data, or EHR data, and customising their treatment plan in accordance with the conclusions drawn from AI models. Precision medicine has benefits, such as lower healthcare expenditures, less adverse drug reactions, and increased drug action efficacy
[21]. Prescribers are assisted by more complex analytical algorithms and decision-making tools in their delivery of precision-focused and targeted therapy for a required patient.
One can avoid overtreatment and pointless surgical removal of malignancies with the use of AI. According to a study in the Journal of the National Cancer Institute, AI algorithms can identify precancerous lesions in cervical pictures and differentiate them from other abnormalities to save patients from receiving unnecessary treatment for minor problems. Glioblastoma is an acute cancer type found in the brain or spinal cord. When treating such aggressive tumors, the doctors may administer higher amount of medication and radiation to shrink the tumor, but within the safe limits for the patient. AI can help the doctors administer safe but accurate doses of medication. Here, AI could aid with the metrics and predictions, whereas IoT can help sensor-driven data generation and insight. Together, AI and IoT can optimize safe drug delivery.
Researchers from the Harvard University and the University of Pennsylvania created a DL algorithm for tumour categorization in order to treat brain cancers. Without intrusive procedures that would usually be necessary, it can identify and characterise the isocitrate dehydrogenase (IDH) mutations from simply MRI scans of gliomas. Today’s clinical practice relies heavily on guided needle biopsies and pathology updates since they can reduce the number of unnecessary surgical excisions. Robotic surgery allows for a quicker recovery and return to regular lives. Using robotic arms that enter the body through tiny incisions, procedures that traditionally required cutting a long incision from the navel to the pubic bone can now be performed on patients who need to have their prostate gland removed because they have prostate cancer. Using a specialised console, the surgeon can control the arms while seeing a magnified view of the surgical site in real time. In the prostatectomy example, a patient could leave the hospital a day after the surgery because the robotic surgery reduces blood loss and pain. While the robotic arms may seem like something out of a sci-fi movie, their delicate, precise movements might make all the difference in a situation where few millimetres could mean the difference between eliminating all malignant tissue and potentially damaging healthy tissue.
Big data and AI enable medical professionals to examine a variety of data about the patient and the cancer cells to develop individualised treatments. The side effects from this kind of therapy will be less severe. Less harm will be done to healthy cells, but it will have a greater effect on cancer cells. Together with Chicago-based AI and precision medicine company Tempus, Cedars Sinai Cancer developed molecular twins of cancer patients for use in cancer treatment. They are practically identical twins of those people. They contain data like DNA, RNA, and proteins, and aid to determine the best course of cancer treatment for a specific patient. The ability to comprehend causality rather than just correlations and weightings of medical data variables is supported by Abzu’s AI technology, which increases the possibility of making new scientific discoveries
[22]. With the help of Abzu’s technology, it has been possible to pinpoint the gene combinations in breast-cancer patients that are more likely to cause mortality. These insights also make it possible to create breast-cancer treatments that are more focused and efficient. By decreasing failure rates in compound-structure activities and employing the understanding they have gained from their explainable models, Abzu’s QLattice has also been beneficial in accelerating the creation of RNA drugs. In addition, the use of AI to evaluate massive amounts of multiomics data, such as exome, transcriptome, and epigenome, along with clinically annotated datasets has produced new insights into the biology of cancer, the discovery of variants, and the prediction of RNA splice sites.
Previously, people believed that only the malignant tissue itself needed to be treated.The tissues of the lungs are treated if someone develops lung cancer. However, in the current situation, oncologists have learned that treating the cancer’s genomes rather than its tissues is more crucial to preventing recurrence, such as identifying which genes in the genome have gone awry. The vast volume of data on this topic creates numerous obstacles for proper interpretation, which AI attempts to overcome.
By offering an easy way to navigate all the research data accessible, a team of Microsoft researchers is utilising ML and NLP to assist the top oncologists in the world in determining the most effective, tailored cancer treatment for their patients
[13][23]. They are working harder to understand how genetics affects cancer development and treatment. According to Fisher, “cancer may not cease to exist but once you learn how to control it, it’s a solved problem.” They contend that the best way to achieve this is through using technology to better understand cancer in general and the biological mechanisms that give rise to malignant cells. Finding the source of the issue and learning how to resolve it come next. Fisher’s objective is to comprehend the cell’s programming, which directs a cell’s actions. Once the operation of a healthy cell is understood, a diseased cell can be contrasted with it. This can assist in identifying the source of problems and their resolution. As part of BMA, a computational model that contrasts the biological functions of a healthy cell with the aberrant functions that take place when illness occurs is created. As a result, researchers may be able to observe the interactions between millions of genes and proteins in the human body that contribute to cancer and swiftly come up with the most effective and safest method of treating each patient specifically. Clinicians might submit all the biological data regarding that patient into the system using BMA. The system may then be used to conduct a variety of tests, comparing the information from the cancer patient with that of a healthy patient, for instance, or simulating how the patient’s body could react to different treatments
[13]. Because there are so many variables among the millions of chemicals, proteins, and genes that interact within the human body, these calculations cannot be performed using straightforward programmes.
Repurposing drugs and innovative drug designing is another significant area where AI has contributed substantially. AI is able to forecast how various drugs would affect malignant cells. This information aids the creation of new anticancer medications and the timing of their use. Drug development is an expensive and time-consuming process, but AI can use neural networks to boost efficiency. Combining DL with reinforcement learning, which approximates the statistical link between potential actions and outcomes, results in a revolutionary approach for the discovery of innovative medicinal compounds. From regulatory processes to pharmacovigilance, AI has the potential to transform the entire pharmaceutical lifecycle. Based on various AI approaches, numerous computational tools have been suggested for cancer-related drug discovery. DeepChem, DeepTox, gene2drug, STITCH, AlphaFold, DeepNeuralNetQSAR, and so on are a few examples of the applications. For example, the Deep Tox algorithm computationally predicts 12,000 medicines and environmental contaminants for 12 different harmful effects in assays that are specially constructed
[24].
According to Tao Wang from the UT Southwestern Medical Center, our immune system’s T cells are continually on the lookout for indications of cancer and other foreign invaders
[15][25]. These cells bind with each other when they detect neoantigens. Neoantigens can cause cancer, yet some of them go unnoticed. The types of neoantigens number in the tens of thousands. It is difficult, expensive, and time-consuming to examine their capacity to activate T cells’ response. This is getting easier with the aid of ML.