
Video Upload Options
The improvement of feed consumption and genetic selection have been the primary areas of poultry research. The control of a variety of microbial infectious diseases caused by Streptococcus, Staphylococcus, Escherichia coli, Pseudomonas, Campylobacter, Salmonella, Yersinia, Bacillus, Clostridium, Mycobacterium, Enterococcus, Klebsiella and Proteus species has been less thoroughly investigated. The immune system of broilers is not fully developed during the first few weeks and therefore it is more susceptible to bacterial infection. Furthermore, it can take up to eight weeks for the gut microbiota to develop and stabilize. The longer the time necessary to reach bacterial homeostasis, the greater the risk of bacterial infection. Poultry are kept in closed facilities to minimize the risk of bacterial infection.
1. Probiotics and Growth Performance
The pathogen most prevalent in the intestines of chickens, particularly broilers, is Salmonella. Therefore, probiotics have been potential candidates for growth promoters in the majority of commercial poultry diets since the withdrawal of antibiotic growth enhancers in poultry nutrition. Antibiotic growth promoters act by blocking the production and secretion of pro-inflammatory cytokine-degrading intermediates in the gastrointestinal tract, resulting in the disturbed gut microbiota [1]. Probiotics, on the other hand, alter the gut environment and strengthen its barrier function through the immune system stimulation, as presented in Table 1 [2]. A total of 280 females of Japanese quails were fed with a mixture of without rapeseed meal, non-fermented post-extraction rapeseed meal (5%, 10%, 15%), and a fermented one (5%, 10%, 15%). The data analysis revealed that the addition of 10% fermented rapeseed meal had the most beneficial effects as such egg quality traits as egg weight, specific gravity, yolk index and color, and albumen pH [3]. In broilers, probiotic non-pathogenic bacteria compete with the pathogenic ones for nutrients in the gut. They also colonize the intestines, preventing pathogenic bacteria from inhabitation and stimulating the secretion of digestive enzymes (e.g., β-galactosidase, α amylase), thus facilitating the absorption of nutrients, and enhancing broiler growth performance [4]. An increased average dietary feed consumption and conversion result in an improved body weight gain, which in turn affects production performance, even though probiotics do not always significantly influence feed consumption and feed conversion. All of the above-mentioned processes depend on several factors, including strain selection and application, time concentration, as well as the absorption of dietary probiotics [5]. An increased average dietary feed consumption and an enhanced feed conversion efficiency are closely attributed to an improvement in body weight gain, which in turn complements production performance [6]. The application of dietary supplementation of probiotics improves body weight gain and feed conversion, even though probiotics do not always significantly enhance feed consumption [7]. The body weight gain, average daily diet consumption, feed conversion efficiency, and production performance of the poultry birds are influenced by several potential factors including strains selection, application, time concentration, and absorption of dietary supplementation of probiotics [8].
Table 1. Summary of the beneficial probiotics on poultry performance.
| Probiotic Strains | Biological Performance | Reference |
|---|---|---|
| Bacillus amyloliquefaciencs | Improve intestinal health and growth performance | [9][10] |
| Bacillus coagulans | Enhances growth performance and gut health | [11] |
| Lactobacillus acidophillus | Improve production performance and helps the immune system and gut histomorphology | [12][13] |
| Lactobacillus bulgaricus | Enhances growth performances and improves immune functions | [14] |
| Pediococcus acidilactici | Improves laying performances and modulates intestinal microflora composition | [15][16] |
| Propionibacterium acidipropionic | Contributes to the better development of intestinal mucosa and microbiota composition | [17] |
| Saccharomyces cerevisiae | Improves growth performance and enhances laying performance | [18] |
| Streptococcus faecium | Avoided the impairment and regulated the stability of the epithelial intestine, and improves the immune functions | [19] |
Wang et al. [20] immunized hatched chicks with a strain of Lactiplantibacillus plantarum LT-113, and found that it protected against Salmonella typhimurium by limiting gastrointestinal invasion and inhibiting tight junction gene expression in intestinal cells. In the control group, Salmonella infection compromised the intestinal mucosal barrier. On the other hand, Olnood et al. [21] revealed that the oral administration of Lactobacillus johnsonii decreased Salmonella and Clostridium perfringens invasion in the gastrointestinal system. Additionally, it has been demonstrated that the combination of xylanase and a multistrain probiotic enhanced dietary energy absorption in the intestine and its preservation in the liver [22]. Energy changes may result from improved nutrient digestibility and feed conversion rate. Probiotics increased the synthesis of short-chain fatty acids, stimulated the immune system and metabolism [23][24].
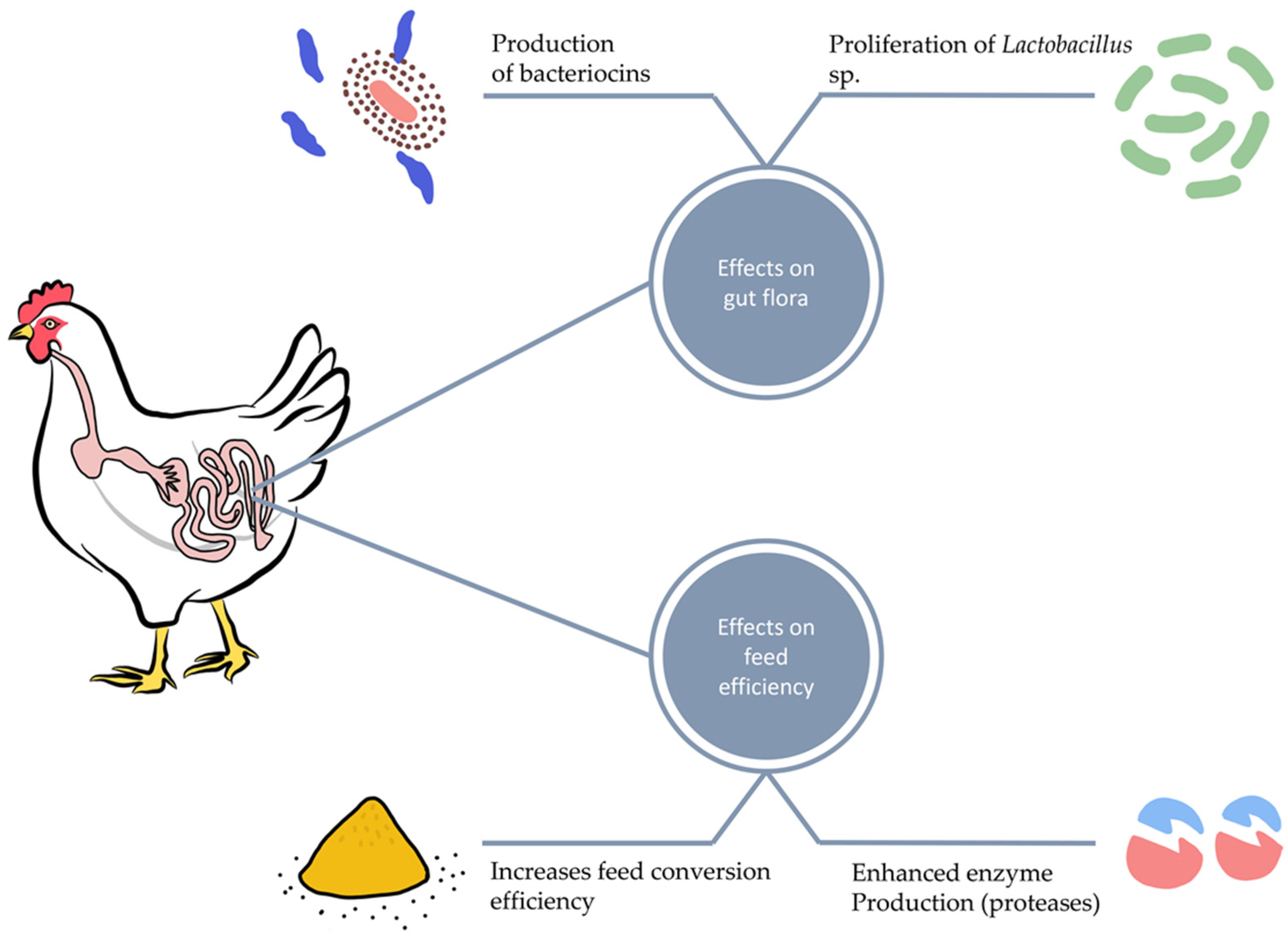
Short-chain fatty acid (SCFA) metabolites produced during microbial carbohydrate fermentation in the gastrointestinal tract impact leukocytes and endothelial cells by stimulating G-protein-coupled receptors and inhibiting histone deacetylase. SCFAs increase the level of IgA produced by B immune cells, impede the NF-κB transcription factors and reduce the production of proinflammatory cytokines [25][26]. Dietary supplementation of poultry with Bacillus licheniformis, a facultative anaerobic bacterium, enhances the gastrointestinal tract absorption rate and surface area. It also stimulates the growth and multiplication of probiotic bacteria such as Lactobacillus, Bifidobacterium, and Aspergillus awamori, as shown in Figure 1 [27].
2. Probiotics and Intestinal Morphology
Zheng et al. [11] exposed broilers to Salmonella enteritidis (SE) and found a significant decline in goblet cell membranes at 7 days post-infection (DPI), as well as a reduction in villus height and villus-crypt ratio in the small intestine. In contrast, birds fed dietary supplementation of Bacillus coagulans had a relatively low crypt depth, a greater villus-crypt ratio, and a larger number of goblet cells in the jejunum at 7 and 17 days post-infection. Gastrointestinal mucous cells synthesize mucin-2, a constituent of mucus, which facilitates the enhancement of barrier activity in Salmonella enteritidis-infected birds. Supplementation with a Bacillus licheniformis-fermented product at 1.25 and 5 g/kg improved cecal morphology and increased the survival rate of broilers and conserve a stable number of goblet cells in the ileum as well as in the caecum under Eimeria tenella challenge. A 1.25 g/kg dose reduced lesions scores in the cecum, while that of 5 g/kg decreased the oocyst-count index. Furthermore, surfactin C isolated from Bacillus licheniformis-fermented products inhibited Eimeria oocyst sporulation and disrupted sporozoite morphology [37].
3. Probiotics and Immune Response
References
- Adedokun, S.A.; Olojede, O.C. Optimizing gastrointestinal integrity in poultry: The role of nutrients and feed additives. Front. Vet. Sci. 2019, 5, 348.
- Al-Khalaifah, H. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018, 97, 3807–3815.
- Wengerska, K.; Czech, A.; Knaga, S.; Drabik, K.; Próchniak, T.; Bagrowski, R.; Gryta, A.; Batkowska, J. The Quality of Eggs Derived from Japanese Quail Fed with the Fermented and Non-Fermented Rapeseed Meal. Foods 2022, 11, 2492.
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395.
- Flores, C.; Duong, T.; Askelson, T.; Dersjant-Li, Y.; Gibbs, K.; Awati, A.; Lee, J. Effects of direct fed-microorganisms and enzyme blend co-administration on growth performance in broilers fed diets with or without antibiotics. J. Appl. Poult. Res. 2019, 28, 1181–1188.
- Cervera, C.; Carmona, J.F. Nutrition and climatic environment. In Nutrition of the Rabbit; CAB International: Wallingford, UK, 2020; pp. 289–307.
- Angelakis, E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017, 106, 162–170.
- Flores, C.; Askelson, T.; Dersjant-Li, Y.; Gibbs, K.; Awati, A.; Duong, T.; Lee, J. Effect of Direct-Fed Microorganisms and enzyme blend co-administration on broilers fed US commercial-type, diets with or without agp. J. Appl. Poult. Res. 2018, 28, 1181–1188.
- Li, Y.; Zhang, H.; Chen, Y.; Yang, M.; Zhang, L.; Lu, Z.; Zhou, Y.; Wang, T. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide-challenged broilers at early age. Poult. Sci. 2015, 94, 1504–1511.
- Mazanko, M.S.; Gorlov, I.F.; Prazdnova, E.V.; Makarenko, M.S.; Usatov, A.V.; Bren, A.B.; Chistyakov, V.A.; Tutelyan, A.V.; Komarova, Z.B.; Mosolova, N.I. Bacillus probiotic supplementations improve laying performance, egg quality, hatching of laying hens, and sperm quality of roosters. Probiotics Antimicrob. Proteins 2018, 10, 367–373.
- Zhen, W.; Shao, Y.; Gong, X.; Wu, Y.; Geng, Y.; Wang, Z.; Guo, Y. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis. Poult. Sci. 2018, 97, 2654–2666.
- Pender, C.M.; Kim, S.; Potter, T.D.; Ritzi, M.M.; Young, M.; Dalloul, R.A. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks. Poult. Sci. 2017, 96, 1052–1062.
- Forte, C.; Acuti, G.; Manuali, E.; Proietti, P.C.; Pavone, S.; Trabalza-Marinucci, M.; Moscati, L.; Onofri, A.; Lorenzetti, C.; Franciosini, M.P. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult. Sci. 2016, 95, 2528–2535.
- Cheng, Y.; Chen, Y.; Li, X.; Yang, W.; Wen, C.; Kang, Y.; Wang, A.; Zhou, Y. Effects of synbiotic supplementation on growth performance, carcass characteristics, meat quality and muscular antioxidant capacity and mineral contents in broilers. J. Sci. Food Agric. 2017, 97, 3699–3705.
- Mikulski, D.; Jankowski, J.; Mikulska, M.; Demey, V. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on productive performance, egg quality, and body composition in laying hens fed diets varying in energy density. Poult. Sci. 2020, 99, 2275–2285.
- Mountzouris, K.; Tsitrsikos, P.; Palamidi, I.; Arvaniti, A.; Mohnl, M.; Schatzmayr, G.; Fegeros, K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010, 89, 58–67.
- Martínez, E.A.; Babot, J.D.; Lorenzo-Pisarello, M.J.; Apella, M.C.; Chaia, A.P. Feed supplementation with avian Propionibacterium acidipropionici contributes to mucosa development in early stages of rearing broiler chickens. Benef. Microbes. 2016, 7, 687–698.
- Hassanein, S.M.; Soliman, N.K. Effect of probiotic (Saccharomyces cerevisiae) adding to diets on intestinal microflora and performance of Hy-Line layers hens. J. Am. Sci. 2010, 6, 159–169.
- Alagawany, M.; El-Hack, A.; Mohamed, E.; Farag, M.R.; Sachan, S.; Karthik, K.; Dhama, K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018, 25, 10611–10618.
- Shafiq, M.; Yao, F.; Bilal, H.; Rahman, S.U.; Zeng, M.; Ali, I.; Zeng, Y.; Li, X.; Yuan, Y.; Jiao, X. Synergistic Activity of Tetrandrine and Colistin against mcr-1-Harboring Escherichia coli. Antibiotics 2022, 11, 1346.
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346.
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.-L.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in poultry manure and their associated health issues: A systematic review. J. Soils Sediments 2020, 20, 486–497.
- Yaqoob, M.U.; Wang, G. An updated review on probiotics as an alternative of antibiotics in poultry. Anim. Biosci. 2022, 35, 1109–1120.
- Abdel-Moneim, A.-M.E.; Shehata, A.M.; Khidr, R.E.; Paswan, V.K.; Ibrahim, N.S.; El-Ghoul, A.A.; Aldhumri, S.A.; Gabr, S.A.; Mesalam, N.M.; Elbaz, A.M. Nutritional manipulation to combat heat stress in poultry—A comprehensive review. J. Therm. Biol. 2021, 98, 102915.
- Coman, M.M.; Mazzotti, L.; Silvi, S.; Scalise, A.; Orpianesi, C.; Cresci, A.; Verdenelli, M.C. Antimicrobial activity of SYNBIO® probiotic formulation in pathogens isolated from chronic ulcerative lesions: In Vitro studies. J. Appl. Microbiol. 2020, 128, 584–597.
- Adedokun, S.A.; Olojede, O.C. Optimizing gastrointestinal integrity in poultry: The role of nutrients and feed additives. Front. Vet. Sci. 2019, 5, 348.
- Al-Khalaifah, H. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018, 97, 3807–3815.
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019, 250, 32–40.
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2.
- Ahmad, R.; Yu, Y.-H.; Hsiao, F.S.-H.; Su, C.-H.; Liu, H.-C.; Tobin, I.; Zhang, G.; Cheng, Y.-H. Influence of Heat Stress on Poultry Growth Performance, Intestinal Inflammation, and Immune Function and Potential Mitigation by Probiotics. Animals 2022, 12, 2297.
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (direct-fed microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: A systematic review. Animals 2020, 10, 1863.
- Khalique, A.; Zeng, D.; Shoaib, M.; Wang, H.; Qing, X.; Rajput, D.S.; Pan, K.; Ni, X. Probiotics mitigating subclinical necrotic enteritis (SNE) as potential alternatives to antibiotics in poultry. AMB Express 2020, 10, 50.
- Robert, H.; Payros, D.; Pinton, P.; Theodorou, V.; Mercier-Bonin, M.; Oswald, I.P. Impact of mycotoxins on the intestine: Are mucus and microbiota new targets? J. Toxicol. Environ. Health Part B 2017, 20, 249–275.
- Bezirtzoglou, E.E.V. Intestinal cytochromes P450 regulating the intestinal microbiota and its probiotic profile. Microb. Ecol. Health Dis. 2012, 23, 1.
- Barkhidarian, B.; Roldos, L.; Iskandar, M.M.; Saedisomeolia, A.; Kubow, S. Probiotic supplementation and micronutrient status in healthy subjects: A systematic review of clinical trials. Nutrients 2021, 13, 3001.
- Meyer, M.M.; Fries-Craft, K.A.; Bobeck, E.A. Composition and inclusion of probiotics in broiler diets alter intestinal permeability and spleen immune cell profiles without negatively affecting performance. J. Anim. Sci. 2020, 98, skz383.
- Yu, Y.-H.; Wu, C.-M.; Chen, W.-J.; Hua, K.-F.; Liu, J.-R.; Cheng, Y.-H. Effectiveness of Bacillus licheniformis-Fermented Products and Their Derived Antimicrobial Lipopeptides in Controlling Coccidiosis in Broilers. Animals 2021, 11, 3576.
- Nochi, T.; Jansen, C.A.; Toyomizu, M.; Eden, W. The well-developed mucosal immune systems of birds and mammals allow for similar approaches of mucosal vaccination in both types of animals. Front. Nutr. 2018, 5, 60.
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090.
- Haykin, H.; Rolls, A. The neuroimmune response during stress: A physiological perspective. Immunity 2021, 54, 1933–1947.
- Nguyen, J.; Pepin, D.M.; Tropini, C. Cause or effect? The spatial organization of pathogens and the gut microbiota in disease. Microbes Infect. 2021, 23, 104815.
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 178.
- Hong, Y.; Cheng, Y.; Guan, L.; Zhou, Z.; Li, X.; Shi, D.; Xiao, Y. Bacillus amyloliquefaciens TL downregulates the ileal expression of genes involved in immune responses in broiler chickens to improve growth performance. Microorganisms 2021, 9, 382.
- Cheng, Y.-H.; Horng, Y.-B.; Dybus, A.; Yu, Y.-H. Bacillus licheniformis-fermented products improve growth performance and intestinal gut morphology in broilers under Clostridium perfringens challenge. Poult. Sci. J. 2021, 58, 30–39.
- Jacquier, V.; Nelson, A.; Jlali, M.; Rhayat, L.; Brinch, K.; Devillard, E. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poult. Sci. 2019, 98, 2548–2554.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.M.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850.
- Yitbarek, A.; Echeverry, H.; Munyaka, P.; Rodriguez-Lecompte, J. Innate immune response of pullets fed diets supplemented with prebiotics and synbiotics. Poult. Sci. 2015, 94, 1802–1811.
- Hatab, M.; Elsayed, M.; Ibrahim, N. Effect of some biological supplementation on productive performance, physiological and immunological response of layer chicks. J. Radiat. Res. Appl. Sci. 2016, 9, 185–192.
- Manafi, M.; Khalaji, S.; Hedayati, M.; Pirany, N. Efficacy of Bacillus subtilis and bacitracin methylene disalicylate on growth performance, digestibility, blood metabolites, immunity, and intestinal microbiota after intramuscular inoculation with Escherichia coli in broilers. Poult. Sci. 2017, 96, 1174–1183.




