
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victor Sans | -- | 3522 | 2022-12-07 15:47:34 | | | |
| 2 | Peter Tang | Meta information modification | 3522 | 2022-12-08 04:27:22 | | |
Video Upload Options
Innovation in materials specially formulated for additive manufacturing is of great interest and can generate new opportunities for designing cost-effective smart materials for next-generation devices and engineering applications. Nevertheless, advanced molecular and nanostructured systems are frequently not possible to integrate into 3D printable materials, thus limiting their technological transferability. In some cases, this challenge can be overcome using polymeric macromolecules of ionic nature, such as polymeric ionic liquids (PILs). Due to their tuneability, wide variety in molecular composition, and macromolecular architecture, they show a remarkable ability to stabilize molecular and nanostructured materials. The technology resulting from 3D-printable PIL-based formulations represents an untapped array of potential applications, including optoelectronic, antimicrobial, catalysis, photoactive, conductive, and redox applications.
1. Introduction
2. 3D Printing Overview
|
Process Principle |
AM Technology |
Materials |
Features |
|---|---|---|---|
|
Vat photopolymerization (VP) |
Stereolithography (SLA) Dynamic light processing (DLP) Continuous Liquid Interface Production (CLIP) |
Photopolymer Ceramic |
High resolution Slow process High cost |
|
Powder bed fusion (PBF) |
Selective laser sintering (SLS) Selective laser melting (SLM) Electron beam melting (EBM) |
Metal Polymer Ceramic |
High resolution Slow process High cost |
|
Material extrusion |
Fused filament fabrication (FFF) Direct ink writing (DIW) |
Polymer Ceramic Biomaterials |
Low resolution Fast process Low cost |
|
Material Jetting (MJ) |
Ink-jetting Thermojet Polyjet |
Photopolymer Wax |
High resolution Fast process |
|
Binder Jetting (BJ) |
Ink-jetting |
Metal Polymer Ceramic |
High resolution Slow process High cost |
|
Direct Energy Deposition (DED) |
Direct Metal Deposition (DMD) Laser Deposition Laser Consolidation |
Metal Powder Wire |
Low resolution Fast process Low cost |
|
Sheet lamination (SL) |
Ultrasonic consolidation Laminated object manufacturing (LOM) |
Hybrids Metallic Ceramic |
Low resolution Fast process Low cost |
3. Polymeric Ionic Liquids
3.1. Properties of Ionic Liquids
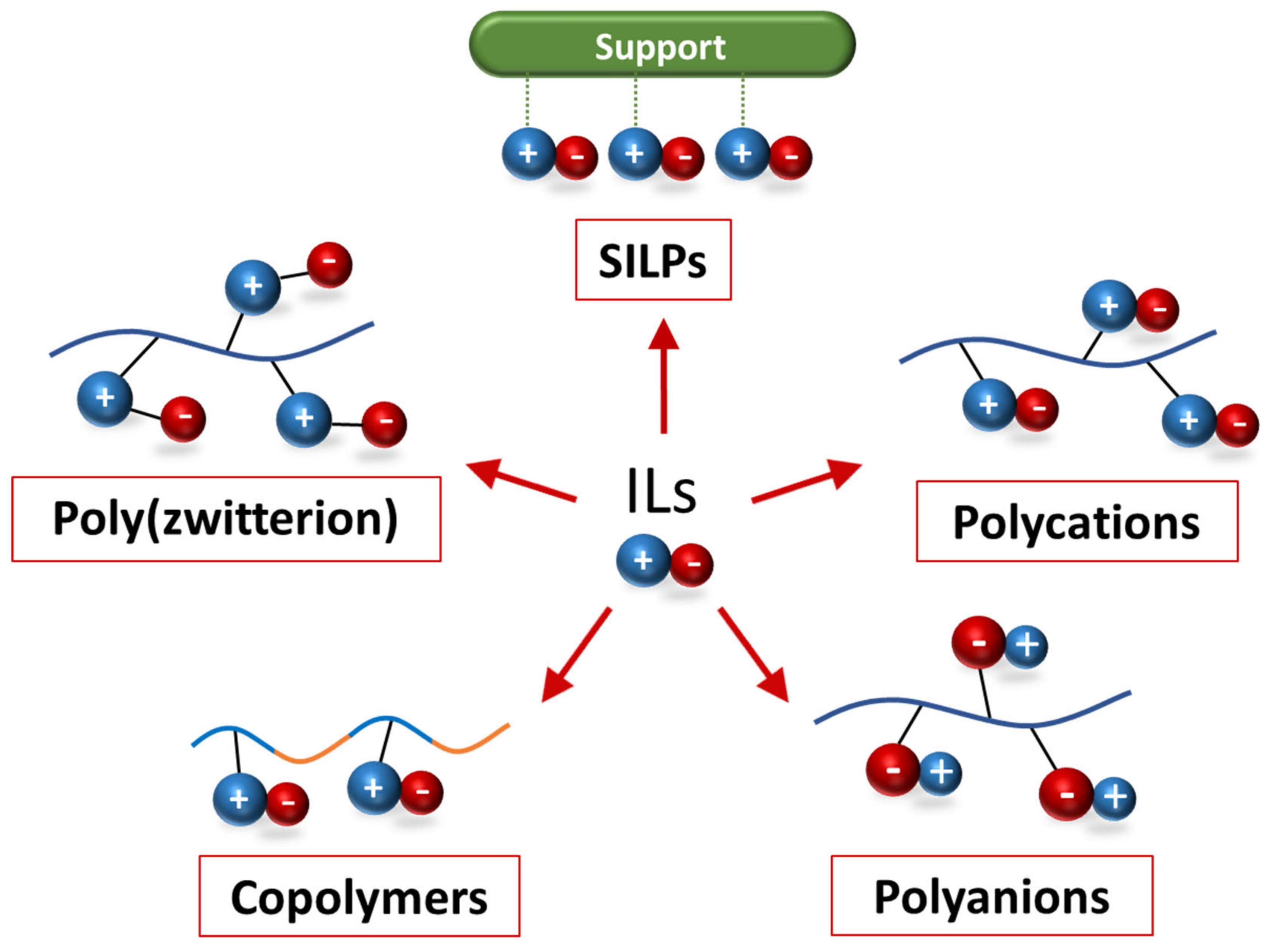
3.2. Properties of Polymeric Ionic Liquids
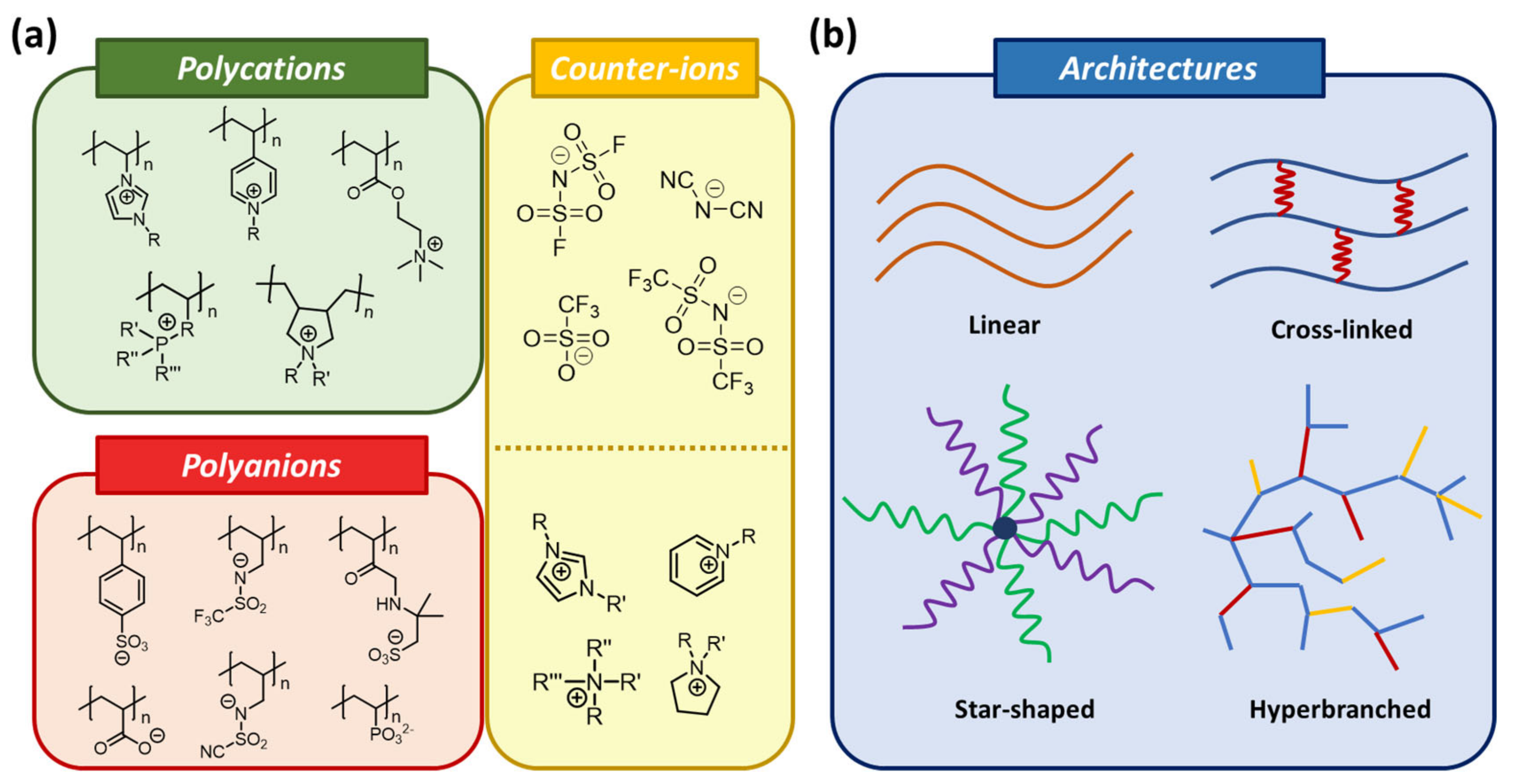
4. Applications of 3D-Printable PILs
References
- Dilberoglu, U.M.; Gharehpapagh, B.; Yaman, U.; Dolen, M. The Role of Additive Manufacturing in the Era of Industry 4.0. Procedia Manuf. 2017, 11, 545–554.
- Bogue, R. Smart materials: A review of capabilities and applications. Assem. Autom. 2014, 34, 16–22.
- Narupai, B.; Nelson, A. 100th Anniversary of Macromolecular Science Viewpoint: Macromolecular Materials for Additive Manufacturing. ACS Macro Lett. 2020, 9, 627–638.
- Shafranek, R.T.; Millik, S.C.; Smith, P.T.; Lee, C.-U.; Boydston, A.J.; Nelson, A. Stimuli-responsive materials in additive manufacturing. Prog. Polym. Sci. 2019, 93, 36–67.
- Lin, Q.; Tang, M.; Ke, C. Thermo-responsive 3D-printed polyrotaxane monolith. Polym. Chem. 2020, 11, 304–308.
- Dharmarwardana, M.; Arimilli, B.S.; Luzuriaga, M.A.; Kwon, S.; Lee, H.; Appuhamillage, G.A.; McCandless, G.T.; Smaldone, R.A.; Gassensmith, J.J. The thermo-responsive behavior in molecular crystals of naphthalene diimides and their 3D printed thermochromic composites. CrystEngComm 2018, 20, 6054–6060.
- Berry, D.R.; Díaz, B.K.; Durand-Silva, A.; Smaldone, R.A. Radical free crosslinking of direct-write 3D printed hydrogels through a base catalyzed thiol-Michael reaction. Polym. Chem. 2019, 10, 5979–5984.
- Appuhamillage, G.A.; Berry, D.R.; Benjamin, C.E.; Luzuriaga, M.A.; Reagan, J.C.; Gassensmith, J.J.; Smaldone, R.A. A biopolymer-based 3D printable hydrogel for toxic metal adsorption from water. Polym. Int. 2019, 68, 964–971.
- Smith, P.T.; Narupai, B.; Tsui, J.H.; Millik, S.C.; Shafranek, R.T.; Kim, D.H.; Nelson, A. Additive Manufacturing of Bovine Serum Albumin-Based Hydrogels and Bioplastics. Biomacromolecules 2020, 21, 484–492.
- Zhang, M.; Li, L.; Lin, Q.; Tang, M.; Wu, Y.; Ke, C. Hierarchical-Coassembly-Enabled 3D-Printing of Homogeneous and Heterogeneous Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 5154–5158.
- Murphy, R.D.; Kimmins, S.; Hibbitts, A.J.; Heise, A. 3D-extrusion printing of stable constructs composed of photoresponsive polypeptide hydrogels. Polym. Chem. 2019, 10, 4675–4682.
- Wong, J.; Gong, A.T.; Defnet, P.A.; Meabe, L.; Beauchamp, B.; Sweet, R.M.; Sardon, H.; Cobb, C.L.; Nelson, A. 3D Printing Ionogel Auxetic Frameworks for Stretchable Sensors. Adv. Mater. Technol. 2019, 4, 1900452.
- Wong, J.; Basu, A.; Wende, M.; Boechler, N.; Nelson, A. Mechano-Activated Objects with Multidirectional Shape Morphing Programmed via 3D Printing. ACS Appl. Polym. Mater. 2020, 2, 2504–2508.
- Wang, Z.; Zhang, J.; Liu, J.; Hao, S.; Song, H.; Zhang, J. 3D Printable, Highly Stretchable, Superior Stable Ionogels Based on Poly(ionic liquid) with Hyperbranched Polymers as Macro-cross-linkers for High-Performance Strain Sensors. ACS Appl. Mater. Interfaces 2021, 13, 5614–5624.
- Boydston, A.J.; Nelson, A. Chemical advances in additive manufacturing. Polym. Chem. 2019, 10, 5948.
- Yin, J.; Lei, Q.; Dong, Y.; Zhao, X. Stimuli Responsive Smart Fluids Based on Ionic Liquids and Poly(ionic liquid)s. In Polymerized Ionic Liquids; The Royal Society of Chemistry: London, UK, 2018; pp. 180–201.
- Tang, Y.; Tang, B.; Wu, P. A polymeric ionic liquid functionalized temperature-responsive composite membrane with tunable responsive behavior. J. Mater. Chem. A 2015, 3, 7919–7928.
- Tudor, A.; Florea, L.; Gallagher, S.; Burns, J.; Diamond, D. Poly(Ionic Liquid) Semi-Interpenetrating Network Multi-Responsive Hydrogels. Sensors 2016, 16, 219.
- Zhao, Q.; Heyda, J.; Dzubiella, J.; Täuber, K.; Dunlop, J.W.C.; Yuan, J. Sensing Solvents with Ultrasensitive Porous Poly(ionic liquid) Actuators. Adv. Mater. 2015, 27, 2913–2917.
- Zhao, Q.; Yin, M.; Zhang, A.P.; Prescher, S.; Antonietti, M.; Yuan, J. Hierarchically Structured Nanoporous Poly(Ionic Liquid) Membranes: Facile Preparation and Application in Fiber-Optic pH Sensing. J. Am. Chem. Soc. 2013, 135, 5549–5552.
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-responsive polymeric materials: Synthesis, self-assembly, and functional applications. Chem. Soc. Rev. 2016, 45, 4391–4436.
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648.
- Marcilla, R.; Blazquez, J.A.; Rodriguez, J.; Pomposo, J.A.; Mecerreyes, D. Tuning the solubility of polymerized ionic liquids by simple anion-exchange reactions. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 208–212.
- Nulwala, H.; Mirjafari, A.; Zhou, X. Ionic liquids and poly(ionic liquid)s for 3D printing—A focused mini-review. Eur. Polym. J. 2018, 108, 390–398.
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159.
- Lv, X.; Wang, J.; Ding, D.; Liang, J.; Zhao, Z.; Liang, Y.; Zhang, Z.; Ye, C.; Chen, Y.; Wei, P.; et al. 3D Printing Conductive Composites with Poly(ionic liquid) as a Noncovalent Intermedia to Fabricate Carbon Circuits. Macromol. Mater. Eng. 2021, 306, 2100560.
- Wales, D.J.; Cao, Q.; Kastner, K.; Karjalainen, E.; Newton, G.N.; Sans, V. 3D-Printable Photochromic Molecular Materials for Reversible Information Storage. Adv. Mater. 2018, 30, 1800159.
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196.
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162.
- Wohlers, T.T.; Campbell, I.; Diegel, O.; Huff, R.; Kowen, J. 3D Printing and Additive Manufacturing Global State of the Industry Annual Worldwide Progress; Wohlers Associates: Fort Collins, CO, USA, 2021.
- Maciel, V.G.; Wales, D.J.; Seferin, M.; Sans, V. Environmental performance of 3D-Printing polymerisable ionic liquids. J. Clean. Prod. 2019, 214, 29–40.
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. US Patent US4575330A, 11 March 1986.
- Zhu, C.; Liu, T.; Qian, F.; Chen, W.; Chandrasekaran, S.; Yao, B.; Song, Y.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; et al. 3D printed functional nanomaterials for electrochemical energy storage. Nano Today 2017, 15, 107–120.
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D printing technologies for electrochemical energy storage. Nano Energy 2017, 40, 418–431.
- Pham, D.T.; Gault, R.S. A comparison of rapid prototyping technologies. Int. J. Mach. Tools Manuf. 1998, 38, 1257–1287.
- Rajaguru, K.; Karthikeyan, T.; Vijayan, V. Additive manufacturing–State of art. Mater. Today: Proc. 2020, 21, 628–633.
- Horn, T.J.; Harrysson, O.L.A. Overview of Current Additive Manufacturing Technologies and Selected Applications. Sci. Prog. 2012, 95, 255–282.
- Miralles-Comins, S.; Alvarez, E.; Lozano, P.; Sans, V. 9 Exothermic advanced manufacturing techniques in reactor engineering: 3D printing applications in flow chemistry. In Flow Chemistry—Applications; De Gruyter: Berlin, Germany, 2021; Volume 2, pp. 259–276.
- Karjalainen, E.; Wales, D.J.; Gunasekera, D.H.A.T.; Dupont, J.; Licence, P.; Wildman, R.D.; Sans, V. Tunable Ionic Control of Polymeric Films for Inkjet Based 3D Printing. ACS Sustain. Chem. Eng. 2018, 6, 3984–3991.
- Ahmed, K.; Kawakami, M.; Khosla, A.; Furukawa, H. Soft, conductive nanocomposites based on ionic liquids/carbon nanotubes for 3D printing of flexible electronic devices. Polym. J. 2019, 51, 511–521.
- Schultz, A.R.; Lambert, P.M.; Chartrain, N.A.; Ruohoniemi, D.M.; Zhang, Z.; Jangu, C.; Zhang, M.; Williams, C.B.; Long, T.E. 3D Printing Phosphonium Ionic Liquid Networks with Mask Projection Microstereolithography. ACS Macro Lett. 2014, 3, 1205–1209.
- Radchenko, A.V.; Duchet-Rumeau, J.; Gérard, J.-F.; Baudoux, J.; Livi, S. Cycloaliphatic epoxidized ionic liquids as new versatile monomers for the development of shape memory PIL networks by 3D printing. Polym. Chem. 2020, 11, 5475–5483.
- Gabriel, S.; Weiner, J. Ueber einige Abkömmlinge des Propylamins. Ber. Der Dtsch. Chem. Ges. 1888, 21, 2669–2679.
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706.
- Dubal, D.P.; Chodankar, N.R.; Kim, D.-H.; Gomez-Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129.
- Smiglak, M.; Pringle, J.M.; Lu, X.; Han, L.; Zhang, S.; Gao, H.; MacFarlane, D.R.; Rogers, R.D. Ionic liquids for energy, materials, and medicine. Chem. Commun. 2014, 50, 9228–9250.
- Evans, D.F.; Chen, S.H.; Schriver, G.W.; Arnett, E.M. Thermodynamics of solution of nonpolar gases in a fused salt. “Hydrophobic bonding” behavior in a nonaqueous system. J. Am. Chem. Soc. 1981, 103, 481–482.
- Fischer, T.; Sethi, A.; Welton, T.; Woolf, J. Diels-Alder reactions in room-temperature ionic liquids. Tetrahedron Lett. 1999, 40, 793–796.
- Badri, M.; Brunet, J.-J.; Perron, R. Ionic liquids as solvents for the regioselective O-alkylation of C/O ambident nucleophiles. Tetrahedron Lett. 1992, 33, 4435–4438.
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398.
- Seddon, K.R. Ionic Liquids for Clean Technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356.
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic liquids are not always green: Hydrolysis of 1-butyl-3- methylimidazolium hexafluorophosphate. Green Chem. 2003, 5, 361–363.
- Thuy Pham, T.P.; Cho, C.W.; Yun, Y.S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372.
- Cevasco, G.; Chiappe, C. Are ionic liquids a proper solution to current environmental challenges? Green Chem. 2014, 16, 2375–2385.
- Welton, T. Solvents and sustainable chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150502.
- Wasserscheid, P.; Keim, W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789.
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Ionic Liquid (Molten Salt) Phase Organometallic Catalysis. Chem. Rev. 2002, 102, 3667–3692.
- Poole, C.F.; Furton, K.G.; Kersten, B.R. Liquid Organic Salt Phases for Gas Chromatography. J. Chromatogr. Sci. 1986, 24, 400–409.
- Zeng, S.; Zhang, X.; Bai, L.; Zhang, X.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673.
- Liu, H.; Liu, Y.; Li, J. Ionic liquids in surface electrochemistry. Phys. Chem. Chem. Phys. 2010, 12, 1685–1697.
- Medetalibeyoğlu, H.; Manap, S.; Yokuş, Ö.A.; Beytur, M.; Kardaş, F.; Akyıldırım, O.; Özkan, V.; Yüksek, H.; Yola, M.L.; Atar, N. Fabrication of Pt/Pd Nanoparticles/Polyoxometalate/Ionic Liquid Nanohybrid for Electrocatalytic Oxidation of Methanol. J. Electrochem. Soc. 2018, 165, F338–F341.
- Majidi, L.; Yasaei, P.; Warburton, R.E.; Fuladi, S.; Cavin, J.; Hu, X.; Hemmat, Z.; Cho, S.B.; Abbasi, P.; Vörös, M.; et al. New Class of Electrocatalysts Based on 2D Transition Metal Dichalcogenides in Ionic Liquid. Adv. Mater. 2019, 31, 1804453.
- Lim, H.-K.; Kwon, Y.; Kim, H.S.; Jeon, J.; Kim, Y.-H.; Lim, J.-A.; Kim, B.-S.; Choi, J.; Kim, H. Insight into the Microenvironments of the Metal–Ionic Liquid Interface during Electrochemical CO2 Reduction. ACS Catal. 2018, 8, 2420–2427.
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189.
- Nancarrow, P.; Mohammed, H. Ionic Liquids in Space Technology—Current and Future Trends. ChemBioEng Rev. 2017, 4, 106–119.
- Radai, Z.; Kiss, N.Z.; Keglevich, G. An Overview of the Applications of Ionic Liquids as Catalysts and Additives in Organic Chemical Reactions. Curr. Org. Chem. 2018, 22, 533–556.
- Lee, J.W.; Shin, J.Y.; Chun, Y.S.; Jang, H.B.; Song, C.E.; Lee, S.-g. Toward Understanding the Origin of Positive Effects of Ionic Liquids on Catalysis: Formation of More Reactive Catalysts and Stabilization of Reactive Intermediates and Transition States in Ionic Liquids. Acc. Chem. Res. 2010, 43, 985–994.
- Zhang, Q.; Zhang, S.; Deng, Y. Recent advances in ionic liquid catalysis. Green Chem. 2011, 13, 2619–2637.
- Wang, D.; Zhao, F.; Zhu, G.; Xia, C. Production of eco-friendly poly(oxymethylene) dimethyl ethers catalyzed by acidic ionic liquid: A kinetic investigation. Chem. Eng. J. 2018, 334, 2616–2624.
- Roman, F.F.; Ribeiro, A.E.; Queiroz, A.; Lenzi, G.G.; Chaves, E.S.; Brito, P. Optimization and kinetic study of biodiesel production through esterification of oleic acid applying ionic liquids as catalysts. Fuel 2019, 239, 1231–1239.
- Vieira, M.O.; Monteiro, W.F.; Neto, B.S.; Ligabue, R.; Chaban, V.V.; Einloft, S. Surface Active Ionic Liquids as Catalyst for CO2 Conversion to Propylene Carbonate. Catal. Lett. 2018, 148, 108–118.
- Dai, J.; Patti, A.F.; Longé, L.; Garnier, G.; Saito, K. Oxidized Lignin Depolymerization using Formate Ionic Liquid as Catalyst and Solvent. ChemCatChem 2017, 9, 2684–2690.
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812.
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629.
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580.
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239.
- Jónsson, E. Ionic liquids as electrolytes for energy storage applications—A modelling perspective. Energy Storage Mater. 2020, 25, 827–835.
- Stettner, T.; Huang, P.; Goktas, M.; Adelhelm, P.; Balducci, A. Mixtures of glyme and aprotic-protic ionic liquids as electrolytes for energy storage devices. J. Chem. Phys. 2018, 148, 193825.
- Sevilla, M.; Ferrero, G.A.; Diez, N.; Fuertes, A.B. One-step synthesis of ultra-high surface area nanoporous carbons and their application for electrochemical energy storage. Carbon 2018, 131, 193–200.
- Chapman Varela, J.; Sankar, K.; Hino, A.; Lin, X.; Chang, W.-s.; Coker, D.; Grinstaff, M. Piperidinium ionic liquids as electrolyte solvents for sustained high temperature supercapacitor operation. Chem. Commun. 2018, 54, 5590–5593.
- Zaccagnini, P.; di Giovanni, D.; Gomez, M.G.; Passerini, S.; Varzi, A.; Lamberti, A. Flexible and high temperature supercapacitor based on laser-induced graphene electrodes and ionic liquid electrolyte, a de-rated voltage analysis. Electrochim. Acta 2020, 357, 136838.
- Suominen, M.; Lehtimäki, S.; Yewale, R.; Damlin, P.; Tuukkanen, S.; Kvarnström, C. Electropolymerized polyazulene as active material in flexible supercapacitors. J. Power Sources 2017, 356, 181–190.
- Navarra, M.A.; Fujimura, K.; Sgambetterra, M.; Tsurumaki, A.; Panero, S.; Nakamura, N.; Ohno, H.; Scrosati, B. New Ether-functionalized Morpholinium- and Piperidinium-based Ionic Liquids as Electrolyte Components in Lithium and Lithium–Ion Batteries. ChemSusChem 2017, 10, 2496–2504.
- Kerner, M.; Johansson, P. Pyrrolidinium FSI and TFSI-Based Polymerized Ionic Liquids as Electrolytes for High-Temperature Lithium-Ion Batteries. Batteries 2018, 4, 10.
- Yang, D.; Zhu, Q.; Han, B. Electroreduction of CO2 in Ionic Liquid-Based Electrolytes. Innovation 2020, 1, 100016.
- Pardal, T.; Messias, S.; Sousa, M.; Machado, A.S.R.; Rangel, C.M.; Nunes, D.; Pinto, J.V.; Martins, R.; da Ponte, M.N. Syngas production by electrochemical CO2 reduction in an ionic liquid based-electrolyte. J. CO2 Util. 2017, 18, 62–72.
- Sharma, T.; Gultekin, B.; Dhapola, P.S.; Sahoo, N.G.; Kumar, S.; Agarwal, D.; Jun, H.K.; Singh, D.; Nath, G.; Singh, P.K.; et al. Ionic liquid doped Poly (methyl methacrylate) for energy applications. J. Mol. Liq. 2022, 352, 118494.
- Hao, X.; Wenren, H.; Wang, X.; Xia, X.; Tu, J. A gel polymer electrolyte based on PVDF-HFP modified double polymer matrices via ultraviolet polymerization for lithium-sulfur batteries. J. Colloid Interface Sci. 2019, 558, 145–154.
- Gao, G.; Wang, J.; Zhang, X.; Li, H.; Wang, L.; Liu, T. An ionic liquid enhanced gel polymer electrolyte for high performance lithium-metal batteries based on sulfurized polyacrylonitrile cathode. Compos. Commun. 2022, 31, 101100.
- Ding, Y.; Zhang, J.; Chang, L.; Zhang, X.; Liu, H.; Jiang, L. Preparation of High-Performance Ionogels with Excellent Transparency, Good Mechanical Strength, and High Conductivity. Adv. Mater. 2017, 29, 1704253.
- Kee, S.; Kim, N.; Kim, B.S.; Park, S.; Jang, Y.H.; Lee, S.H.; Kim, J.; Kim, J.; Kwon, S.; Lee, K. Controlling Molecular Ordering in Aqueous Conducting Polymers Using Ionic Liquids. Adv. Mater. 2016, 28, 8625–8631.
- De Izarra, A.; Park, S.; Lee, J.; Lansac, Y.; Jang, Y.H. Ionic Liquid Designed for PEDOT:PSS Conductivity Enhancement. J. Am. Chem. Soc. 2018, 140, 5375–5384.
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150.
- Mehnert, C.P. Supported Ionic Liquid Catalysis. Chem. A Eur. J. 2005, 11, 50–56.
- Karbass, N.; Sans, V.; Garcia-Verdugo, E.; Burguete, M.I.; Luis, S.V. Pd(0) supported onto monolithic polymers containing IL-like moieties. Continuous flow catalysis for the Heck reaction in near-critical EtOH. Chem. Commun. 2006, 2006, 3095–3097.
- Burguete, M.I.; Galindo, F.; Garcia-Verdugo, E.; Karbass, N.; Luis, S.V. Polymer supported ionic liquid phases (SILPs) versus ionic liquids (ILs): How much do they look alike. Chem. Commun. 2007, 2007, 3086–3088.
- Patil, R.V.; Chavan, J.U.; Dalal, D.S.; Shinde, V.S.; Beldar, A.G. Biginelli Reaction: Polymer Supported Catalytic Approaches. ACS Comb. Sci. 2019, 21, 105–148.
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513.
- Hirao, M.; Ito, K.; Ohno, H. Preparation and polymerization of new organic molten salts; N-alkylimidazolium salt derivatives. Electrochim. Acta 2000, 45, 1291–1294.
- Yoshizawa, M.; Ogihara, W.; Ohno, H. Novel polymer electrolytes prepared by copolymerization of ionic liquid monomers. Polym. Adv. Technol. 2002, 13, 589–594.
- Ogihara, W.; Washiro, S.; Nakajima, H.; Ohno, H. Effect of cation structure on the electrochemical and thermal properties of ion conductive polymers obtained from polymerizable ionic liquids. Electrochim. Acta 2006, 51, 2614–2619.
- Yoshizawa, M.; Hirao, M.; Ito-Akita, K.; Ohno, H. Ion conduction in zwitterionic-type molten salts and their polymers. J. Mater. Chem. 2001, 11, 1057–1062.
- He, X.; Yang, Y.; Song, H.; Wang, S.; Zhao, H.; Wei, D. Polyanionic Composite Membranes Based on Bacterial Cellulose and Amino Acid for Antimicrobial Application. ACS Appl. Mater. Interfaces 2020, 12, 14784–14796.
- Nishimura, N.; Ohno, H. 15th anniversary of polymerised ionic liquids. Polymer 2014, 55, 3289–3297.
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036.
- Kausar, A. Research Progress in Frontiers of Poly(Ionic Liquid)s: A Review. Polym. -Plast. Technol. Eng. 2017, 56, 1823–1838.
- Eftekhari, A.; Saito, T. Synthesis and properties of polymerized ionic liquids. Eur. Polym. J. 2017, 90, 245–272.
- Lu, W.; Fadeev, A.G.; Qi, B.; Smela, E.; Mattes, B.R.; Ding, J.; Spinks, G.M.; Mazurkiewicz, J.; Zhou, D.; Wallace, G.G.; et al. Use of ionic liquids for π-conjugated polymer electrochemical devices. Science 2002, 297, 983–987.
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M.A. The Design of Polymeric Ionic Liquids for the Preparation of Functional Materials. Polym. Rev. 2009, 49, 339–360.
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448.
- Qiu, B.; Lin, B.; Si, Z.; Qiu, L.; Chu, F.; Zhao, J.; Yan, F. Bis-imidazolium-based anion-exchange membranes for alkaline fuel cells. J. Power Sources 2012, 217, 329–335.
- Qiu, B.; Lin, B.; Qiu, L.; Yan, F. Alkaline imidazolium- and quaternary ammonium-functionalized anion exchange membranes for alkaline fuel cell applications. J. Mater. Chem. 2012, 22, 1040–1045.
- Lin, B.; Qiu, L.; Lu, J.; Yan, F. Cross-Linked Alkaline Ionic Liquid-Based Polymer Electrolytes for Alkaline Fuel Cell Applications. Chem. Mater. 2010, 22, 6718–6725.
- Trigueiro, J.P.C.; Lavall, R.L.; Silva, G.G. Supercapacitors based on modified graphene electrodes with poly(ionic liquid). J. Power Sources 2014, 256, 264–273.
- Ayalneh Tiruye, G.; Muñoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. All-solid state supercapacitors operating at 3.5 V by using ionic liquid based polymer electrolytes. J. Power Sources 2015, 279, 472–480.
- Shaplov, A.S.; Ponkratov, D.O.; Vygodskii, Y.S. Poly(ionic liquid)s: Synthesis, properties, and application. Polym. Sci. Ser. B 2016, 58, 73–142.
- Prabhu Charan, K.T.; Pothanagandhi, N.; Vijayakrishna, K.; Sivaramakrishna, A.; Mecerreyes, D.; Sreedhar, B. Poly(ionic liquids) as “smart” stabilizers for metal nanoparticles. Eur. Polym. J. 2014, 60, 114–122.
- Sun, J.-K.; Kochovski, Z.; Zhang, W.-Y.; Kirmse, H.; Lu, Y.; Antonietti, M.; Yuan, J. General Synthetic Route toward Highly Dispersed Metal Clusters Enabled by Poly(ionic liquid)s. J. Am. Chem. Soc. 2017, 139, 8971–8976.
- Liu, W.; Wang, D.; Duan, Y.; Zhang, Y.; Bian, F. Palladium supported on poly (ionic liquid) entrapped magnetic nanoparticles as a highly efficient and reusable catalyst for the solvent-free Heck reaction. Tetrahedron Lett. 2015, 56, 1784–1789.
- Pourjavadi, A.; Hosseini, S.H.; AghayeeMeibody, S.A.; Hosseini, S.T. Poly(basic ionic liquid) coated magnetic nanoparticles: High-loaded supported basic ionic liquid catalyst. Comptes Rendus Chim. 2013, 16, 906–911.
- Vijayakrishna, K.; Charan, K.T.P.; Manojkumar, K.; Venkatesh, S.; Pothanagandhi, N.; Sivaramakrishna, A.; Mayuri, P.; Kumar, A.S.; Sreedhar, B. Ni Nanoparticles Stabilized by Poly(Ionic Liquids) as Chemoselective and Magnetically Recoverable Catalysts for Transfer Hydrogenation Reactions of Carbonyl Compounds. ChemCatChem 2016, 8, 1139–1145.
- Li, M.; Liu, Y.; Ding, S.; Zhu, A.; Shi, G. In situ synthesis of poly(ionic liquid)–Pt nanoparticle composite in glass capillary for the electrocatalytic reduction of oxygen. Analyst 2014, 139, 5964–5969.
- Isik, M.; Fernandes, A.M.; Vijayakrishna, K.; Paulis, M.; Mecerreyes, D. Preparation of poly(ionic liquid) nanoparticles and their novel application as flocculants for water purification. Polym. Chem. 2016, 7, 1668–1674.
- Yu, L.; Zhang, Y.; Wang, Y.; Zhang, H.; Liu, J. High flux, positively charged loose nanofiltration membrane by blending with poly (ionic liquid) brushes grafted silica spheres. J. Hazard. Mater. 2015, 287, 373–383.
- Chatterjee, P.; Nofen, E.M.; Xu, W.; Hom, C.; Jiang, H.; Dai, L.L. Pyrrole-based poly(ionic liquids) as efficient stabilizers for formation of hollow multi-walled carbon nanotubes particles. J. Colloid Interface Sci. 2017, 504, 140–148.
- Chen, S.; Xiang, Y.; Banks, M.K.; Peng, C.; Xu, W.; Wu, R. Polyoxometalate-coupled MXene nanohybrid via poly(ionic liquid) linkers and its electrode for enhanced supercapacitive performance. Nanoscale 2018, 10, 20043–20052.
- Marcilla, R.; Ochoteco, E.; Pozo-Gonzalo, C.; Grande, H.; Pomposo, J.A.; Mecerreyes, D. New Organic Dispersions of Conducting Polymers Using Polymeric Ionic Liquids as Stabilizers. Macromol. Rapid Commun. 2005, 26, 1122–1126.
- Kim, T.Y.; Lee, T.H.; Kim, J.E.; Kasi, R.M.; Sung, C.S.P.; Suh, K.S. Organic solvent dispersion of poly(3,4-ethylenedioxythiophene) with the use of polymeric ionic liquid. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6872–6879.
- Kim, T.; Tung, T.T.; Lee, T.; Kim, J.; Suh, K.S. Poly(ionic liquid)-mediated hybridization of single-walled carbon nanotubes and conducting polymers. Chem Asian J 2010, 5, 256–260.
- Tung, T.T.; Kim, T.Y.; Shim, J.P.; Yang, W.S.; Kim, H.; Suh, K.S. Poly(ionic liquid)-stabilized graphene sheets and their hybrid with poly(3,4-ethylenedioxythiophene). Org. Electron. 2011, 12, 2215–2224.
- Hong, S.H.; Tung, T.T.; Huyen Trang, L.K.; Kim, T.Y.; Suh, K.S. Preparation of single-walled carbon nanotube (SWNT) gel composites using poly(ionic liquids). Colloid Polym. Sci. 2010, 288, 1013–1018.
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular Ordering of Organic Molten Salts Triggered by Single-Walled Carbon Nanotubes. Science 2003, 300, 2072.
- Grollmisch, A.; Kragl, U.; Großeheilmann, J. Enzyme Immobilization in Polymerized Ionic Liquids-based Hydrogels for Active and Reusable Biocatalysts. SynOpen 2018, 02, 0192–0199.
- Hosseini, S.H.; Hosseini, S.A.; Zohreh, N.; Yaghoubi, M.; Pourjavadi, A. Covalent Immobilization of Cellulase Using Magnetic Poly(ionic liquid) Support: Improvement of the Enzyme Activity and Stability. J. Agric. Food Chem. 2018, 66, 789–798.
- Santana, J.L.; Oliveira, J.M.; Nascimento, J.S.; Mattedi, S.; Krause, L.C.; Freitas, L.S.; Cavalcanti, E.B.; Pereira, M.M.; Lima, Á.S.; Soares, C.M.F. Continuous flow reactor based with an immobilized biocatalyst for the continuous enzymatic transesterification of crude coconut oil. Biotechnol. Appl. Biochem. 2020, 67, 404–413.
- Texter, J. Anion Responsive Imidazolium-Based Polymers. Macromol. Rapid Commun. 2012, 33, 1996–2014.
- Gupta, N.; Liang, Y.N.; Hu, X. Thermally responsive ionic liquids and polymeric ionic liquids: Emerging trends and possibilities. Curr. Opin. Chem. Eng. 2019, 25, 43–50.
- Green, M.D.; Long, T.E. Designing Imidazole-Based Ionic Liquids and Ionic Liquid Monomers for Emerging Technologies. Polym. Rev. 2009, 49, 291–314.
- Xiang, S.; He, X.; Zheng, F.; Lu, Q. Multifunctional flexible sensors based on ionogel composed entirely of ionic liquid with long alkyl chains for enhancing mechanical properties. Chem. Eng. J. 2022, 439, 135644.
- Döbbelin, M.; Arias, G.; Loinaz, I.; Llarena, I.; Mecerreyes, D.; Moya, S. Tuning Surface Wettability of Poly(3-sulfopropyl methacrylate) Brushes by Cationic Surfactant-Driven Interactions. Macromol. Rapid Commun. 2008, 29, 871–875.
- Tiruye, G.A.; Muñoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. Performance of solid state supercapacitors based on polymer electrolytes containing different ionic liquids. J. Power Sources 2016, 326, 560–568.
- Ponkratov, D.O.; Lozinskaya, E.I.; Vlasov, P.S.; Aubert, P.-H.; Plesse, C.; Vidal, F.; Vygodskii, Y.S.; Shaplov, A.S. Synthesis of novel families of conductive cationic poly(ionic liquid)s and their application in all-polymer flexible pseudo-supercapacitors. Electrochim. Acta 2018, 281, 777–788.
- Teodoro, R.M.; Tomé, L.C.; Mantione, D.; Mecerreyes, D.; Marrucho, I.M. Mixing poly(ionic liquid)s and ionic liquids with different cyano anions: Membrane forming ability and CO2/N2 separation properties. J. Membr. Sci. 2018, 552, 341–348.
- Fdz De Anastro, A.; Casado, N.; Wang, X.; Rehmen, J.; Evans, D.; Mecerreyes, D.; Forsyth, M.; Pozo-Gonzalo, C. Poly(ionic liquid) iongels for all-solid rechargeable zinc/PEDOT batteries. Electrochim. Acta 2018, 278, 271–278.
- Sen, S.; Goodwin, S.E.; Barbará, P.V.; Rance, G.A.; Wales, D.; Cameron, J.M.; Sans, V.; Mamlouk, M.; Scott, K.; Walsh, D.A. Gel–Polymer Electrolytes Based on Poly(Ionic Liquid)/Ionic Liquid Networks. ACS Appl. Polym. Mater. 2021, 3, 200–208.
- Marcilla, R.; Alcaide, F.; Sardon, H.; Pomposo, J.A.; Pozo-Gonzalo, C.; Mecerreyes, D. Tailor-made polymer electrolytes based upon ionic liquids and their application in all-plastic electrochromic devices. Electrochem. Commun. 2006, 8, 482–488.
- Jeon, N.; Hwang, D.K.; Kang, Y.S.; Im, S.S.; Kim, D.-W. Quasi-solid-state dye-sensitized solar cells assembled with polymeric ionic liquid and poly(3,4-ethylenedioxythiophene) counter electrode. Electrochem. Commun. 2013, 34, 1–4.
- Vidal, F.; Plesse, C.; Teyssie, D.; Chevrot, C. Long-life air working conducting semi-IPN/ionic liquid based actuator. Synth. Met. 2004, 182, 247.
- Sans, V.; Karbass, N.; Burguete, M.I.; Compan, V.; Garcia-Verdugo, E.; Luis, S.V.; Pawlak, M. Polymer-supported ionic-liquid-like phases (SILLPs): Transferring ionic liquid properties to polymeric matrices. Chemistry 2011, 17, 1894–1906.
- Appetecchi, G.B.; Kim, G.T.; Montanino, M.; Carewska, M.; Marcilla, R.; Mecerreyes, D.; De Meatza, I. Ternary polymer electrolytes containing pyrrolidinium-based polymeric ionic liquids for lithium batteries. J. Power Sources 2010, 195, 3668–3675.
- Balli, B.; Şavk, A.; Şen, F. Graphene and polymer composites for supercapacitor applications. In Nanocarbon and Its Composites: Preparation, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–151.





