Innovation in materials specially formulated for additive manufacturing is of great interest and can generate new opportunities for designing cost-effective smart materials for next-generation devices and engineering applications. Nevertheless, advanced molecular and nanostructured systems are frequently not possible to integrate into 3D printable materials, thus limiting their technological transferability. In some cases, this challenge can be overcome using polymeric macromolecules of ionic nature, such as polymeric ionic liquids (PILs). Due to their tuneability, wide variety in molecular composition, and macromolecular architecture, they show a remarkable ability to stabilize molecular and nanostructured materials. The technology resulting from 3D-printable PIL-based formulations represents an untapped array of potential applications, including optoelectronic, antimicrobial, catalysis, photoactive, conductive, and redox applications.
- polymeric ionic liquids
- 3D printing
- advanced materials
- catalysis
- photoactive
- antimicrobial
- electronic
1. Introduction
2. 3D Printing Overview
|
Process Principle |
AM Technology |
Materials |
Features |
|---|---|---|---|
|
Vat photopolymerization (VP) |
Stereolithography (SLA) Dynamic light processing (DLP) Continuous Liquid Interface Production (CLIP) |
Photopolymer Ceramic |
High resolution Slow process High cost |
|
Powder bed fusion (PBF) |
Selective laser sintering (SLS) Selective laser melting (SLM) Electron beam melting (EBM) |
Metal Polymer Ceramic |
High resolution Slow process High cost |
|
Material extrusion |
Fused filament fabrication (FFF) Direct ink writing (DIW) |
Polymer Ceramic Biomaterials |
Low resolution Fast process Low cost |
|
Material Jetting (MJ) |
Ink-jetting Thermojet Polyjet |
Photopolymer Wax |
High resolution Fast process |
|
Binder Jetting (BJ) |
Ink-jetting |
Metal Polymer Ceramic |
High resolution Slow process High cost |
|
Direct Energy Deposition (DED) |
Direct Metal Deposition (DMD) Laser Deposition Laser Consolidation |
Metal Powder Wire |
Low resolution Fast process Low cost |
|
Sheet lamination (SL) |
Ultrasonic consolidation Laminated object manufacturing (LOM) |
Hybrids Metallic Ceramic |
Low resolution Fast process Low cost |
3. Polymeric Ionic Liquids
3.1. Properties of Ionic Liquids
ILs are organic molten salts in which the ions are poorly coordinated and the salts melt below 100 °C, or even at room temperature. The first IL, ethanol ammonium nitrate, was reported in 1888 [43][82], but it was few decades ago when the vast potential of these compounds was noticed. The renewed interest in them was due to their unique characteristics: chemical and thermal stability, high viscosity, wide electrochemical windows, low vapor pressure, specific solvating ability, good tunable solubility, wide solid to liquid range, and high ionic conductivity. The number of publications related to ILs has been increasing sharply every year for the last two decades, indicating the relevance of these materials [44][45][46][83,84,85]. Initially, these materials were mainly used as solvents for organic synthesis and catalysis, since they were more desirable than conventional volatile solvents [47][48][49][86,87,88]. Indeed, due to their low flammability, negligible vapor pressure, low melting point, and non-volatility properties, ILs were considered “green solvents” [50][51][89,90], although some concerns about their toxicity have appeared [52][53][54][91,92,93]. Nevertheless, these ionic compounds can contribute to making chemical production and usage more sustainable [55][94]. Furthermore, they can create a biphasic system, having the catalyst solubilized, while the products are insoluble in the IL [56][57][95,96], a feature that has also been widely used in analytical chemistry for separation procedures [58][97]. Progressively, the uses of ILs have broadened, being used in CO2 capture and separation systems [59][98], in electrocatalysis [60][61][62][63][99,100,101,102], in pharmaceutical industries [64][103], and even in space technology [65][104]. In particular, these chemicals have shown a lot of promise in the development of green catalytic technologies [66][105]. ILs can play the role of solvent in catalytic reactions, since they are able to dissolve a wide range of organometallic and inorganic compounds. Furthermore, the ionic behavior of these materials can stabilize the catalytic species and intermediates [67][68][106,107]. Moreover, the ILs themselves have been used as the catalyst in a variety of reactions, from biodiesel production, to CO2 conversion [69][70][71][108,109,110]. In some studies, ILs acted as both a solvent and a catalyst simultaneously [72][111]. Likewise, they have been extensively used in electrochemical applications as their charge density is much higher than that of a traditional salt solution [73][74][112,113]. Electrolytes based on ILs offer several benefits over aqueous and organic electrolytes, making them attractive for use in flexible and stretchable energy storage systems [75][76][77][78][79][114,115,116,117,118], high temperature supercapacitors [80][81][82][119,120,121], lithium-ion batteries [83][84][122,123], and electrochemical CO2 reductions [85][86][124,125]. Doping IL into polymers is another process that has recently been investigated, yielding significant changes in both conductivity and overall mechanical properties [87][88][126,127]. Pure polymer electrolytes generally have a limited ionic conductivity, thus adding an ionic moisture can be very beneficial [89][90][128,129]. The addition of ILs can benefit, not only neutral polymers, but also conductive polymers [91][92][130,131]. Despite ILs being very popular in both the academic community and industry, due to their well-known advantages [93][132], some downsides have been highlighted. The main problematics in using ILs are related to processing difficulties. For example, in catalysis or as a solvent the employment of ionic moisture can complicate the purification procedure of the product, and it is almost impossible to recycle them. Moreover, ILs cannot be used in fixed bed reactors, which, along with certain toxicity concerns, are critical challenges to overcome for future scale-up. Additionally, these compounds are liquids, which means that they can liquify and leach over time. An initial approach to overcoming these disadvantages was to use supported ionic liquid phases (SILPs) prepared by immobilizing the ILs onto solid supports [94][95][96][133,134,135]. In many cases the performance was improved, since the properties of ILs were transferred to the solid support, and simultaneously working with a solid material offered additional benefits [96][97][98][135,136,137]. Further methods have been developed to prepare IL-based polymers such as in situ polymerization of neutral vinyl monomers with non-polymerizable ILs or directly polymerizing an ionic monomer of IL structure [99][100][101][102][138,139,140,141]. A variety of polymeric IL systems, including polycation-type ILs [102][141], polyanion-type ILs [103][142], copolymer [100][139], and poly(zwitterion) [102][141], have been reported, as summarized in Figure 1.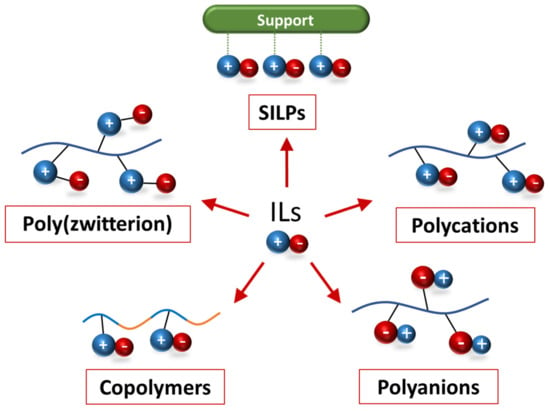
3.2. Properties of Polymeric Ionic Liquids
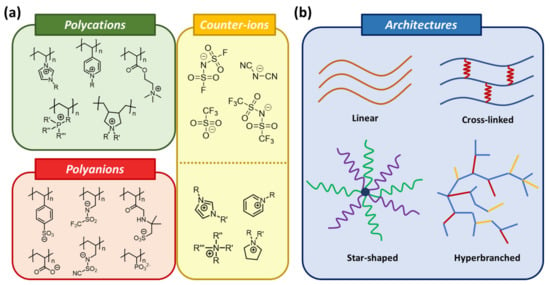
Polymeric ionic liquids/poly(ionic liquids) (PILs) are the union of multiple anionic or cationic monomers, forming a polymer backbone with its counterion species bonded to it. These high-molecular-mass compounds combine attractive IL properties with polymer properties (i.e., durability, low toxicity, and easy polymer processing). In other words, during the development of PILs, it is possible to preserve the specific features of ILs and improve other ones by taking advantage of the polymer’s nature, including mechanical robustness, thermal stability, hydrophilicity, rapid manipulation, optimum assembly, and stabilizing properties [104][143]. Another unique feature of PILs is that the properties of the polymers can be controlled by changing the corresponding ions. Depending on the ion pairs it is possible to vary the polymer’s solubility, fusibility, hydrophilicity, ionic conductivity, molecular mass, glass transition temperature, heat resistance, and thermal stability in a wide range. Most PILs are the nitrogen-based polycationic type, namely imidazolium, pyridine, pyridinium, quaternary ammonium, and cholinium; although phosphorous-containing cationic PILs are gaining popularity (Figure 2a). While ILs can only undergo structural variation through ion metathesis or cation/anion exchange, PILs can be modified by selecting cations, anions, side chains, and functionalities. Even when using the same ionic species and the same number of monomers, different PILs can be built by only changing the architecture: linear, hyperbranched, or star-shaped polymers; obtaining an unlimited library of species (Figure 2b) [105][144]. Homopolymerization is an important route to prepare PILs; however, other types of polymers can be added covalently to the PIL to form PIL block copolymers.