Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Biljana Šljukić | -- | 2324 | 2022-12-07 13:59:23 | | | |
| 2 | Vivi Li | -1 word(s) | 2323 | 2022-12-08 03:50:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gusmão, F.M.B.; Mladenović, D.; Radinović, K.; Santos, D.M.F.; Šljukić, B. Polyoxometalates as Electrocatalysts. Encyclopedia. Available online: https://encyclopedia.pub/entry/38205 (accessed on 07 February 2026).
Gusmão FMB, Mladenović D, Radinović K, Santos DMF, Šljukić B. Polyoxometalates as Electrocatalysts. Encyclopedia. Available at: https://encyclopedia.pub/entry/38205. Accessed February 07, 2026.
Gusmão, Filipe M. B., Dušan Mladenović, Kristina Radinović, Diogo M. F. Santos, Biljana Šljukić. "Polyoxometalates as Electrocatalysts" Encyclopedia, https://encyclopedia.pub/entry/38205 (accessed February 07, 2026).
Gusmão, F.M.B., Mladenović, D., Radinović, K., Santos, D.M.F., & Šljukić, B. (2022, December 07). Polyoxometalates as Electrocatalysts. In Encyclopedia. https://encyclopedia.pub/entry/38205
Gusmão, Filipe M. B., et al. "Polyoxometalates as Electrocatalysts." Encyclopedia. Web. 07 December, 2022.
Copy Citation
Polyoxometalates (POMs) are polyatomic ions with closed 3D frameworks and unique structure containing a large number of redox active sites that make them promising electrocatalysts for electrochemical energy conversion and storage applications.
polyoxometalates
electrocatalysis
energy conversion and storage
hydrogen evolution reaction
oxygen evolution reaction
oxygen reduction reaction
1. Introduction
Today’s society faces one of its biggest challenges in the form of climate change. This makes the pursuit of energy storage and production options that do not emit greenhouse gases mandatory for all developed countries [1][2]. This endeavor takes several forms: the replacement of fossil-fueled vehicles with electrical ones, the production of electricity via renewable sources for all forms of electrical consumption, and finally, efficient energy storage. This last facet is a mandatory part of the equation when it comes to renewable energy production since it tends to fluctuate greatly during the day and throughout the year in terms of energy produced.
In the endeavor to solve these issues, a potentially viable option has been found. The concept of the “Hydrogen Economy”, based on using hydrogen as an energy carrier, has now become a reality. However, most hydrogen comes from hydrocarbon reforming, a source of global pollution [3][4], which is certainly not sustainable. Using water electrolysis cells powered by renewable energy sources produces green hydrogen gas, a clean and efficient form of energy storage. Coupled with the fact that hydrogen can be fed to fuel cells to generate electricity, this makes the concept very interesting from a circular economy perspective [5].
Of course, this technology does not come without its limitations. Core reactions in water electrolyzers and fuel cells, oxygen evolution (OER) and oxygen reduction (ORR), as well as hydrogen evolution reaction (HER), have slow kinetics, requiring high overpotentials to obtain reasonable currents. Electrocatalysts are used to reduce the energy consumption in these reactions, with ruthenium oxide and platinum being the most efficient for OER and ORR/HER, respectively, but also high-cost materials. The ongoing search for low-cost, high-performance catalysts has proven to be difficult; most of these only operate in current ranges below 100 mA cm−2, which is not satisfactory for production on an industrial scale. Another problem in testing these materials is the difference between temperature, pressure, and electrolyte concentration values of small-scale lab cells compared with industry-scale ones, which will lead to significant divergence in electrolyte conductivity, ion migratory flux, and catalyst structural stability [6]. For this reason, the present entry focuses on the use of polyoxometalates (POMs) to overcome the aforementioned problems. POMs have shown interesting properties for OER/ORR and HER electrocatalysis, with good activity and low cost. Furthermore, they were shown to lead to promising performance of different types of batteries and supercapacitors when employed as electrode materials.
POMs are polyatomic ions composed of three or more transition metal (e.g., Mo, V, W) oxyanions linked together by shared oxygen atoms to form closed three-dimensional frameworks, Figure 1. The unique structure of POMs, which leads to a large number of redox active sites, makes them promising for energy conversion and storage applications. The study of POMs has been very in-depth, and applications have been found in the fields of material sciences, medical applications, and catalysis, which this entry will cover more closely. Concerning this last field, important properties have been observed in these materials, such as high thermal stability, high sensitivity to electricity, and resistance to oxidative decomposition, making them prime candidates for electrocatalytic study.
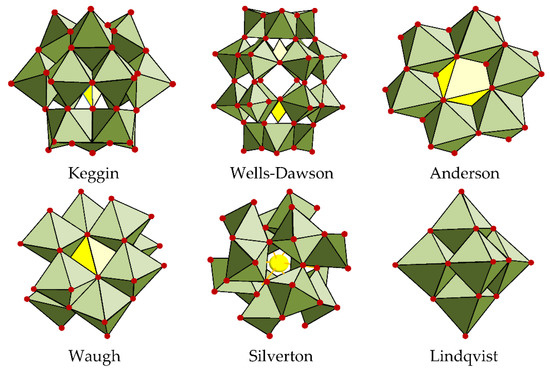
Figure 1. Polyoxometalates (POM) structures in polyhedral representations [7].
POMs demonstrate distinctive redox characteristics and the capability of reversibly uptaking as much as 24 electrons per cluster unit [PMo12O40]3− in the solid state [8][9][10][11][12]. This clearly indicates POMs as materials applicable for multi-electron transfer processes. The electrochemical behavior of POMs in terms of redox potential and number of electrons stored can be tailored additionally by, for example, the incorporation of redox-active metal centers through a chemical modification; such tailored-made POMs might be suitable for various electrochemical and electroanalytical applications [10].
POMs are normally insulators with only a few POMs demonstrating (semi-)conducting behavior. However, high electronic conductivity, often along with ionic conductivity, mainly protonic conductivity, is essential for the use of POMs as electrocatalysts in electrochemical energy conversion and storage devices. Thus, to use POMs in these devices, it is crucial to ensure adequate charge flow. Anchoring POMs through covalent or non-covalent means on nanostructured carbon materials, including carbon nanotubes (CNTs) and graphene, as conductive and high-surface area substrates, enables the mentioned charge flow [13]. “Wiring” of POMs to conductive organic polymers has also been suggested as a way to overcome the issue of their low conductivity [10].
2. POMs as Electrocatalysts for the Oxygen Evolution Reaction (OER)
One of the reactions involved in water electrolysis is the oxygen evolution reaction (OER), in which water is oxidized to O2. This reaction is further essential for the operation of metal-air batteries and unitized regenerative fuel cells. It has different mechanisms depending on the pH of the electrolyte solution, as shown in Figure 2. In any case, OER encompasses all the problems that plague the production of hydrogen via electrolysis cells, as shown in Figure 3. It has high thermodynamic demands, and its kinetic obstacles lead to high activation energies. This translates into high overpotentials required for the reaction to start, making it evident that developing an efficient catalyst is mandatory when pursuing research in this field.
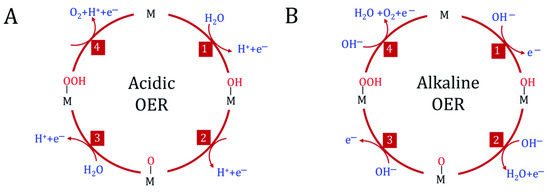
Figure 2. The schematic representation of the OER mechanism in acidic (A) and alkaline (B) media [14]. Each of the 4 steps corresponds to a single electron transfer.
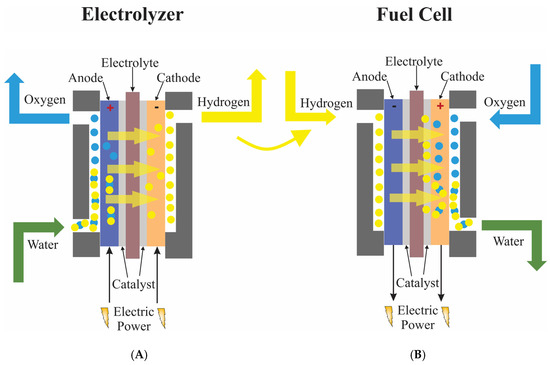
Figure 3. Schematic representation of a water electrolyzer (A) and a fuel cell (B).
Good performance when using POMs as catalysts for the OER has already been reported [15]. Removing metal oxygen entities creates empty spaces in the POM structure, creating so-called lacunary POMs. These are good inorganic ligands that stabilize multi-metal oxide clusters, creating a very robust structure that can be used to assemble catalytically active transitional metal-oxo clusters. So, different POMs can be studied for the desired catalytic effect, with various metals serving as the active sites.
2.1. Ruthenium POMs
One of the transition metals that can act as an active site of POMs is ruthenium (Ru). Even though Ru is a noble metal and, consequently, expensive, it should be mentioned that it has been demonstrated to be a powerful oxidant in a near-neutral pH. For example, [Ru4O4(OH)2(H2O)4(SiW10O36)2]10−, or simply (Ru4Si2), has been reported as being highly active for OER [14][15], having a ratio of product formed per molecule of catalyst (turn-over number, TON) of up to 180 and initial d(TON)/dt (turn-over frequency, TOF) of up to 288 h−1 [16][17]. [Ru4(O)5(OH)(H2O)4(PW10O36)2]9−, or (Ru4P2) was also shown to be able to catalyze the OER, but at a slightly lower efficiency [18].
Furthermore, studies involving Ru4Si2 combined with conductive materials have also been done. For example, anchoring the material to a conductive bed of multi-walled carbon nanotubes (MWCNTs) leads to higher electrocatalytic activity when compared to simply Ru4Si2-functionalized amorphous carbon [19]; this is most likely due to the enhanced electron transfer in MWCNTs. With these materials, a TOF of 300 h−1 was reached at an overpotential of 0.6 V [20].
Composite materials based on electrostatic immobilization of Ru4Si2 onto graphene followed by electrochemical deposition on glassy carbon have also been studied by Guo et al. [21]. This material showed good catalytic activity and stability under near neutral pH, with currents comparable to that of IrO2 at an overpotential of 0.35 V.
According to Quintana et al. [22], another approach to increase the electrocatalytic performance of Ru4Si2 was to use graphene, covalently functionalized with organic, hydrogen-bonding, cations. This hybrid material shows an overpotential of 0.3 V to reach a current density of 0.150 mA cm−2, and a negligible loss of performance even after 4 h of testing, all at neutral pH, performing at a higher efficiency than both isolated Ru4Si2 and its nanotube analogue.
2.2. Cobalt POMs
Cobalt (Co) is somewhat better than Ru in terms of higher abundance, but not particularly more sustainable. Namely, most of its reserves are located in countries with fewer regulations. which further leads to less emphasis on controlling the pollutants from the extraction of this metal [23]. Thus, Co may be considered a midway between the more sustainable transition metal-based POMs and the noble metal-based POMs.
Limani et al. [24] studied the viability of four cobalt phosphotungstate materials (MWCNT_N8_Co4, GF_N8_Co4, GF_ND8_Co4, and GF_NS8_Co4) as electrocatalysts. In alkaline media, with a pH of 13, all composites showed good performance, particularly GF_N8_Co4, which had an onset potential of 0.34 V vs. RHE and a maximum current of 70 mA cm−2 at 2 V vs. RHE, while also maintaining around 73–82% of its current after about 5.5 h. It is important to notice that these data mean that this electrocatalyst outperforms state-of-the-art IrO2.
2.3. Manganese POMs
As mentioned, although Co is more abundant and consequently cheaper than Ru, its use comes with some drawbacks. So, better alternatives are yet to be found, for example, manganese (Mn). However, only a small number of Mn-POMs have been studied. The lack of Mn examples is not exclusive to POMs, because Mn oxide is generally much less active than the corresponding Co or Ni analogs when considering heterogeneous catalysis [25]. A good place to start the search for Mn-POMs that exhibit OER activity seems to be the Mn-analogue of the well-studied Co4P2, Mn4(H2O)2(PW9O34)2]10−, or simply (Mn4) [26]. Even though electrochemical water oxidation experiments show initial activity comparable to the Co counterpart, the current density decreased very rapidly, becoming negligible in 30 min with the formation of an inactive MnOx layer on the electrode.
Wu et al. [27] also studied another six POMs comprising different Mn-O clusters and Mn in different oxidation states: (Mn2-POM), (Mn4-POM), (Mn6-POM-1), (Mn6-POM-4), (Mn14-POM), and (Mn19-POM), both with these materials in solution, and then deposited on the surface of indium tin oxide (ITO) electrodes to form the composite films. It was found that Mn14-POM displays the highest electrocatalytic performance toward oxygen evolution. It was also concluded that the oxidation state of Mn and the cubic structure of Mn-O cluster are important factors impacting the POMs electrocatalytic performance for OER.
2.4. Nickel POMs
Extensive studies of Ni-based electrocatalysts for electrochemical energy-related applications showed that their performance often overcomes that of the benchmark electrocatalysts [28]. Nanostructured Ni-based materials have especially shown promising activity for oxygen electrode reactions, as well as HER.
When it comes to POMS, nickel POMs are some of the most recent ones; [Ni5(OH)6(OH2)3(SiW9O33)2]12− or (Ni5Si2) was only first reported in 2012 by Zhu et al. [29]. The research into this type of material continued with Singh et al. [30] who in 2018 reported a hybrid POM-supported NiII coordination complex, [(NiII(bpy)2(H2O))(HCoIIWVI12O40)]23−, with NiII metal ion acting as the active center. This catalyst was highly stable and robust for OER at pH 7 having a high TOF of 18.49 (mol of O2)(mol of Ni(II))−1 s−1 and a Tafel slope of 168.41 mV dec−1.
A simple one-step hydrothermal deposition of microcrystals of a Dexter–Silverton POM, [Co6.8Ni1.2W12O42(OH)4(H2O)8], on a commercial Ni foam, resulted in an industrially viable/applicable composite electrode for OER in alkaline electrolytes [31]. Namely, the overpotential to reach a current density of 10 mA cm−2 under OER polarization conditions was observed to be as low as 360 mV. The material’s good activity towards OER was further reflected in the Tafel slope value of 126 mV dec−1, accompanied by a faradaic efficiency of over 90%. Furthermore, the material demonstrated high mechanical and chemical stability and no detachment of the POM microcrystals from the metal foam support.
Similarly, (C5H7N2)6[NiW12O44] showed an overpotential of 347 mV to reach 10 mA cm−2 complemented by long-term stability (up to 96 h) under OER polarization conditions in alkaline media [32]. NiO and WOx (x = 1 or 2), with activity for OER, were generated in situ, further contributing to the electrocatalysis of OER.
Ni-POMs have also been shown to have a high photocatalytic activity to drive efficient water oxidation under visible light. Thus, Na24[Ni12(OH)9(CO3)3(PO4)(SiW9O34)3]·56H2O, Na25[Ni13(H2O)3(OH)9(PO4)4(SiW9O34)3]·50H2O, and Na50[Ni25(H2O)2OH)18(CO3)2(PO4)6(SiW9O34)6]·85H2O demonstrated high O2 evolution TON of 128.2, 147.6, and 204.5, respectively [33]. The studied compounds’ photocatalytic activity was attributed to the band gap structures, numerous active sites, and the favorable structural design of POMs.
2.5. Copper and Iron POMs
Yu et al. [34][35] studied two Cu-POMs clusters, where [Cu3(H2O)3(SbW9O33)2]12− was shown to catalyze OER at neutral pH without decomposition under homogeneous electrochemical conditions. Interestingly, [Cu5(OH)4(H2O)2(SiW9O33)2]10−, the POM that could photochemically catalyze the same reaction, showed negligible electrochemical activity under the same conditions.
Azmani et al. reported the activity of Fe-POMs in relation to Co-POMs in 2021 [36], comparing [FeIII4(H2O)2(B-α-PW9O34)2]6− (Fe4-WS) to its cobalt analog [CoII4(H2O)2(B-α-PW9O34)2]10− (Co4-WS). Still, it was found that FeIII derivatives display lower OER activity than CoII-POMs. These results are in agreement with the theoretical considerations, as the lower Tafel slope exhibited by Co4-WS pointed to faster OER kinetics than in the case of Fe4-WS. This owes to the lower activation barrier in the case of Co4-WS. These activation barriers were determined using the computation of the transition state of one water molecule forming a hydrogen bond with a bridging oxygen [36].
Han et al. synthesized sub-nanometric heterometallic CoW and FeCoW clusters by a molecule-to-cluster approach starting from several different POMs (i.e., [{Co4(OH)3PO4}4(SiW9O34)4]32−, [{Fe2Co2(OH)3PO4}4(SiW9O34)4]24−, and [{FeCo3(OH)3PO4}4(SiW9O34)4]28−) as precursors [37]. The amount of Fe in the FeCoW clusters could be controlled by using POM precursors comprising a different number of Fe atoms. Outstanding OER activity with overpotential to reach a current density of 10 mA cm−2, η10, as low as 192 mV, and a Tafel slope as low as 36 mV dec−1 was recorded for the most promising material.
References
- Santos, D.M.F.; Šljukić, B. Advanced materials for electrochemical energy conversion and storage devices. Materials 2021, 14, 7711.
- Badwal, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79.
- Santos, D.M.F.; Šljukić, B.; Sequeira, C.A.C.; Macció, D.; Saccone, A.; Figueiredo, J.L. Electrocatalytic approach for the efficiency increase of electrolytic hydrogen production: Proof-of-concept using platinum-dysprosium alloys. Energy 2013, 50, 486–492.
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762.
- Chen, J.; Xiao, R.; Fu, K.; Wu, Y.; Guo, Y.; Yang, S.; Li, H.; Zheng, J.; Li, X. Metal hydride mediated water splitting: Electrical energy saving and decoupled H2/O2 generation. Mater. Today 2021, 47, 16–24.
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrog. Energy 2020, 45, 26036–26058.
- Stamate, A.E.; Pavel, O.D.; Zavoianu, R.; Marcu, I.C. Highlights on the catalytic properties of polyoxometalate-intercalated layered double hydroxides: A review. Catalysts 2020, 10, 57.
- Wang, H.; Hamanaka, S.; Nishimoto, Y.; Irle, S.; Yokoyama, T.; Yoshikawa, H.; Awaga, K. In operando X-ray absorption fine structure studies of polyoxometalate molecular cluster batteries: Polyoxometalates as electron sponges. J. Am. Chem. Soc. 2012, 134, 4918–4924.
- Kawasaki, N.; Wang, H.; Nakanishi, R.; Hamanaka, S.; Kitaura, R.; Shinohara, H.; Yokoyama, T.; Yoshikawa, H.; Awaga, K. Nanohybridization of polyoxometalate clusters and single-wall carbon nanotubes: Applications in molecular cluster batteries. Angew. Chemie-Int. Ed. 2011, 50, 3471–3474.
- López, X.; Fernández, J.A.; Poblet, J.M. Redox properties of polyoxometalates: New insights on the anion charge effect. J. Chem. Soc. Dalt. Trans. 2005, 6, 1162–1167.
- Wang, H.; Kawasaki, N.; Yokoyama, T.; Yoshikawa, H.; Awaga, K. Molecular cluster batteries of nano-hybrid materials between Keggin POMs and SWNTs. Dalt. Trans. 2012, 41, 9863–9866.
- López, X.; Bo, C.; Poblet, J.M. Electronic properties of polyoxometalates: Electron and proton affinity of mixed-addenda Keggin and Wells-Dawson anions. J. Am. Chem. Soc. 2002, 124, 12574–12582.
- Ji, Y.; Huang, L.; Hu, J.; Streb, C.; Song, Y.F. Polyoxometalate-functionalized nanocarbon materials for energy conversion, energy storage and sensor systems. Energy Environ. Sci. 2015, 8, 776–789.
- Yan, Z.; Liu, H.; Hao, Z.; Yu, M.; Chen, X.; Chen, J. Electrodeposition of (hydro)oxides for an oxygen evolution electrode. Chem. Sci. 2020, 11, 10614–10625.
- Lv, H.; Geletii, Y.V.; Zhao, C.; Vickers, J.W.; Zhu, G.; Luo, Z.; Song, J.; Lian, T.; Musaev, D.G.; Hill, C.L. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589.
- Geletii, Y.V.; Botar, B.; Kögerler, P.; Hillesheim, D.A.; Musaev, D.G.; Hill, C.L. An all-inorganic, stable, and highly active tetraruthenium homogeneous catalyst for water oxidation. Angew. Chemie. Int. Ed. 2008, 47, 3896–3899.
- Geletii, Y.V.; Huang, Z.; Hou, Y.; Musaev, D.G.; Lian, T.; Hill, C.L. Homogeneous light-driven water oxidation catalyzed by a tetraruthenium complex with all inorganic ligands. J. Am. Chem. Soc. 2009, 131, 7522–7523.
- Besson, C.; Huang, Z.; Geletii, Y.V.; Lense, S.; Hardcastle, K.I.; Musaev, D.G.; Lian, T.; Proust, A.; Hill, C.L. Cs9, a new all-inorganic, soluble catalyst for the efficient visible-light-driven oxidation of water. Chem. Commun. 2010, 46, 2784–2786.
- Toma, F.M.; Sartorel, A.; Iurlo, M.; Carraro, M.; Parisse, P.; MacCato, C.; Rapino, S.; Gonzalez, B.R.; Amenitsch, H.; Da Ros, T.; et al. Efficient water oxidation at carbon nanotube-polyoxometalate electrocatalytic interfaces. Nat. Chem. 2010, 2, 826–831.
- Horn, M.R.; Singh, A.; Alomari, S.; Goberna-Ferrón, S.; Benages-Vilau, R.; Chodankar, N.; Motta, N.; Ostrikov, K.; Macleod, J.; Sonar, P.; et al. Polyoxometalates (POMs): From electroactive clusters to energy materials. Energy Environ. Sci. 2021, 14, 1652–1700.
- Guo, S.X.; Liu, Y.; Lee, C.Y.; Bond, A.M.; Zhang, J.; Geletii, Y.V.; Hill, C.L. Graphene-supported 10- for highly efficient electrocatalytic water oxidation. Energy Environ. Sci. 2013, 6, 2654–2663.
- Quintana, M.; López, A.M.; Rapino, S.; Toma, F.M.; Iurlo, M.; Carraro, M.; Sartorel, A.; MacCato, C.; Ke, X.; Bittencourt, C.; et al. Knitting the catalytic pattern of artificial photosynthesis to a hybrid graphene nanotexture. ACS Nano 2013, 7, 811–817.
- How the Race for Cobalt Risks Turning It from Miracle Metal to Deadly Chemical. Available online: https://www.theguardian.com/global-development/2019/dec/18/how-the-race-for-cobalt-risks-turning-it-from-miracle-metal-to-deadly-chemical (accessed on 20 May 2022).
- Limani, N.; Marques, I.S.; Jarrais, B.; Fernandes, A.J.S.; Freire, C.; Fernandes, D.M. Cobalt Phosphotungstate-based composites as bifunctional electrocatalysts for oxygen reactions. Catalysts 2022, 12, 357.
- Zaharieva, I.; Chernev, P.; Risch, M.; Klingan, K.; Kohlhoff, M.; Fischer, A.; Dau, H. Electrosynthesis, functional, and structural characterization of a water-oxidizing manganese oxide. Energy Environ. Sci. 2012, 5, 7081–7089.
- Goberna-Ferrón, S.; Soriano-López, J.; Galán-Mascarós, J.R. Activity and stability of the tetramanganese polyanion 10− during electrocatalytic water oxidation. Inorganics 2015, 3, 332–340.
- Wu, Y.; Pei, J.; Yu, X.; Bi, L. Study on catalytic water oxidation properties of polynuclear manganese containing polyoxometalates. Catalysts 2022, 12, 160.
- Vij, V.; Sultan, S.; Harzandy, A.; Meena, A.; Tiwari, J.; Lee, W.; Yoon, T.; Kim, K. Nickel–Based Electrocatalysts for Energy Related Applications: Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. ACS Catalysis. 2017, 7, 7196–7225.
- Zhu, G.; Glass, E.N.; Zhao, C.; Lv, H.; Vickers, J.W.; Geletii, Y.V.; Musaev, D.G.; Song, J.; Hill, C.L. A nickel containing polyoxometalate water oxidation catalyst. Dalt. Trans. 2012, 41, 13043–13049.
- Singh, C.; Mukhopadhyay, S.; Das, S.K. Polyoxometalate-supported Bis(2,2′-bipyridine)mono(aqua)nickel(II) coordination complex: An efficient electrocatalyst for water oxidation. Inorg. Chem. 2018, 57, 6479–6490.
- Luo, W.; Hu, J.; Diao, H.; Schwarz, B.; Streb, C.; Song, Y. Robust polyoxometalate/nickel foam composite electrodes for sustained electrochemical oxygen evolution at high pH. Angew. Chem. Int. Ed. 2017, 56, 4941–4944.
- Joshi, A. A rare polyoxometalate cluster 14- based solid as a pre-catalyst for efficient and long-term oxygen evolution. Nanoscale Adv. 2022.
- Han, X.; Li, Y.; Zhang, Z.; Tan, H.; Lu, Y.; Wang, E. Polyoxometalate-based nickel clusters as visible light-driven water oxidation catalysts. J. Am. Chem. Soc. 2015, 137, 5486–5493.
- Yu, L.; Lin, J.; Zheng, M.; Chen, M.; Ding, Y. Homogeneous electrocatalytic water oxidation at neutral pH by a robust trinuclear copper(II)-substituted polyoxometalate. Chem. Commun. 2018, 54, 354–357.
- Yu, L.; Du, X.; Ding, Y.; Chen, H.; Zhou, P. Efficient visible light-driven water oxidation catalyzed by an all-inorganic copper-containing polyoxometalate. Chem. Commun. 2015, 51, 17443–17446.
- Azmani, K.; Besora, M.; Soriano-López, J.; Landolsi, M.; Teillout, A.L.; de Oliveira, P.; Mbomekallé, I.M.; Poblet, J.M.; Galán-Mascarós, J.R. Understanding polyoxometalates as water oxidation catalysts through iron vs. cobalt reactivity. Chem. Sci. 2021, 12, 8755–8766.
- Han, X.B.; Tang, X.Y.; Lin, Y.; Gracia-Espino, E.; Liu, S.G.; Liang, H.W.; Hu, G.Z.; Zhao, X.J.; Liao, H.G.; Tan, Y.Z.; et al. Ultrasmall abundant metal-based clusters as oxygen-evolving catalysts. J. Am. Chem. Soc. 2019, 141, 232–239.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
09 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




