
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pantu Kumar Roy | -- | 2728 | 2022-12-07 10:16:57 | | | |
| 2 | Vivi Li | Meta information modification | 2728 | 2022-12-08 03:24:44 | | |
Video Upload Options
Biofilm is a complex matrix made up of extracellular polysaccharides, DNA, and proteins that protect bacteria against physical, chemical, and biological stresses and allow them to survive in harsh environments. Safe and healthy foods are mandatory for saving lives. However, foods can be contaminated by pathogenic microorganisms at any stage from farm to fork. The contaminated foods allow pathogenic microorganisms to form biofilms and convert the foods into stigmatized poison for consumers. Biofilm formation by pathogenic microorganisms in agri-farm industries is still poorly understood and intricate to control. In biofilms, pathogenic bacteria are dwelling in a complex manner and share their genetic and physicochemical properties making them resistant to common antimicrobial agents. Therefore, finding the appropriate antibiofilm approaches is necessary to inhibit and eradicate the mature biofilms from foods and food processing surfaces. Advanced studies have already established several emerging antibiofilm approaches including plant- and microbe-derived biological agents, and they proved their efficacy against a broad-spectrum of foodborne pathogens.
1. Introduction
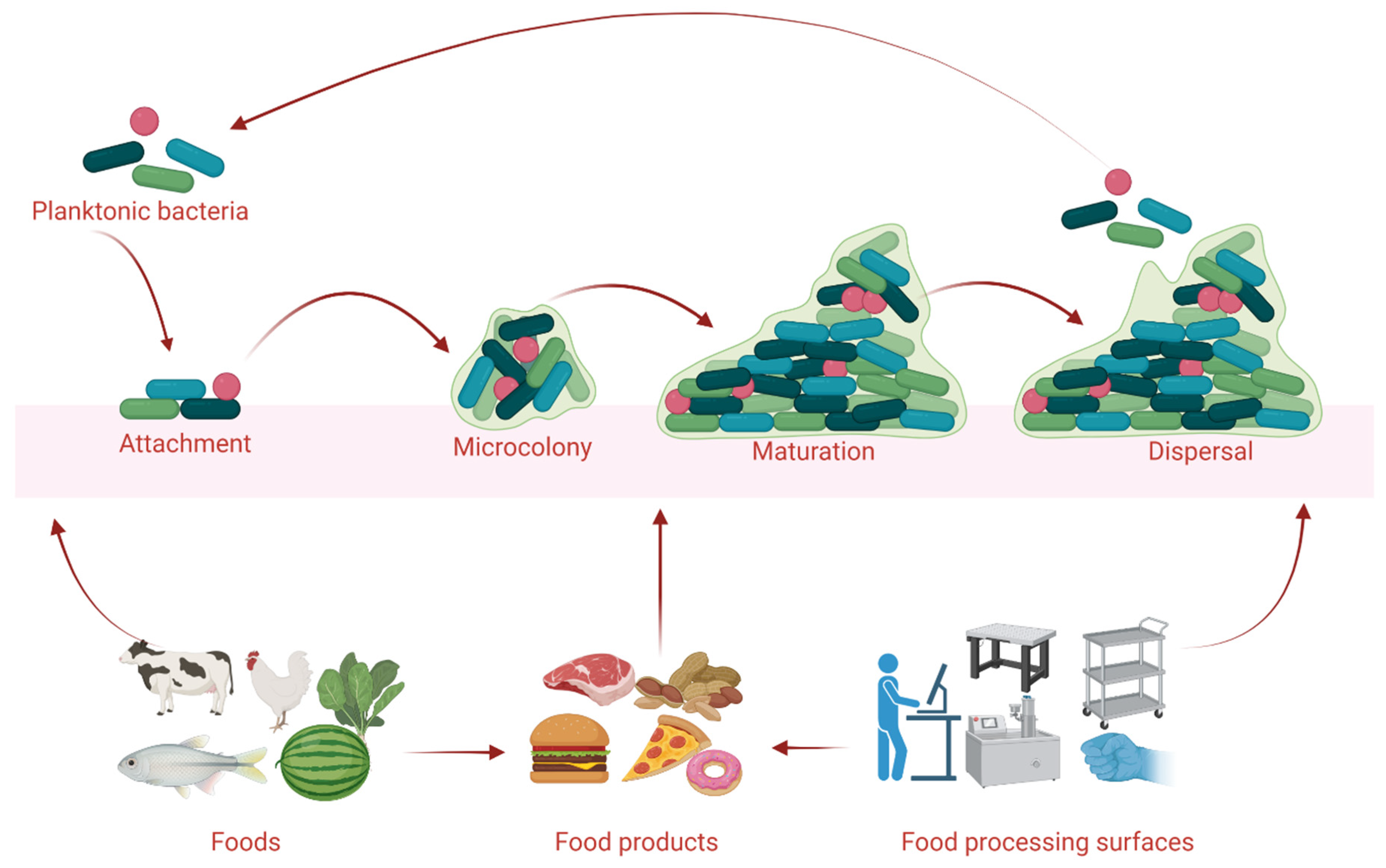
2. Microbial Biofilms in Food Processing Industries
2.1. Biofilm Associated Problems in the Dairy Industry
Biofilm Control Strategies in the Dairy Industry
2.2. Biofilm-Associated Problems in the Meat Processing Industry
2.3. Biofilm Associated Problems in the Aquatic Industry
2.4. Biofilm Associated Problems in the Agricultural Industry
References
- Bogino, P.; Abod, A.; Nievas, F.; Giordano, W. Water-limiting conditions alter the structure and biofilm-forming ability of bacterial multispecies communities in the Alfalfa rhizosphere. PLoS ONE 2013, 8, e79614.
- Seviour, T.; Derlon, N.; Dueholm, M.S.; Flemming, H.-C.; Girbal-Neuhauser, E.; Horn, H.; Kjelleberg, S.; van Loosdrecht, M.C.M.; Lotti, T.; Malpei, M.F.; et al. Extracellular polymeric substances of biofilms: Suffering from an identity crisis. Water Res. 2019, 151, 1–7.
- Toushik, S.H.; Park, J.-H.; Kim, K.; Ashrafudoulla, M.; Senakpon Isaie Ulrich, M.; Mizan, M.F.R.; Roy, P.K.; Shim, W.-B.; Kim, Y.-M.; Park, S.H.; et al. Antibiofilm efficacy of Leuconostoc mesenteroides J.27-derived postbiotic and food-grade essential oils against Vibrio parahaemolyticus, Pseudomonas aeruginosa, and Escherichia coli alone and in combination, and their application as a green preservative in the seafood industry. Food Res. Int. 2022, 156, 111163.
- Hossain, M.I.; Rahaman Mizan, M.F.; Toushik, S.H.; Roy, P.K.; Jahid, I.K.; Park, S.H.; Ha, S.-D. Antibiofilm effect of nisin alone and combined with food-grade oil components (thymol and eugenol) against Listeria monocytogenes cocktail culture on food and food-contact surfaces. Food Control 2022, 135, 108796.
- Liu, Z.; Hong, C.J.; Yang, Y.; Dai, L.; Ho, C.L. Advances in bacterial biofilm management for maintaining microbiome homeostasis. Biotechnol. J. 2020, 15, 1900320.
- Roy, P.K.; Ha, A.J.-W.; Mizan, M.F.R.; Hossain, M.I.; Ashrafudoulla, M.; Toushik, S.H.; Nahar, S.; Kim, Y.K.; Ha, S.-D. Effects of environmental conditions (temperature, pH, and glucose) on biofilm formation of Salmonella enterica serotype Kentucky and virulence gene expression. Poult. Sci. 2021, 100, 101209.
- Toushik, S.H.; Mizan, M.F.R.; Hossain, M.I.; Ha, S.-D. Fighting with old foes: The pledge of microbe-derived biological agents to defeat mono- and mixed-bacterial biofilms concerning food industries. Trends Food Sci. Technol. 2020, 99, 413–425.
- Galie, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898.
- Roy, P.K.; Mizan, M.F.R.; Hossain, M.I.; Han, N.; Nahar, S.; Ashrafudoulla, M.; Toushik, S.H.; Shim, W.-B.; Kim, Y.-M.; Ha, S.-D. Elimination of Vibrio parahaemolyticus biofilms on crab and shrimp surfaces using ultraviolet C irradiation coupled with sodium hypochlorite and slightly acidic electrolyzed water. Food Control 2021, 128, 108179.
- Hossain, M.I.; Kim, K.; Rahaman Mizan, M.F.; Toushik, S.H.; Ashrafudoulla, M.; Roy, P.K.; Nahar, S.; Jahid, I.K.; Choi, C.; Park, S.H.; et al. Comprehensive molecular, probiotic, and quorum-sensing characterization of anti-listerial lactic acid bacteria, and application as bioprotective in a food (milk) model. J. Dairy Sci. 2021, 104, 6516–6534.
- Ashrafudoulla, M.; Na, K.W.; Hossain, M.I.; Mizan, M.F.R.; Nahar, S.; Toushik, S.H.; Roy, P.K.; Park, S.H.; Ha, S.-D. Molecular and pathogenic characterization of Vibrio parahaemolyticus isolated from seafood. Mar. Pollut. Bull. 2021, 172, 112927.
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15.
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11.
- Hossain, M.I.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.; Ha, S.-D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110595.
- Hossain, M.I.; Sadekuzzaman, M.; Ha, S.-D. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: A review. Food Res. Int. 2017, 100, 63–73.
- Roy, P.K.; Song, M.G.; Jeon, E.B.; Kim, S.H.; Park, S.Y. Antibiofilm efficacy of quercetin against Vibrio parahaemolyticus biofilm on food-contact surfaces in the food industry. Microorganisms 2022, 10, 1902.
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New weapons to fight old enemies: Novel strategies for the (bio) control of bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 1641.
- Mogha, K.V.; Shah, N.P.; Prajapati, J.B.; Chaudhari, A.R. Biofilm-a threat to dairy industry. Indian J. Dairy Sci. 2014, 67, 459–466.
- Burke, N.; Zacharski, K.A.; Southern, M.; Hogan, P.; Ryan, M.P.; Adley, C.C. The Dairy Industry: Process, Monitoring, Standards, and Quality. In Descriptive Food Science; IntechOpen: London, UK, 2018; pp. 1–25.
- Abril, A.G.; Villa, T.G.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus exotoxins and their detection in the dairy industry and mastitis. Toxins 2020, 12, 537.
- Marchand, S.; De Block, J.; De Jonghe, V.; Coorevits, A.; Heyndrickx, M.; Herman, L. Biofilm formation in milk production and processing environments; influence on milk quality and safety. Compr. Rev. Food Sci. Food Saf. 2012, 11, 133–147.
- Huellemeier, H.A.; Eren, N.M.; Payne, T.D.; Schultz, Z.D.; Heldman, D.R. Monitoring and characterization of milk fouling on stainless steel using a high-pressure high-temperature quartz crystal microbalance with dissipation. Langmuir 2022, 38, 9466–9480.
- Palabiyik, I.; Atik, D.S.; Sivri, G.T.; Uzun, S.; Kahyaoglu, L.N.; Koc, Y.; Celebi, E.; Calisir, K.; Boluk, E. Optimization of temperature for effective cleaning with a novel cleaning rig: Influence of soil and surface types. Food Bioprod. Process. 2022, 136, 36–46.
- Toté, K.; Horemans, T.; Berghe, D.V.; Maes, L.; Cos, P. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 2010, 76, 3135–3142.
- Wang, R. Biofilms and meat safety: A mini-review. J. Food Prot. 2018, 82, 120–127.
- Dourou, D.; Beauchamp, C.S.; Yoon, Y.; Geornaras, I.; Belk, K.E.; Smith, G.C.; Nychas, G.-J.E.; Sofos, J.N. Attachment and biofilm formation by Escherichia coli O157:H7 at different temperatures, on various food-contact surfaces encountered in beef processing. Int. J. Food Microbiol. 2011, 149, 262–268.
- Habimana, O.; Heir, E.; Langsrud, S.; Åsli, A.W.; Møretrø, T. Enhanced surface colonization by Escherichia coli O157: H7 in biofilms formed by an Acinetobacter calcoaceticus isolate from meat-processing environments. Appl. Environ. Microbiol. 2010, 76, 4557–4559.
- Hathroubi, S.; Hancock, M.; Bosse, J.; Langford, P.; Tremblay, Y.; Labrie, J.; Jacques, M. Surface polysaccharide mutants reveal that absence of O antigen reduces biofilm formation of Actinobacillus pleuropneumoniae. Infect. Immun. 2015, 84, 127–137.
- Oliveira, C.J.B.; Hisrich, E.R.; Moura, J.F.P.; Givisiez, P.E.N.; Costa, R.G.; Gebreyes, W.A. On farm risk factors associated with goat milk quality in Northeast Brazil. Small Rumin. Res. 2011, 98, 64–69.
- Lyra, D.G.; Sousa, F.G.; Borges, M.F.; Givisiez, P.E.; Queiroga, R.C.; Souza, E.L.; Gebreyes, W.A.; Oliveira, C.J. Enterotoxin-encoding genes in Staphylococcus spp. from bulk goat milk. Foodborne Pathog. Dis. 2013, 10, 126–130.
- Kim, Y.K.; Roy, P.K.; Ashrafudoulla, M.; Nahar, S.; Toushik, S.H.; Hossain, M.I.; Mizan, M.F.R.; Park, S.H.; Ha, S.-D. Antibiofilm effects of quercetin against Salmonella enterica biofilm formation and virulence, stress response, and quorum-sensing gene expression. Food Control 2022, 137, 108964.
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540.
- CDC. List of Multistate Foodborne Outbreak Notices; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/foodsafety/outbreaks/lists/outbreaks-list.html (accessed on 12 August 2022).
- Gazal, L.E.D.S.; Medeiros, L.P.; Dibo, M.; Nishio, E.K.; Koga, V.L.; Gonçalves, B.C.; Grassotti, T.T.; de Camargo, T.C.L.; Pinheiro, J.J.; Vespero, E.C. Detection of ESBL/AmpC-producing and fosfomycin-resistant Escherichia coli from different sources in poultry production in Southern Brazil. Front. Microbiol. 2021, 11, 3387.
- Elmali, M.; CAN, H.Y.; Yaman, H. Prevalence of Listeria monocytogenes in poultry meat. Food Sci. Technol. 2015, 35, 672–675.
- Heidemann Olsen, R.; Christensen, H.; Kabell, S.; Bisgaard, M. Characterization of prevalent bacterial pathogens associated with pododermatitis in table egg layers. Avian Pathol. 2018, 47, 281–285.
- FAO. Fishery and Aquaculture Statistics 2019/FAO Annuaire; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; Available online: https://www.fao.org/fishery/static/Yearbook/YB2019_USBcard/index.htm (accessed on 16 September 2022).
- Pandey, P.; Bharti, V.; Kumar, K. Biofilm in aquaculture production. Afr. J. Microbiol. Res. 2014, 8, 1434–1443.
- Dumen, E.; Ekici, G.; Ergin, S.; Bayrakal, G.M. Presence of foodborne pathogens in seafood and risk ranking for pathogens. Foodborne Pathog. Dis. 2020, 17, 541–546.
- Toushik, S.H.; Kim, K.; Ashrafudoulla, M.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Kim, Y.; Ha, S.-D. Korean kimchi-derived lactic acid bacteria inhibit foodborne pathogenic biofilm growth on seafood and food processing surface materials. Food Control 2021, 129, 108276.
- Banaszkiewicz, S.; Calland, J.K.; Mourkas, E.; Sheppard, S.K.; Pascoe, B.; Bania, J. Genetic diversity of composite enterotoxigenic Staphylococcus epidermidis pathogenicity islands. Genome Biol. Evol. 2019, 11, 3498–3509.
- Ham, H.-J.; Kim, S.-E.; Ryu, S.-H.; Hwang, Y.-O.; Choi, S.-M. Bacterial distributions of Escherichia coli and Bacillus cereus etc. isolated from dried seasoned marine products in Garak fishery wholesale market in Seoul, 2009. J. Food Hyg. Saf. 2010, 25, 10–15.
- Moon, H.-J.; Min, K.-J.; Park, N.-Y.; Park, H.-J.; Yoon, K.-S. Survival of Staphylococcus aureus in dried fish products as a function of temperature. Food Sci. Biotechnol. 2017, 26, 823–828.
- Skowron, K.; Kwiecińska-Piróg, J.; Grudlewska, K.; Świeca, A.; Paluszak, Z.; Bauza-Kaszewska, J.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. The occurrence, transmission, virulence and antibiotic resistance of Listeria monocytogenes in fish processing plant. Int. J. Food Microbiol. 2018, 282, 71–83.
- Velmourougane, K.; Prasanna, R.; Saxena, A.K. Agriculturally important microbial biofilms: Present status and future prospects. J. Basic Microbiol. 2017, 57, 548–573.
- Motaung, T.E.; Peremore, C.; Wingfield, B.; Steenkamp, E. Plant-associated fungal biofilms—Knowns and unknowns. FEMS Microbiol. Ecol. 2020, 96, fiaa224.
- Berger, C.N.; Sodha, S.V.; Shaw, R.K.; Griffin, P.M.; Pink, D.; Hand, P.; Frankel, G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ. Microbiol. 2010, 12, 2385–2397.
- Toushik, S.H.; Lee, K.-T.; Lee, J.-S.; Kim, K.-S. Functional applications of lignocellulolytic enzymes in the fruit and vegetable processing industries. J. Food Sci. 2017, 82, 585–593.
- Iwu, C.D.; Okoh, A.I. Preharvest transmission routes of fresh produce associated bacterial pathogens with outbreak potentials: A review. Int. J. Environ. Res. Public Health 2019, 16, 4407.
- EFSA; ECDC. The European Union one health 2019 zoonoses report. EFSA J. 2021, 19, e06406.
- EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, e05077.




