Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hisashi Kato-Noguchi | -- | 2465 | 2022-12-07 01:12:28 | | | |
| 2 | Rita Xu | Meta information modification | 2465 | 2022-12-07 03:23:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kato-Noguchi, H.; Kato, M. Allelopathy of S. canadensis. Encyclopedia. Available online: https://encyclopedia.pub/entry/38148 (accessed on 07 February 2026).
Kato-Noguchi H, Kato M. Allelopathy of S. canadensis. Encyclopedia. Available at: https://encyclopedia.pub/entry/38148. Accessed February 07, 2026.
Kato-Noguchi, Hisashi, Midori Kato. "Allelopathy of S. canadensis" Encyclopedia, https://encyclopedia.pub/entry/38148 (accessed February 07, 2026).
Kato-Noguchi, H., & Kato, M. (2022, December 07). Allelopathy of S. canadensis. In Encyclopedia. https://encyclopedia.pub/entry/38148
Kato-Noguchi, Hisashi and Midori Kato. "Allelopathy of S. canadensis." Encyclopedia. Web. 07 December, 2022.
Copy Citation
Solidago canadensis L. is native to North America and have naturalized many other continents including Europa and Asia. Their species is an aggressive colonizer and forms thick monospecific stands. Allelopathy is the chemical interaction between donor plants and recipient plants through allelochemicals. Allelochemicals are produced in some plant parts and released into the vicinity of the donor plants, including their rhizosphere soil either by the root exudation, rainfall leachates, volatilization from the plant parts or decomposition processes of plant residues.
allelochemical
invasive species
monospecific stand
mycorrhizal colonization
1. Introduction
Solidago canadensis sensu lato (s.l.), belonging Asteraceae, is an erect rhizomatous perennial plant, native to North America. S. canadensis s.l. was introduced to Europe as an ornamental plant in the 17th to 18th centuries. The species spread from the gardens to the natural environments, and has extended its habitats in Central and Eastern Europe. It expanded its habitat at a rate of 741 km2 per year in Europe [1]. The species has also been introduced and naturalized in many other countries such as Australia, Brazil, China, India, New Zealand and Japan [2][3][4][5][6].
The species expands its habitat though seed distribution and rhizome expansion. The rhizomes arise near the base of the shoots in autumn and produce aerial stems from their apex in the following spring. The stems are not branched, and bear triple-nerved, lanceolate, alternate leaves which are found along the stems and roots at the base of the shoots. The rhizome systems contribute to expanding the species’ community and to form thick monospecific stands [7][8]. Shoot density in the established stands of the species was reported to be 309 shoots per m2 [3]. In addition, oil-filled cavities, which contain terpenes and/or lipids, were randomly distributed in the rhizomes [9]. These compounds may have some biological functions such as allelopathy. The species is a prolific seed producer. Its inflorescence forms broad pyramidal panicles, which contain numerous florets (Figure 1). A single plant produces 1000–20,000 light-winged achenes which contain seeds. The achenes disperse easily by wind, water and human activities. The germination rate is 30–75%, depending on the conditions [7][8][10][11]. The seed distribution may contribute to establishing the populations of S. canadensis s.l. in new habitats.

Figure 1. Solidago canadensis s.l.
This species has adapted to a wide range of soil fertility and water potential [8][11][12], as well as been colonized into disturbed areas such as abandoned fields, roadsides, riverbanks and forest edges [11][13][14]. The species established its population on an agriculture field in two years after the abandonment [15][16]. Once established, the population remained dominant over 30 years [8][17][18][19][20]. It was also reported that the species showed great impact on the native plant diversity in the introduced ranges [21][22][23]. Owing to the potential of the species for the rapid expansion and the formation of thick monospecific stands in the introduced ranges, as well as its impact on the environments, S. canadensis s.l. has been designated as a harmful invasive plant species [4][24].
The S. canadensis complex is a highly variable species. S. canadensis s.l. contains S. canadensis L. as S. canadensis subsp. canadensis (L.), and S. altissima L. as S. canadensis subsp. altissima (L.) O.Bolòs et Vigo [2][25][26]. S. canadensis and S. altissima are very similar taxa. The field experiments also showed that the competitive abilities of S. altissima and S. canadensis against other plant species was similar [27]. However, they could be distinguished by their morphological traits such as shoot length and flowering time [2][26][28][29]. The chromosome number between S. canadensis (diploid; 2n = 18) and S. altissima (hexaploid; 2n = 54) is also different [7][29][30]. The native ranges of both species in North America are not exactly the same [8][26]. In addition, the experimental crossing of S. canadensis and S. altissima could not bear viable seeds [30], which indicates a genetic barrier between both taxa. However, owing to the lack of consistency in the identification of the species, the separation of both species has been considered to be very problematic [2][28][29]. For example, most European populations of both species were described to be S. altissima [3][25], although macro-morphological analyses indicate that S. canadensis is a common species in Europa [29][31]. S. altissima was also mentioned as a synonym of S. canadensis [32][33]. There may have been a misidentification of the species.
It was reported that invasive plants are often allelopathic and inhibit the germination and growth of the native plant species in the invasive ranges through specific secondary metabolites defined as allelochemicals [34][35][36][37][38][39][40][41][42][43]. S. canadensis showed an allelopathic activity on the sugar maple seedlings in the field, greenhouse and laboratory conditions [44]. The cis-Dehydromatricaria ester was identified in the extracts of S. altissima as its allelochemical [45]. The evidence of the allelopathy of S. canadensis and S. altissima has been accumulated since the late 20th century, and their allelopathy was often implicated in the potential of their invasiveness and naturalization.
2. Allelopathy of S. canadensis
Allelopathy is the chemical interaction between donor plants and recipient plants through allelochemicals. Allelochemicals are produced in some plant parts and released into the vicinity of the donor plants, including their rhizosphere soil either by the root exudation, rainfall leachates, volatilization from the plant parts or decomposition processes of plant residues [46][47][48][49]. Several investigations in field conditions showed that S. canadensis reduced the number and biodiversity of the native plant community in its invaded ranges [50]. The invasion level of S. canadensis correlated negatively with the taxonomic diversity of the native plant community, and positively with the invasibility of the community [51]. Those observations may imply the involvement of allelopathy in the interaction between S. canadensis and native plant species to some extent. Many researchers have evaluated the allelopathic activity of the root exudates, rhizosphere soil, residues and plant extracts of S. canadensis (Table 1).
Table 1. Allelopathic activities of exudates, rhizosphere soil, residues and plant extracts of S. canadensis.
| Source | Inhibition | Target Plant Species | Reference | |||
|---|---|---|---|---|---|---|
| Germination | Growth | Chlorophyll | Mycorrhizal colonization | |||
| Root exudate | ✓ | Gnaphalium affine, Xanthium sibiricum, Conyza canadensis, Celosia argentea, Aster subulatus, Sesbania cannabina, Eclipta prostrata | [52] | |||
| ✓ | Arabidopsis thaliana, | [53] | ||||
| Rhizosphere soil | ✓ | ✓ | Dactylis glomerata, Lythrum salicaria, Stachys officinalis, Trifolium pratense | [53] | ||
| Soil extract | ✓ | ✓ | Digitaria sanguinalis, Amaranthus retroflexus | [54] | ||
| Residue | ✓ | ✓ | Raphanus sativus, Triticum aestivum | [55] | ||
| Plant extract | ||||||
| Whole part | ✓ | ✓ | Kummerowia striata | [56] | ||
| Leaf | ✓ | ✓ | Raphanus sativus, Lactuca sativa | [57] | ||
| ✓ | ✓ | Triticum aestivum, Setaria viridi | [58] | |||
| ✓ | Raphanus sativus | [59] | ||||
| ✓ | Trifolium pratense | [60] | ||||
| Leaf and stem | ✓ | ✓ | Raphanus sativus, Triticum aestivum | [55] | ||
| Above-ground part | ✓ | ✓ | Lactuca sativa | [61] | ||
| ✓ | ✓ | Digitaria sanguinalis (L.) Scop. and Amaranthus retroflexus | [54] | |||
| Above-ground part, root | ✓ | ✓ | Zoysia japonica | [62] | ||
| Stem, root, blossom, seed | ✓ | ✓ | Brassica napus, Lolium perenne | [63] | ||
| Leaf, stem, rhizome | ✓ | ✓ | Morus alba, Pharbitis nil, Triticum aestivum, Brassica campestris | [64] | ||
| Root, rhizome | ✓ | Raphanus sativus, Lactuca sativa | [57] | |||
| ✓ | ✓ | Trifolium repens, Trifolium pratense, Medicago lupulina, Suaeda glauca, Plantago virginica, Kummerowia stipulacea, Festuca arundinacea, Ageratum conyzoides, Portulaca oleracea, Amaranthus spinosus | [65] | |||
| Rhizome | ✓ | Echinochloa crus-galli, Kummerowia striata, Ageratum conyzoides | [66] |
2.1. Allelopathy of Root Exudate and Plant Residue
Root exudates of S. canadensis, which were obtained from its aeroponic culture, significantly suppressed the growth of two Asian original plant species; Gnaphalium affine D.Don and Xanthium sibiricum Patrin ex Widder., two of America origin; Conyza canadensis (L.) Cronquist and Celosia argentea L., two of tropical origin; Aster subulatus Michx. and Sesbania cannabina (Renz.) Poir. and a cosmopolitan species; Eclipta prostrata (L.) L. The suppression rate was similar in all plant species [52]. Root exudates of S. canadensis also showed the growth inhibition of Arabidopsis thaliana (L.) Heynh. [53]. When the seeds of seven European native plant species were sown into the S. canadensis cultivated soils with or without activated carbon, the germination of five species such as Dactylis glomerata L., Lythrum salicaria L., Stachys officinalis (L.) Trevis. and Trifolium pratense L. were significantly suppressed in activated carbon-free plots than in activated carbon plots. Although the germination rate was not significantly different between both plots, the biomass of Arrhenatherum elatius (L.) P.Beauv. ex J. et C.Presl in the activated carbon plots after three months of sowing was two times greater than that in the activated carbon-free plots [53]. Activated carbon is a widely used material to investigate allelopathy because it adsorbs allelochemicals in the plant rhizosphere soil [34][67]. In addition, aqueous extracts of the rhizosphere soil of S. canadensis inhibited the germination and growth of Digitaria sanguinalis (L.) Scop. and Amaranthus retroflexus L., and the inhibitory activity was greater in the extracts of the soil obtained from the invasive ranges of S. canadensis (China) than that from its native ranges (USA) [54]. These observations suggest that certain allelochemicals, which may cause growth inhibition, would be released into the rhizosphere soil as root exudates of S. canadensis, and the released allelochemicals in the soil may be greater in the invasive ranges than those in the native ranges.
Crushed stems, leaves and rhizomes of S. canadensis were mixed with soil and water and kept at 20/15 °C (12/12 h light/dark condition), and the mixture was filtered after 45 days. The obtained filtrate suppressed the germination and growth of Raphanus sativus L. and Triticum aestivum L. [55]. This observation also suggests that certain allelochemicals may be released into the rhizosphere soil during the decomposition process of plant residues of S. canadensis.
2.2. Allelopathy of Plant Extract
Some plant tissues may contain allelochemicals, since allelochemicals are synthesized and stored in certain plant tissues until their release into the environment [46][47][48][49]. Many investigations on the allelopathic activity of the extracts from different plant parts of S. canadensis have been conducted. Aqueous extracts of the leaves of S. canadensis inhibited the germination and root growth of Raphanus sativus L. and Lactuca sativa L. [57], as well as those of Triticum aestivum L. and Setaria viridis (L.)P.Beauv. [58]. The extracts also suppressed the germination, growth and chlorophyll content of Trifolium pratense L. and Raphanus sativus L., and increased their electrolyte leakage from the cell membrane of the seedlings [59][60].
The fresh leaves and stems of S. canadensis were soaked in water for 48 h, and the obtained soaking water showed the inhibitory activity on the germination and growth of Raphanus sativus L. and Triticum aestivum L. [67]. Aqueous extracts of the above-ground parts of S. canadensis suppressed the germination and growth of Lactuca sativa L. [61], as well as those of Digitaria sanguinalis (L.) Scop. and Amaranthus retroflexus L. [54]. The inhibitory activity was greater in the plant extracts obtained from the heavily invaded stands than in those obtained from the lightly invaded stands [62], and in the plant extracts obtained from the invasive ranges than those from the native ranges [54].
Aqueous extracts of the above-ground parts and roots of S. canadensis inhibited the germination and growth of Zoysia japonica Steud, and the extracts of the above-ground parts significantly stimulated malondialdehyde and peroxidase activity [62]. The extracts of the stems, roots, blossoms and seeds of S. canadensis suppressed the germination and growth of Brassica napus L. and Lolium perenne L. [63], and the extracts of the roots and rhizomes of S. canadensis also inhibited the root growth of Raphanus sativus L. and Lactuca sativa L. [57].
Aqueous ethanol extracts of the roots and rhizomes of S. canadensis inhibited the germination and growth of Trifolium repens L., Trifolium pratense L., Medicago lupulina L., Suaeda glauca (Brunge) Brunge, Plantago virginica L., Kummerowia stipulacea (Maxium.) Makino, Festuca arundinacea Schreb., Ageratum conyzoides L., Portulaca oleracea L. and Amaranthus spinosus L. [68]. Aqueous ethanol extracts of the above- and below-ground parts of S. canadensis suppressed the germination of Kummerowia striata (Thunb.) Schindl., and the inhibitory activity was greater in the plant extracts collected from the invasive ranges of S. canadensis than those from its native ranges [56]. Aqueous and ethanol extracts of the leaves, stems and rhizomes of S. canadensis inhibited the germination and growth of Morus alba L., Pharbitis nil (L.) Roth, Triticum aestivum L. and Brassica campestris L., and the inhibition was grater in the ethanol extracts than in the aqueous extracts [64].
Investigations on the aqueous and ethanol extracts of every part of S. canadensis showed the allelopathic activity on the germination, growth, chlorophyll content, electrolyte leakage and/or some enzyme activities of several plant species, including the native plant species. The inhibitory activity was greater in the plant extracts obtained from the invasive ranges of S. canadensis than in those from its native ranges, and in the extracts collected from the heavily invaded stands than in those collected from the lightly invaded stands. These observations suggest that whole parts of S. canadensis may contain water and ethanol extractable allelochemicals, which may cause the inhibition. In addition, the plants grown in the invasive ranges and heavily invaded stands may contain more allelochemicals than the plants in the native ranges and lightly invaded stands.
2.3. Effects of the Extract on Arbuscular Mycorrhizal Fungi
The rhizomes of S. canadensis were soaked in water for 24 h, and the obtained soaking water caused the suppression of the arbuscular mycorrhizal colonization of Echinochloa crus-galli (L.) P.Beauv., Kummerowia striata (Thnb.) Schindl. and Ageratum conyzoides L. [66]. The field and greenhouse investigations also showed that S. canadensis altered the composition of the arbuscular mycorrhizal fungal population in its rhizosphere soil through the inhibition of some dominant species and the stimulation of other species. The established arbuscular mycorrhizal community increased the competitive ability and the biomass of S. canadensis [65][66][69][70][71]. This altered arbuscular mycorrhizal community also increased the mycorrhizal-mediated 15N uptake in S. canadensis, as well as decreased the 15N uptake in the native species Kummerowia statrica (Thunb.) Schindl. [72]. In addition, the aqueous ethanol extract of the roots and rhizomes of S. canadensis also suppressed the population of the soilborne pathogens, namely Pythium ultimum Trow and Rhizoctonia solani J.G. Kühn [73]. These observations indicate that the aqueous extracts of S. canadensis may alter the arbuscular mycorrhizal population and suppress the colonization of the native plant species. The established arbuscular mycorrhizal community enhanced the competitive ability of S. canadensis. Certain compounds in the extracts may be involved in the alteration of the arbuscular mycorrhizal community.
2.4. Allelochemicals
As described above, the inhibitory activity of the extracts of the plants and rhizosphere soil of S. canadensis obtained from the invasive ranges was greater than that obtained from the native ranges [54][56]. The concentrations of total phenolics, total flavones and total saponins in S. canadensis and its rhizosphere soil obtained from the invasive ranges were also greater than those from the native ranges [54][56]. These concentrations in the soil obtained from S. canadensis-infested stands were also greater than those in the soil obtained from S. canadensis-free stands [74].
Major compounds identified in the aqueous methanol extracts of the leaves and inflorescences of S. canadensis were chlorogenic acid, quercitrin and rutin (quercetin-3-O-β-rutinoside) (Figure 2) [75]. A fatty acid, n-hexadecanonic acid, was isolated from the aqueous ethanol extract of the stems and leaves of S. canadensis as an allelopathic agent. n-Hexadecanonic acid significantly inhibited the growth of Triticum aestivum L. [55]. A flavonoid, kaempferol-3-O-D-glucoside, was isolated from the aqueous ethanol extract of the S. canadensis straw, and the compound inhibited the growth of Echinochloa colona (L.) Link [75]. In addition, the concentration of rutin in the leaves of S. canadensis was greater than that of other Solidago species [76][77]. Some flavonoids were also identified in the aerial parts of S. canadensis [78].
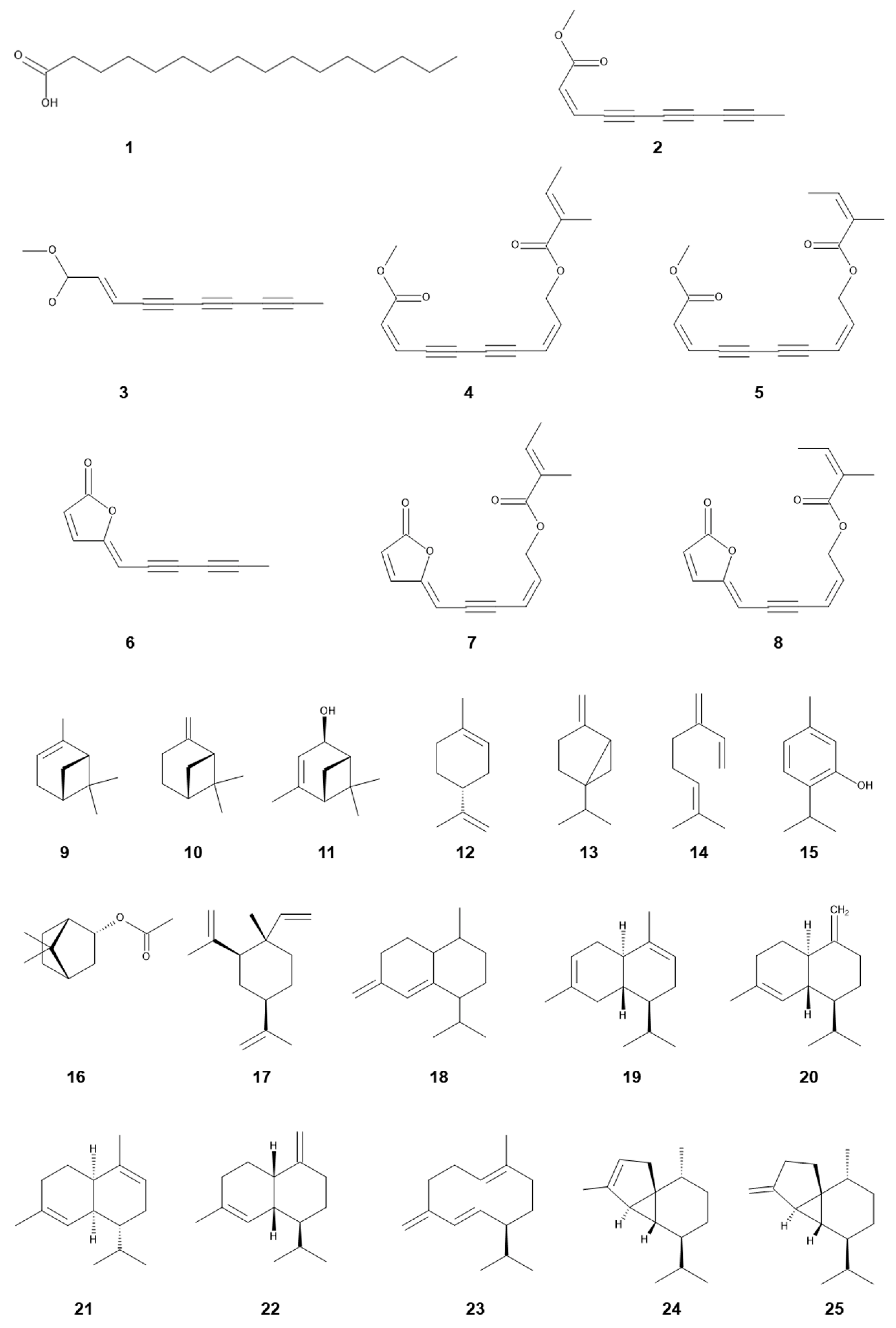

Figure 2. Allelochemicals identified in S. canadensis and S. altissima.
References
- Weber, E. The dynamics of plant invasions: A case study of three exotic goldenrod species (Solidago L.) in Europe. J. Biogeogr. 1998, 25, 147–154.
- Weber, E. Morphological variation of the introduced perennial Solidago canadensis L. sensu lato (Asteraceae) in Europe. Bot. J. Linn. Soc. 1997, 123, 197–210.
- Weber, E. Biological flora of Central Europe: Solidago altissima L. Flora 2000, 195, 123–134.
- Invasive Species Compendium, Solidago canadensis. Available online: https://www.cabi.org/isc/datasheet/50599 (accessed on 12 September 2022).
- Royal Botanical Gardens, Kew, Solidago canadensis. Solidago altissima L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:249454-1 (accessed on 12 September 2022).
- Royal Botanical Gardens, Kew, Solidago altissima L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:285347-2 (accessed on 12 September 2022).
- Croat, T. Solidago canadensis complex of the great plains. Brittonia 1972, 24, 317–326.
- Werner, P.A.; Bradbury, I.K.; Gross, R.S. The biology of Canadian weeds. 45. Solidago canadensis L. Can. J. Plant Sci. 1980, 60, 1393–1409.
- Curtis, J.D.; Lersten, N.R. Oil reservorirs in stem, rhizomes, and root of Solidago canadensis (Asteraceas, tribe Astereae). Nor. J. Bot. 1990, 10, 443–449.
- Meyer, A.H.; Schmid, B. Seed dynamics and seedling establishment in the invading perennial Solidago altissima under different experimental treatments. J. Ecol. 1999, 87, 28–41.
- Cornelius, R. The strategies of Solidago canadensis L. in relation to urban habitats. I. Resource requirements. Acta Oecol. 1990, 11, 19–34.
- Huang, H.; Guo, S.; Chen, G. Reproductive biology in an invasive plant Solidago canadensis. Front. Biol. China 2007, 2, 196–204.
- Follak, S.; Eberius, M.; Essl, F.; Fürdös, A.; Sedlacek, N.; Trognitz, F. Invasive alien plants along roadsides in Europe. EPPO Bull. 2018, 48, 256–265.
- Park, J.S.; Choi, D.; Kim, Y. Potential Distribution of Goldenrod (Solidago altissima L.) during Climate Change in South Korea. Sustainability 2020, 12, 6710.
- Kotowska, D.; Pärt, T.; Żmihorski, M. Evaluating google street view for tracking invasive alien plants along roads. Ecol. Indi. 2021, 121, 107020.
- Shimoda, M.; Nakamoto, M. Vegetation and threatened plant dynamics of wet abandoned rice fields in Nakaikemi, Fukui, Prefecture, Japan. Jpn. J. Ecol. 2003, 53, 197–217.
- Shimoda, S.; Wagai, R. Ecosystem dynamics after abandonment of rice paddy fields; Does aaien plant invasion enhance carbon storage? Ecosystems 2020, 23, 617–629.
- Newell, S.J.; Tramer, E.J. Reproductive strategies in herbaceous plant communities during succession. Ecology 1978, 59, 228–234.
- Bakelaar, R.G.; Odum, E.P. Community and population level response to fertilization in an old-field ecosystem. Ecology 1978, 59, 660–665.
- Maddox, G.D.; Cook, R.E.; Wimberger, P.H.; Gardescu, S. Clone structure in four Solidago altissima (Asteraceae) population: Rhizome connections within genotypes. Am. J. Bot. 1989, 76, 318–326.
- Dong, L.J.; Yu, H.W.; He, W.M. What determines positive, neutral and negative impacts of Solidago canadensis invasion on native plant species richness? Sci. Rep. 2015, 5, 16804.
- Fenesi, A.; Vágási, C.I.; Beldean, M.; Földesi, R.; Kolcsár, L.P.; Shapiro, J.T.; Török, E.; Kovács-Hostyánszki, A. Solidago canadensis impacts on native plant and pollinator communities in different-aged old fields. Basic Appl. Ecol. 2015, 16, 335–346.
- Zagurskaya, Y.V. Study issues of invasive species of the genus Solidago. Ecosyst. Transform. 2022, 5, 42–54.
- EPPO. PQR database. Paris, France: European and Mediterranean Plant Protection Organization. Available online: https://gd.eppo.int/taxon/SOOCA (accessed on 12 September 2022).
- Weber, E.; Schmid, B. Latitudinal population differentiation in two species of Solidago (Asteraceae) introduced into Europe. Am. J. Bot. 1998, 85, 1110–1121.
- iNatuarist, Identifying Solidago altissima & Solidago canadensis. Available online: https://www.inaturalist.org/posts/19288-identifying-solidago-altissima-solidago canadensis (accessed on 12 September 2022).
- Szymura, M.; Szymura, T.H. Interactions between alien goldenrods (Solidago and Euthamia species) and comparison with native species in Central Europe. Flora 2016, 218, 51–61.
- Semple, J.C.; Rahman, H.; Bzovsky, S.; Sorour, M.K.; Kornobis, K.; Laphitz, R.L.; Tong, L. A multivariate morphometric study of the Solidago altissima complex and S. canadensis (Asteraceae: Astereae). Phytoneuron 2015, 10, 1–31.
- Verloove, F.; Zonneveld, B.J.M.; Semple, J.C.S. First evidence for the presence of invasive Solidago altissima (Asteraceae) in Europe. Willdenowia 2017, 47, 69–75.
- Melville, M.R.; Morton, J.K. A biosystematics study of the Solidago canadensis (Compositae) complex. I. The Ontario populations. Can. J. Bot. 1982, 60, 976–997.
- Szymura, M.; Szymura, T.H.; Kreitschitz, A. Morphological and cytological diversity of goldenrods (Solidago L. and Euthamia Nutt.) from south-western Poland. Biodiv. Res. Conserv. 2015, 38, 41–49.
- Pisula, N.; Meiners, S.J. Allelopatyhic effcts of goldenrod species on turnover in successional communities. Am. Midl. Nat. 2010, 163, 161–172.
- Lawson, S.K.; Sharp, L.G.; Powers, C.N.; McFeeters, R.L.; Satyal, P.; Setzer, W.N. Volatile Compositions and Antifungal Activities of Native American Medicinal Plants: Focus on the Asteraceae. Plants 2020, 9, 126.
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523.
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 419–426.
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193.
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia. Engin. 2011, 18, 240–246.
- Meiners, S.J.; Kong, C.H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant. Ecol. 2012, 213, 1861–1867.
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766.
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028.
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2022, 11, 3.
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672.
- Kato-Noguchi, H. Allelopathy and Allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551.
- Fisher, R.F.; Woods, R.A.; Glavicic, M.R. Allelopathic effects of golderrod and aster on young sugar maple. Can. J. Forest Res. 1978, 8, 1–9.
- Kobayashi, A.; Morimoto, S.; Shibata, Y.; Yamashita, K.; Numata, M. C10-Polyacetylenes as allelopathic substances in dominants in early stages of secondary succession. J. Chem. Ecol. 1980, 6, 119–131.
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422.
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266.
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578.
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326.
- Bielecka, A.; Borkowska, L.; Królak, E. Environmental changes caused by the clonal invasive plant Solidago canadensis. Ann. Bot. Fennici. 2019, 57, 33–48.
- Wang, C.; Cheng, H.; Wanga, S.; Weia, M.; Du, D. Plant community and the influence of plant taxonomic diversity on community stability and invasibility: A case study based on Solidago canadensis L. Sci. Total Environ. 2021, 768, 144518.
- Yang, B.; Li, J. Phytotoxicity of toot exudates of invasive Solidago canadensis on co-occurring native and invasive plant species. Pak. J. Bot. 2022, 54, 1019–1024.
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? J. Ecol. 2008, 96, 993–1001.
- Weißhuhn, K.; Prati, D. ; Activated carbon may have undesired side effects for testing allelopathy in invasive plants. Basic Appl. Ecol. 2009, 10, 500–507.
- Yang, X.; Cheng, J.; Yao, B.; Lu, H.; Zhang, Y.; Xu, J.; Song, X.; Qiang, S. Polyploidy-promoted phenolic metabolism confers the increased competitive ability of Solidago canadensis. Oikos 2021, 130, 1014–1025.
- Zhang, D.; Ye, Y.; Li, L.; Domg, L. Allelopathic pathways, isolation and identification of an allelopathic substance from Solidago canadensis L. Allelopath. J. 2014, 33, 201–212.
- Butcko, V.M.; Jensen, R.J. Evidence of tissue-specific allelopathic activity in Euthamia graminifolia and Solidago canadensis (Asteraceae). Am. Midl. Nat. 2002, 148, 253–262.
- Li, S.L.; Li, Z.H.; Wang, Y.F.; Xiao, R.; Pan, C.D.; Wang, Q. Preliminary study for the allelopathic effect of water extracts from Solidago canadensis leaves. Adv. Mater. Res. 2013, 699, 340–348.
- Możdżeń, K.; Barabasz-Krasny, B.; Zandi, P.; Kliszcz, A.; Puła, J. Effect of aqueous extracts from Solidago canadensis L. Leaves on germination and early growth stages of three cultivars of Raphanus Sativus L. var. Radicula Pers. Plants 2020, 9, 1549.
- Zandi, P.; Barabasz-Krasny, B.; Stachurska-Swakoń, A.; Puła, J.; Możdżeń, K. Allelopathic effect of invasive Canadian goldenrod (Solidago canadensis L.) on early growth of red clover (Trifolium pratense L.). Not. Bot. Horti. Agrobo. 2020, 48, 2060–2071.
- Yu, Y.; Cheng, H.; Xu, Z.; Zhong, S.; Wang, C.; Guo, E. Invasion intensity modulates the allelopathic impact of Solidago canadensis L. leaves and roots against Lactuca sativa L. during germination and early seedling stage. Int. J. Environ. Res. 2022, 16, 48.
- Sun, J.F.; Liang, Q.J.; Wu, N.; Javed, Q.; Huang, P.; Du, D.L. Allelopathic effects of aqueous extracts from different plant parts of Canada goldenrod (Solidago canadensis L.) on seed germination and seedling growth of Korean lawngrass (Zoysia japonica Steud.). Appl. Ecol. Environ. Res. 2022, 20, 1009–1022.
- Baležentien, L. Secondary metabolite accumulation and phytotoxicity of invasive species Solidago canadensis L. during the growth period. Allelopath. J. 2015, 35, 217–226.
- Yang, R.Y.; Mei, L.X.; Tang, J.J.; Chen, X. Allelopathic effects of invasive Solidago canadensis L. on germination and growth of native Chinese plant species. Allelopath. J. 2007, 19, 241–248.
- Yuan, Y.; Wang, B.; Zhang, S.; Tang, J.; Tu, C.; Hu, S.; Yong, J.W.H.; Chen, X. Enhanced allelopathy and competitive ability of invasive plant Solidago canadensis in its introduced range. J. Plant Ecol. 2013, 6, 253–263.
- Sun, B.Y.; Tan, J.Z.; Wan, Z.G.; Gu, F.G.; Zhu, M.D. Allelopathic effects of extracts from Solidago canadensis L. against seed germination and seedling growth of some plants. J. Environ. Sci. 2006, 18, 304–309.
- Zhang, Q.; Yao, L.J.; Yang, R.Y.; Yang, X.Y.; Tang, J.J.; Chin, X. Potential allelopathic effects of an invasive species Solidago canadensis on the mycorrhizae of native plant species. Allelopath. J. 2007, 20, 71–78.
- Sun, Z.K.; He, W.M. Evidence for enhanced mutualism hypothesis: Solidago canadensis plants from regular soils perform better. PLoS ONE 2010, 5, e15418.
- Betekhtina, A.A.; Mukhacheva, T.A.; Kovaleva, S.Y.; Gusevb, A.P.; Veselkin, D.V. Abundance and diversity of arbuscular mycorrhizal fungi in invasive Solidago canadensis and indigenous S. virgaurea. Russ. J. Ecol. 2016, 47, 575–579.
- Dong, L.J.; Ma, L.N.; He, W.M. Arbuscular mycorrhizal fungi help explain invasion success of Solidago canadensis. Appl. Soil. Ecol. 2021, 167, 103763.
- Řezáčová, V.; Řezáč, M.; Gryndler, M.; Hršelová, H.; Gryndlerová, H.; Michalová, T. Plant invasion alters community structure and decreases diversity of arbuscular mycorrhizal fungal communities. Appl. Soil. Ecol. 2021, 167, 104039.
- Yang, R.; Zhou, G.; Zan, S.; Guo, F.; Su, N.; Li, J. Arbuscular mycorrhizal fungi facilitate the invasion of Solidago canadensis L. in southeastern China. Acta Oecol. 2014, 61, 74–77.
- Zhang, S.; Jin, Y.; Tang, J.; Chen, X. The invasive plant Solidago canadensis L. Suppresses local soil pathogens through allelopathy. Appl. Soil. Ecol. 2009, 41, 215–222.
- Zhang, S.; Zhu, W.; Wang, B.; Tang, J.; Chen, X. Secondary metabolites from the invasive Solidago canadensis L. Accumulation in soil and contribution to inhibition of soil pathogen Pythium ultimum. Appl. Soil. Ecol. 2011, 48, 280–286.
- Jun, L.; Yonghao, Y.; Hongwu, H.; Liyao, D. Kaempferol-3-O-β-D-glucoside, a potential allelochemical isolated from Solidago canadensis. Allelopath. J. 2011, 28, 259–266.
- Radusiene, J.; Marska, M.; Ivanauskas, L.; Jakstas, J.; Karpaviciene, B. Assessment of phenolic compound accumulation in two widespread goldenrods. Ind. Crops Prod. 2015, 63, 158–166.
- Likhanov, A.; Oliinyk, M.; Pashkevych, N.; Churilov, A.; Kozyr, M. The role of flavonoids in invasion strategy of Solidago canadensis L. Plants 2021, 10, 1748.
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Assessment of flavonoids and phenolic compound accumulation in invasive Solidago сanadensis L. in Slovakia. Slovak J. Food Sci. 2020, 14, 587–594.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
905
Revisions:
2 times
(View History)
Update Date:
07 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




