Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jinwang Li | -- | 2479 | 2022-12-06 08:07:05 | | | |
| 2 | Camila Xu | -110 word(s) | 2369 | 2022-12-06 08:49:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, X.; Wang, Y.; Zhao, G.; Liu, G.; Wang, P.; Li, J. Intestinal Microorganisms Enhence Utilization of Sulforaphane. Encyclopedia. Available online: https://encyclopedia.pub/entry/38099 (accessed on 07 February 2026).
Li X, Wang Y, Zhao G, Liu G, Wang P, Li J. Intestinal Microorganisms Enhence Utilization of Sulforaphane. Encyclopedia. Available at: https://encyclopedia.pub/entry/38099. Accessed February 07, 2026.
Li, Xiude, Yihan Wang, Guoping Zhao, Guangmin Liu, Pengjie Wang, Jinwang Li. "Intestinal Microorganisms Enhence Utilization of Sulforaphane" Encyclopedia, https://encyclopedia.pub/entry/38099 (accessed February 07, 2026).
Li, X., Wang, Y., Zhao, G., Liu, G., Wang, P., & Li, J. (2022, December 06). Intestinal Microorganisms Enhence Utilization of Sulforaphane. In Encyclopedia. https://encyclopedia.pub/entry/38099
Li, Xiude, et al. "Intestinal Microorganisms Enhence Utilization of Sulforaphane." Encyclopedia. Web. 06 December, 2022.
Copy Citation
Sulforaphane (SFN) was generated by the hydrolysis of glucoraphanin under the action of myrosinase. However, due to the instability of SFN, the bioavailability of SFN was limited. Meanwhile, the gut flora obtained the ability to synthesize myrosinase and glucoraphanin, which could be converted into SFN in the intestine. However, the ability of microorganisms to synthesize myrosinase in the gut was limited. Therefore, microorganisms with myrosinase synthesis ability need to be supplemented. With the development of research, microorganisms with high levels of myrosinase synthesis could be obtained by artificial selection and gene modification.
sulforaphane
microorganisms
myrosinase synthesis
1. Introduction
Sulforaphane (1-isothiocyanate-4-(methylsulfonyl) butane, SFN) is produced by the degradation of glucoraphanin under the action of myrosinase (β-D-thioglucosidase) [1][2][3]. SFN and its precursor (glucoraphanin) are important safeguard substances used by plants to defend themselves against insects, pathogens, and herbivores [4]. Recently, researchers have found that SFN possesses antioxidant, anti-inflammatory, and antibacterial functions [5][6]. In addition, SFN could prevent and cure cancer [7][8][9], improve the nervous system [10][11][12], and prevent coronavirus disease 2019 [13]. Therefore, SFN has attracted extensive attention from researchers around the world.
Although SFN has shown great positive effects on human health, it is an unstable compound. Therefore, it is difficult to obtain SFN directly from cruciferous plant tissues owing to its structural instability [14][15][16][17]. It has always been a research topic of interest to maintain the function of SFN stably in the human body. Exogenous hydrolysis and transformation research of glucoraphanin have suggested that SFN could be obtained outside plant tissues with high biological activity. Meanwhile, the key controlling factors for the exogenous transformation are glucoraphanin concentration and the activity of myrosinase [18][19]. Triska et al. found that SFN formation was controlled by a temperature-specific epithiospecifier protein (a myrosinase cofactor). The most suitable exogenous transformation condition is to ensure sufficient radish sprout content to continuously add myrosinase and to maintain a stable transformation temperature during the process [20].
However, external factors can also affect myrosinase synthesis during the cultivation of cruciferous plants. Polyethylene glycol can enhance the expression of the myrosinase gene, which can increase myrosinase synthesis [21]. Lower planting temperatures can also result in higher activity of the myrosinase obtained from cruciferous plants and increase the ability of myrosinase to convert glucoraphanin into SFN [22]. Although the production of plant-derived SFN is increased through exogenous environmental regulation, it is difficult for the human body to take up enough SFN from plant tissues due to the instability of the SFN structure [23]. Therefore, determining how to enhance the absorption rate of SFN in intestinal tissues has attracted the attention of researchers.
Studies show that the gut microbiome not only helps digest food ingested into the gastrointestinal tract, but also converts dietary pairs into more active products [24][25][26]. Lai et al. showed that after feeding F344 rats with cooked broccoli (without myrosinase), the microbes in the gut of the rats converted glucoraphanin into SFN, and SFN was detected in the blood [27]. However, studies on humans show that there are individual differences in the ability of intestinal flora to produce SFN [28][29].
Meanwhile, studies have reported that in the microbial conversion of glucoraphanin, probiotics, which are a class of active microorganisms that colonize the human intestinal environment, could improve the composition of human intestinal flora and enhance intestinal digestion [30][31]. Probiotics have also shown preventive and therapeutic effects on colorectal cancer, mental disorders, diabetes, and other diseases [32][33]. Therefore, probiotics are widely used in health products, dietary supplements, and prebiotics [34][35][36]. In contrast, studies have shown that lactic acid bacteria (LAB) can convert glucoraphanin from broccoli into SFN under suitable conditions, and high SFN concentrations were found in fermented broccoli puree [37][38]. The LAB fermentation results also indicated that, in addition to the mammalian gut flora, microorganisms from other sources could also degrade glucoraphanin and produce SFN.
Although microorganisms can convert glucoraphanin into SFN in the gut, the production of SFN is limited due to the insufficient number of microorganisms that can synthesize myrosinase in the intestine [4]. With the discovery of exogenous myrosinase, and the ability of LAB to ferment broccoli puree to produce high concentrations of SFN, researchers attempted to replicate this process in vivo [37][38].
2. Intestinal Microorganisms Can Enhance the Utilization of SFN
2.1. Structure and Biochemical Characteristics of SFN, Glucoraphanin, and Myrosinase
SFN (1-isothiocyanate-4-(methylsulfonyl) is an isothiocyanates (a general formula R-N=C=S). Due to the presence of an active electrophilic carbon atom in the SFN group (-N=C=S), SFN is easily reversible with thiols under physiological conditions, resulting in pH-sensitive dithiocarbamates reacting with amines and forming thiourea [1][39][40]. This is the reason why SFN is sensitive to temperature and other conditions. SFN is converted by its precursor glucoraphanin under the enzymatic hydrolysis reaction of myrosinase. Meanwhile, glucoraphanin (4-methylsulfinyl butyl glucosinolate) is a methionine-derived aliphatic glucosinolate. Glucoraphanin is widely found in cruciferous plants, especially broccoli. Glucoraphanin is a water-soluble sulfur-containing anionic secondary metabolite consisting of a β-glucosinolate N-hydroxysulfate with a side chain center and a β-D-glucopyranose residue [41][42][43]. Glucoraphanin demonstrates no physiological activity. Therefore, the best form of utilization for SFN is to preserve.
Myrosinase, also known as β-glucosidase, is a ubiquitous enzyme in cruciferous plants that can efficiently degrade glucosinolates. The essence of myrosinase is a glycoprotein, and currently found in plants, aphids, and other myrosinase of the glycoside hydrolase family 1 [44][45]. The differences in myrosinase from different sources are mainly reflected in molecular weight, subunit number, and side chain sugar content, which leads to the different ability of myrosinase to degrade glucoraphanin.
2.2. Increasing the Intestinal SFN Production Rate Is a Scientific Approach to Enhance SFN Utilization
Although SFN has strong anticancer properties, it is very unstable and loses its biological activity under certain conditions, such as the presence of oxygen, which could reduce the utilization of SFN [46][47][48]. To improve SFN utilization, researchers considered the possibility of directly producing SFN in vivo and conducted in vitro simulation studies [49]. Xu et al. found that glucoraphanin could be converted into SFN under a simulated gastrointestinal environment in vitro, and the maximum conversion rate could reach 46.2%. Moreover, when glucoraphanin was directly fed to germ-free and human-microbiota-associated mice, SFN degradation products were found in the urine of the mice, which indicated that SFN could be produced in the intestinal environment and that SFN could be utilized [50].
Lai et al. used male F344 rats as an animal model and demonstrated that the cecum can also degrade glucoraphanin and produce SFN [27]. Once glucoraphanin was directly gavaged into male F344 rats, SFN was detected in the plasma of the rats after 120 min, and the level of SFN in the plasma remained constant for 1 h. In addition, a study indicated that the intestinal environment not only is a limiting factor for SFN utilization, but also has a promoting effect on SFN utilization [29]. However, humans can consume glucoraphanin-rich Brassica vegetables directly instead of consuming some glucoraphanin. Raw broccoli is used for studies on humans, and the results indicate that glucosinolates in broccoli are degraded in the human body and the degradation products of glucosinolates are detected in the blood and urine of volunteers [51]. Therefore, the studies on humans suggest that SFN can be produced in the human intestinal environment.
Meanwhile, the bioavailability of SFN in raw broccoli could reach 37%, which is significantly higher than that in cooked broccoli, and the consumption of cooked broccoli would delay the absorption of SFN [52]. Egner et al. found that the bioavailability of SFN was far superior to glucoraphanin in the human body [53]. Another study showed that the main reason for the higher bioavailability of SFN than sulforaphane is that glucoraphanin must be hydrolyzed to be absorbed [54].
However, because humans consume more cooked than raw vegetables, researchers have used cooked white cabbage to simulate the in vivo degradation of glucoraphanin in a rat duodenal model [55]. The results show that 82% of glucoraphanin is released from white cabbage seeds after 10 min, but no degradation of glucoraphanin is detected. However, the in vitro simulation results for the rat duodenum are different from the results from the study on male F344 rats. Therefore, Wu et al. explored the possibility of SFN production by the gut microbiota [28][56]. Research using a male C57BL/6 mouse shows that glucoraphanin could be degraded to SFN in the intestine, and the production of SFN is related to the intestinal flora. Hwang et al. show that 13 pmol/g fresh weight of SFN is produced in the gut after 120 min, and approximately 29% of the SFN is taken up and utilized by cells, indicating that the intestinal environment has a promoting effect on the production and utilization of SFN [57].
Meanwhile, Sangkret et al. found that the main elements affecting SFN production were myrosinase activity, temperature, pH, and reaction time [14]. Recently, researchers obtained a new myrosinase-producing bacterium from marine sediment (Marine Bacterium Shewanella baltica Myr-37) [58]. Once the reaction temperature is 40 °C and pH = 7.0, myrosinase can efficiently degrade sulforaphane to SFN in 25 min, the yield is 0.57 mg/mL, and the corresponding SFN conversion efficiency is 89%. However, intestinal myrosinase is also affected by epithiospecifier protein (EP) and sulfur–selenium interaction(S–Se) in the process of SFN formation [48][59][60]. EP interferes with the production of SFN, while S–Se induces the expression of myrosinase gene to produce more myrosinase.
The intestinal environment can degrade glucoraphanin to produce SFN without the action of plant-derived myrosinase, and the main factor for SFN production is the effect of the intestinal flora. However, the mechanisms by which the gut microbiota degrades glucoraphanin to produce SFN, and the gut microbes involved, are not clear. Therefore, researchers investigated the mechanisms of the microbial transformation of glucoraphanin.
2.3. Microorganisms Converted Glucoraphanin into SFN Using Myrosinase Synthesis
Myrosinase is a beta-thioglucosidase glucohydrolase that was originally discovered in cruciferous plants; it can resist in vitro damage and degrade glucoraphanin [61][62][63]. Studies have found that some microorganisms can also synthesize myrosinase (Table 1) [64][65][66]. Naoki Tani et al. first isolated a species of Enterobacter cloacae, which could synthesize myrosinase, but its molecular weight was smaller than the endogenous myrosinase of the plant. Meanwhile, Bacteroides thetaiotaomicron (another dominant species derived from the human colon) could convert glucosinolates into allyl isothiocya-nate [67]. With the further development of research on SFN production by intestinal flora, it was discovered that a variety of intestinal strains can degrade glucoraphanin to produce SFN [68].
Table 1. Microorganisms with myrosinase synthesis function.
| Strain | Source | Substrate | Products | Transformation Ability | References |
|---|---|---|---|---|---|
| Lactobacillus agilis R16 | NS | Glucoiberin/glucoraphanin | NS | 10% | [68] |
| Enterococcus casseliflavus CP1 | Human feces | Glucoiberin/glucoraphanin | Iberin/SFN | 40–50% | |
| Escherichia coli VL8 | Human feces | Glucoiberin/glucoraphanin | Glucoiberverin/glucoerucin | 80–90% | |
| Enterococcus gallinarum HG001 | Mouse feces | Glucosinolate | Isothiocyanate | 39.54% | [69] |
| Escherichia coli HG002 | Mouse feces | Glucosinolate | Isothiocyanate | 29.17% | |
| L. plantarum KW30 | NS | Glucoraphanin, etc. | SFN, etc. | 30–33% | [70] |
| Lactococcus lactis subsp. lactis KF147 | NS | Glucoraphanin, etc. | SFN, etc. | 30–33% | |
| E. coli Nissle 1917 | NS | Glucoraphanin, etc. | Glucoerucin, etc. | 65–78% | |
| Bacteroides thetaiotaomicron | Human feces | Sinigrin | Allyl isothiocyanate | NS | [71] |
| Companilactobacillus farciminis KB1089 | Pickles | Sinigrin | Allyl isothiocyanate | NS | [72] |
| Citrobacter Wye1 | Soil | Sinigrin | Allylcyanide | NS | [73] |
| Leclercia adecarboxylata | Soil | Sinigrin | Allylcyanide | NS | [74] |
| Aspergillus sp. NR46F13 | Soil | Sinigrin | NS | [75] | |
| LAB | Broccoli | Glucoraphanin | SFN | NS | [37] |
| LAB | Broccoli | Glucoraphanin | SFN | NS | [38] |
| Pediococcus pentosaceus | Natural fermented cherry juice | Glucoraphanin | SFN | NS | [76] |
| Aspergillus sp. NR-4201 | NS | Glucosinolate | Allylcyanide | NS | [77] |
| Lactobacillus agilis R16 | NS | Sinigrin | Allyl isothiocyanate | NS | [70] |
Note: Not explicitly stated (NS).
With the development of research on human intestinal enzyme-producing flora, researchers successfully isolated Enterococcus gallinarum HG001 and Escherichia coli HG002, which could synthesize myrosinase from the intestines of C57BL/6 mice [69]. However, the mechanisms by which intestinal myrosinase produces SFN remain unclear. Watanabe et al., studying LAB as research objects, found that intestinal myrosinase may be involved in the metabolism of glucoraphanin through the β-glucoside-specific IIB, IIC, and IIA phosphotransferase system components (Figure 1) [72]. With the intestinal myrosinase synthesis mechanisms becoming clear, researchers further investigated the myrosinase synthesis capabilities of microorganisms to identify the types of microorganisms that synthesize myrosinase.
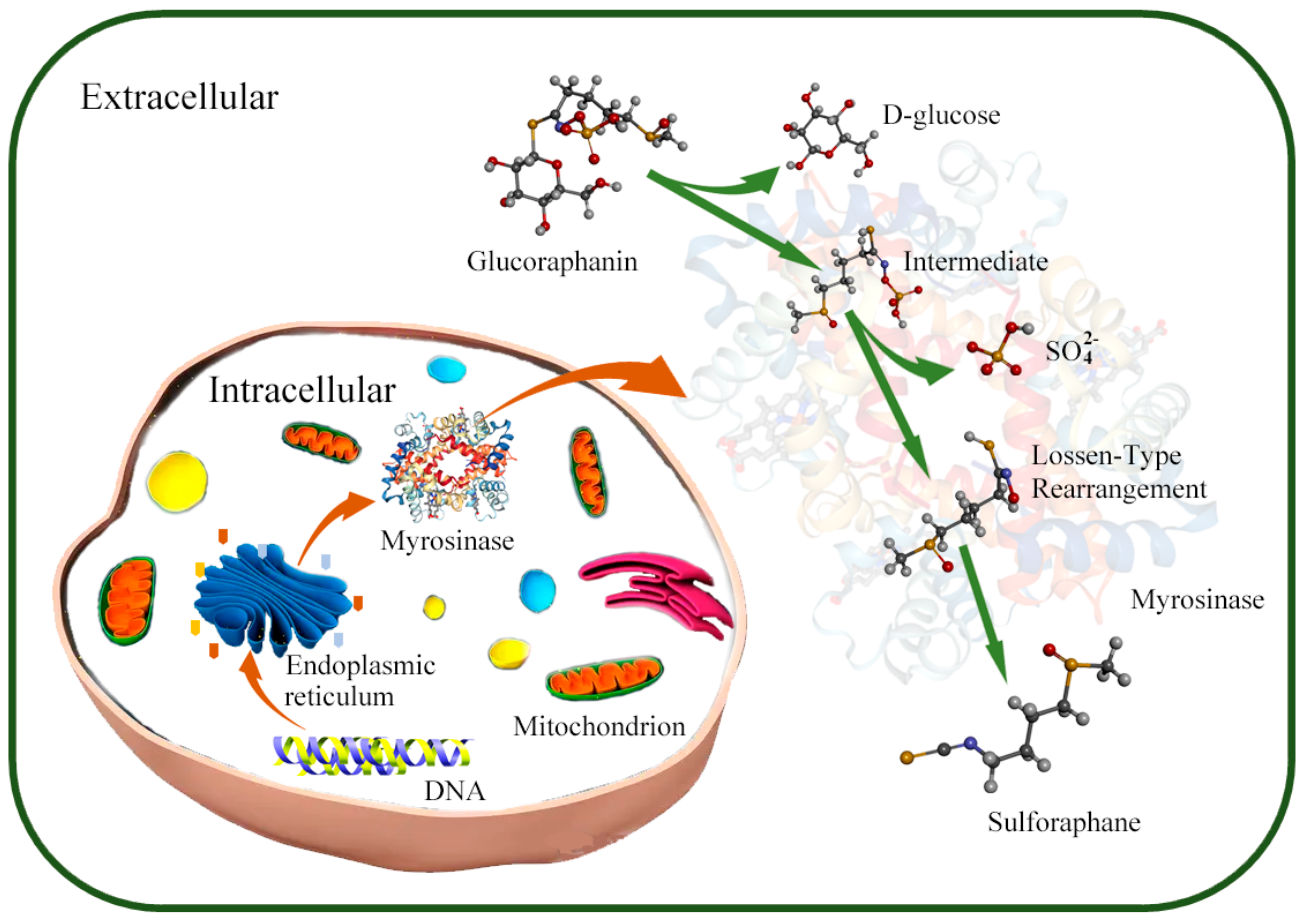
Figure 1. Mechanism of SFN production by microorganisms. In microorganisms, myrosinase is synthesized and secreted to environment through ribosome and endoplasmic reticulum under the myrosinase gene regulation; the glucoraphanin is converted into sulforaphane under the action of myrosinase in extracellular environment.
Researchers turned to fungal microbes that are ubiquitous. Rakariyatham et al. investigated the ability of Aspergillus sp. NR-4201 to synthesize myrosinase [77]. Aspergillus sp. NR-4201 converted all glucosinolates into allyl cyanide within 32 h, indicating it can synthesize myrosinase. Subsequently, Nuansri et al. also explored whether Aspergillus sp. NR46F13 could synthesize myrosinase [75], and their results show that 3.19 U mL–1 myrosinase was isolated from the medium of this strain after 48 h of culture. With the continuous exploration of microorganisms, researchers isolated Leclercia adecarboxylata and Citrobacter Wye1, which could synthesize myrosinase, from the soil [73][74].
Palop et al. investigated the potential of 42 Lactobacillus species to degrade glucosinolates, and the results indicate that strain R16 shows a strong ability to degrade glucosinolates [78]. In addition, Mullaney et al. explored the different abilities of L. plantarum KW30, Lactococcus lactis subsp. lactis KF147, and E. coli Nissle 1917, while E. cloacae was used to degrade glucoraphanin [70]. The comparative research of myrosinase obtained from different microbial sources shows that the myrosinase of the intestinal flora is more capable of degrading glucoraphanin than those of plant-derived microorganisms. Some researchers investigated the feasibility of using LAB fermentation as an alternative method to maintain myrosinase activity and enhance the bioconversion of glucosides into SFN [37]. The fermentation results show that the fermentation of LAB could achieve stable SFN production.
Some researchers further explored the effect of high-temperature sterilization or preheating broccoli puree on the fermentation of LAB to produce SFN [38][71]. The results show that high-temperature sterilization and preheating broccoli puree enhances the yields of SFN produced by LAB fermentation, and the SFN yield from broccoli puree that was preheated in advance is 16 times higher than that of non-preheated broccoli puree. Xu et al. show that using Pediococcus pentosaceus for fermentation in broccoli juice also produces more SFN [76]. Exogenous myrosinase could stably produce SFN under the condition of sufficient raw materials, which provides a new route for SFN production. In addition, the results indicate that microbial-derived myrosinase has the same efficacy as plant-derived myrosinase, but microbial-derived myrosinase is easier to obtain in vitro.
Microorganisms that could synthesize myrosinase have also been isolated from soil and broccoli, in addition to those isolated from the intestinal environment. However, the myrosinase-synthesizing abilities of the microorganisms were restricted by the culture conditions and their gene sequences; therefore, it was necessary to further investigate the factors that restrict the synthesis of high-yield myrosinase enzymes.
References
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 1029.
- Abukhabta, S.; Ghawi, S.K.; Karatzas, K.A.; Charalampopoulos, D.; McDougall, G.J.; Allwood, J.W.; Verrall, S.R.; Lavery, S.; Latimer, C.; Pourshahidi, L.K.; et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur. J. Nutr. 2021, 60, 1263–1276.
- Hafezian, S.M.; Azizi, S.N.; Biparva, P.; Bekhradni, A. High-efficiency purification of sulforaphane from the broccoli extract by nanostructured SBA-15 silica using solid-phase extraction method. J. Chromatogr. B 2019, 1108, 1–10.
- Janczewski, L. Sulforaphane and Its Bifunctional Analogs: Synthesis and Biological Activity. Molecules 2022, 27, 125.
- Melrose, J. The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 2019, 7, 821.
- Zhang, Y.-J.; Wu, Q. Sulforaphane protects intestinal epithelial cells against lipopolysaccharide-induced injury by activating the AMPK/SIRT1/PGC-1alpha pathway. Bioengineered 2021, 12, 4349–4360.
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Wozniak, K.; Daglia, M.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405.
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 5618.
- Daily, J.W.; Byoung-Seob, K.; Jina, R.; Meiling, L.; Weijun, Z.; Park, S. Isothiocyanate from broccoli, sulforaphane, and its properties. J. Med. Food 2019, 22, 121–126.
- Kim, J. Pre-Clinical Neuroprotective Evidences and Plausible Mechanisms of Sulforaphane in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 201.
- Ho-Sub, P.; Eun-Sang, H.; Ga-Young, C.; Hyun-Bum, K.; Kyun-Seob, P.; Jai-Yoon, S.; Yoonjin, H.; Geun Wook, C.; Byung Il, K.; Hyunwoo, P.; et al. Sulforaphane enhances long-term potentiation and ameliorate scopolamine-induced memory impairment. Physiol. Behav. 2021, 238, 113467.
- Bobermin, L.D.; Weber, F.B.; dos Santos, T.M.; Bello-Klein, A.; Wyse, A.T.S.; Goncalves, C.-A.; Quincozes-Santos, A. Sulforaphane Induces Glioprotection After LPS Challenge. Cell Mol. Neurobiol. 2022, 42, 829–846.
- Ordonez, A.A.; Bullen, C.K.; Villabona-Rueda, A.F.; Thompson, E.A.; Turner, M.L.; Merino, V.F.; Yan, Y.; Kim, J.; Davis, S.L.; Komm, O.; et al. Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice. Commun. Biol. 2022, 5, 1666.
- Sangkret, S.; Pongmalai, P.; Devahastin, S.; Chiewchan, N. Enhanced production of sulforaphane by exogenous glucoraphanin hydrolysis catalyzed by myrosinase extracted from Chinese flowering cabbage (Brassica rapa var. parachinensis). Sci. Rep. 2019, 9, 101.
- Zhang, S.; Ying, D.Y.; Cheng, L.J.; Bayrak, M.; Jegasothy, H.; Sanguansri, L.; Augustin, M.A. Sulforaphane in broccoli-based matrices: Effects of heat treatment and addition of oil. LWT Food Sci. Technol. 2020, 128, 1464.
- Li, C.; Song, S.; He, Y.; Zhang, X.; Liu, H. CaCl2-HCl electrolyzed water affects glucosinolate metabolism and improves the quality of broccoli sprouts. Food Res. Int. 2021, 150, 1901.
- Chen, W.; Wang, Y.; Xu, L.; Dong, J.; Zhu, X.; Ying, J.; Wang, Q.; Fan, L.; Li, C.; Liu, L. Methyl jasmonate, salicylic acid and abscisic acid enhance the accumulation of glucosinolates and sulforaphane in radish (Raphanus sativus L.) taproot. Sci. Hortic. 2019, 250, 159–167.
- Triska, J.; Balik, J.; Houska, M.; Novotna, P.; Magner, M.; Vrchotova, N.; Hic, P.; Jilek, L.; Thorova, K.; Snurkovic, P.; et al. Factors Influencing Sulforaphane Content in Broccoli Sprouts and Subsequent Sulforaphane Extraction. Foods 2021, 10, 745.
- Goharrizi, K.J.; Fatehi, F.; Nazari, M.; Salehi, F.; Maleki, M. Assessment of changes in the content of sulforaphane and expression levels of CYP79F1 and myrosinase genes and proteomic profile of Lepidium draba plant under water-deficit stress induced by polyethylene glycol. Acta Physiol. Plant 2020, 42, 991.
- Oloyede, O.O.; Wagstaff, C.; Methven, L. Influence of Cabbage (Brassica oleracea) Accession and Growing Conditions on Myrosinase Activity, Glucosinolates and Their Hydrolysis Products. Foods 2021, 10, 717.
- Chen, X.; Li, Z.; Sun, X.; Ma, H.; Chen, X.; Ren, J.; Hu, K. New Method for the Synthesis of Sulforaphane and Related Isothiocyanates. Synthesis 2011, 1, 3991–3996.
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659.
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1789.
- Ren-Hau, L.; Miller, M.J.; Jeffery, E. Glucoraphanin hydrolysis by microbiota in the rat cecum results in sulforaphane absorption. Food Funct. 2010, 1, 161–166.
- Li, F.; Hullar, M.A.J.; Beresford, S.A.A.; Lampe, J.W. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br. J. Nutr. 2011, 106, 408–416.
- Angelino, D.; Jeffery, E. Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: Focus on glucoraphanin. J. Funct. Foods 2014, 7, 67–76.
- Zhang, C.; Zhang, Y.; Li, H.; Liu, X. The potential of proteins, hydrolysates and peptides as growth factors for Lactobacillus and Bifidobacterium: Current research and future perspectives. Food Funct. 2020, 11, 1946–1957.
- Xinzhou, W.; Peng, Z.; Xin, Z. Probiotics regulate gut microbiota: An effective method to improve immunity. Molecules 2021, 26, 6076.
- Monika, Y.; Mandeep; Pratyoosh, S. Probiotics of diverse origin and their therapeutic applications: A review. J. Am. Coll. Nutr. 2020, 39, 469–479.
- Gang, T.; Linyu, Z. Update on strategies of probiotics for the prevention and treatment of colorectal cancer. Nutr. Cancer 2022, 74, 27–38.
- How, Y.; Pui, L. Effect of prebiotics encapsulated with probiotics on encapsulation efficiency, microbead size, and survivability: A review. J. Food. Meas. Charac. 2021, 15, 4899–4916.
- Monika, Y.; Pratyoosh, S. Efficient engineered probiotics using synthetic biology approaches: A review. Biotechnol. Appl. Biochem. 2020, 67, 22–29.
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10.
- Yan Xue, C.; Ji Hui, W.; McAuley, C.; Augustin, M.A.; Terefe, N.S. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods 2019, 61, 103461.
- Jian-Hui, Y.; Long-Yue, H.; Terefe, N.S.; Augustin, M.A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 2019, 286, 616–623.
- Boehm, J.; Davis, R.; Murar, C.E.; Li, T.; McCleland, B.; Dong, S.; Yan, H.; Kerns, J.; Moody, C.J.; Wilson, A.J.; et al. Discovery of a crystalline sulforaphane analog with good solid-state stability and engagement of the Nrf2 pathway in vitro and in vivo. Bioorg. Med. Chem. 2019, 27, 579–588.
- Zambrano, V.; Bustos, R.; Mahn, A. Insights about stabilization of sulforaphane through microencapsulation. Heliyon 2019, 5, 2899.
- Jeon, M.; Lee, J.; Lee, H.K.; Cho, S.; Lim, J.H.; Choi, Y.; Pak, S.; Jeong, H.J. Sulforaphane mitigates mast cell-mediated allergic inflammatory reactions in in silico simulation and in vitro models. Immunopharmacol. Immunotoxicol. 2020, 42, 74–83.
- Ge, M.; Zhang, L.; Cao, L.; Xie, C.; Li, X.; Li, Y.; Meng, Y.; Chen, Y.; Wang, X.; Chen, J.; et al. Sulforaphane inhibits gastric cancer stem cells via suppressing sonic hedgehog pathway. Int. J. Food Sci. Nutr. 2019, 70, 570–578.
- Doheny-Adams, T.; Redeker, K.; Kittipol, V.; Bancroft, I.; Hartley, S.E. Development of an efficient glucosinolate extraction method. Plant Methods 2017, 13, 17.
- Favela-Gonzalez, K.M.; Hernandez-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 3, e13414.
- Yang, H.; Qin, J.; Wang, X.; Ei-Shora, H.M.; Yu, B. Production of plant-derived anticancer precursor glucoraphanin in chromosomally engineered Escherichia coli. Microbiol. Res. 2020, 238, 126484.
- Francis, F.; Lognay, G.; Wathelet, J.P.; Haubruge, E. Characterisation of aphid myrosinase and degradation studies of glucosinolates. Arch. Insect Biochem. Physiol. 2002, 50, 173–182.
- Roman, J.; Castillo, A.; Cottet, L.; Mahn, A. Kinetic and structural study of broccoli myrosinase and its interaction with different glucosinolates. Food Chem. 2018, 254, 87–94.
- Lei, W.; Rose, D.; Pingfan, R.; Yue, Z. Development of prolamin-based composite nanoparticles for controlled release of sulforaphane. J. Agric. Food Chem. 2020, 68, 13083–13092.
- Wei, L.; Liu, C.; Zheng, H.; Zheng, L. Melatonin treatment affects the glucoraphanin-sulforaphane system in postharvest fresh-cut broccoli (Brassica oleracea L.). Food Chem. 2020, 307, 299.
- Wu, Y.; Lv, C.; Zou, L.; Sun, J.; Song, X.; Zhang, Y.; Mao, J. Approaches for enhancing the stability and formation of sulforaphane. Food Chem. 2021, 345, 128771.
- Xinxing, X.; Mei, D.; Fei, L.; Fang, C.; Xiaosong, H.; Yuping, L.; Jihong, W. Effect of glucoraphanin from broccoli seeds on lipid levels and gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2020, 68, 103858.
- Budnowski, J.; Hanske, L.; Schumacher, F.; Glatt, H.; Platz, S.; Rohn, S.; Blaut, M. Glucosinolates Are Mainly Absorbed Intact in Germfree and Human Microbiota-Associated Mice. J. Agric. Food Chem. 2015, 63, 8418–8428.
- Sun, J.; Charron, C.S.; Novotny, J.A.; Peng, B.; Yu, L.; Chen, P. Profiling glucosinolate metabolites in human urine and plasma after broccoli consumption using non-targeted and targeted metabolomic analyses. Food Chem. 2020, 4, 309.
- Martijn Vermeulen, I.W.M.; Armstrong, K.P.-K.; Van Den Berg, R.; Vaes, W.H.J. Bioavailability and Kinetics of Sulforaphane in Humans after Consumption of Cooked versus Raw Broccoli. J Agric Food Chem. 2008, 56, 10505–10509.
- Egner, P.A.; Chen, J.G.; Wang, J.B.; Wu, Y.; Sun, Y.; Lu, J.H.; Zhu, J.; Zhang, Y.H.; Chen, Y.S.; Friesen, M.D.; et al. Bioavailability of Sulforaphane from two broccoli sprout beverages: Results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev. Res. 2011, 4, 384–395.
- Shapiro, T.A.; Jhones, W.F.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective Glucosinolates and Isothiocyanates of Broccoli Sprouts: Metabolism and Excretion in Humans1. Cancer Epidemiol. Biomark. Prev. 2001, 10, 501–508.
- Kuljarachanan, T.; Fu, N.; Chiewchan, N.; Devahastin, S.; Chen, X.D. In vitro digestion using dynamic rat stomach-duodenum model as an alternative means to assess bioaccessibility of glucosinolates in dietary fiber powder from cabbage. LWT Food Sci. Technol. 2021, 2, 151.
- Yuanfeng, W.; Yuke, S.; Ye, Z.; Mupunga, J.; Ligen, Z.; Chao, L.; Shiwang, L.; Jianwei, M. Broccoli ingestion increases the glucosinolate hydrolysis activity of microbiota in the mouse gut. Int. J. Food Sci. Nutr. 2019, 70, 585–594.
- Hwang, E.-S.; Bornhorst, G.M.; Oteiza, P.I.; Mitchell, A.E. Assessing the Fate and Bioavailability of Glucosinolates in Kale (Brassica oleracea) Using Simulated Human Digestion and Caco-2 Cell Uptake Models. J. Agric. Food Chem. 2019, 67, 9492–9500.
- Ye, Q.; Fang, Y.; Li, M.; Mi, H.; Liu, S.; Yang, G.; Lu, J.; Zhao, Y.; Liu, Q.; Zhang, W.; et al. Characterization of a Novel Myrosinase with High Activity from Marine Bacterium Shewanella baltica Myr-37. Int. J. Mol. Sci. 2022, 5, 23.
- Mao, S.; Wang, J.; Wu, Q.; Liang, M.; Yuan, Y.; Wu, T.; Liu, M.; Wu, Q.; Huang, K. Effect of selenium-sulfur interaction on the anabolism of sulforaphane in broccoli. Phytochemistry 2020, 179, 112499.
- Matusheski, N.V.; Ron, S.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E.H. Epithiospecifier Protein from Broccoli (Brassica oleracea L. ssp. italica) Inhibits Formation of the Anticancer Agent Sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076.
- Sporer, T.; Koernig, J.; Wielsch, N.; Gebauer-Jung, S.; Reichelt, M.; Hupfer, Y.; Beran, F. Hijacking the Mustard-Oil Bomb: How a Glucosinolate-Sequestering Flea Beetle Copes With Plant Myrosinases. Front. Plant Sci. 2021, 12, 1009.
- Feng, Q.; Li, L.; Liu, Y.; Shao, X.; Li, X. Jasmonate regulates the FAMA/mediator complex subunit 8-THIOGLUCOSIDE GLUCOHYDROLASE 1 cascade and myrosinase activity. Plant Physiol. 2021, 187, 963–980.
- Jafari, S.; Ryde, U.; Irani, M. QM/MM Study of the Catalytic Reaction of Myrosinase; Importance of Assigning Proper Protonation States of Active-Site Residues. J. Chem. Theory Comput. 2021, 17, 1822–1841.
- Sicong, T.; Xiaodong, L.; Peng, L.; Xiaohong, Z.; Yujuan, S. Microbiota: A mediator to transform glucosinolate precursors in cruciferous vegetables to the active isothiocyanates. J. Sci. Food Agric. 2018, 98, 1255–1260.
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2556.
- Fahey, J.W.; Kensler, T.W. The Challenges of Designing and Implementing Clinical Trials With Broccoli Sprouts horizontal ellipsis and Turning Evidence Into Public Health Action. Front. Nutr. 2021, 8, 7290.
- Elfoul, L.; Rabot, S.; Khelifa, N.; Quinsac, A.; Duguay, A.; Rimbault, A. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol. Lett. 2001, 197, 99–103.
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl- glucosinolates by a selection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883.
- Zhang, Y.; Huang, S.; Sun, J.; Song, X.; Jiang, C.; Wu, Y. Isolation and Characterization of Glucosinolate-Hydrolysis Enterococcus gallinarum HG001 and Escherichia coli HG002 from C57BL/6 Mouse Microbiota. Indian J. Microbiol. 2022, 62, 273–279.
- Mullaney, J.A.; Kelly, W.J.; McGhie, T.K.; Ansell, J.; Heyes, J.A. Lactic Acid Bacteria Convert Glucosinolates to Nitriles Efficiently Yet Differently from Enterobacteriaceae. J. Agric. Food Chem. 2013, 61, 3039–3046.
- Yan Xue, C.; Augustin, M.A.; Jegasothy, H.; Ji Hui, W.; Terefe, N.S. Mild heat combined with lactic acid fermentation: A novel approach for enhancing sulforaphane yield in broccoli puree. Food Funct. 2020, 11, 779–786.
- Watanabe, H.; Usami, R.; Kishino, S.; Osada, K.; Aoki, Y.; Morisaka, H.; Takahashi, M.; Izumi, Y.; Bamba, T.; Aoki, W.; et al. Enzyme systems involved in glucosinolate metabolism in Companilactobacillus farciminis KB1089. Sci. Rep. 2021, 11, 267.
- Cebeci, F.; Mayer, M.J.; Rossiter, J.T.; Mithen, R.; Narbad, A. Molecular Cloning, Expression and Characterisation of a Bacterial Myrosinase from Citrobacter Wye1. Protein J. 2022, 41, 131–140.
- Tie, Y.; Zhu, W.; Zhang, C.; Yin, L.; Zhang, Y.; Liu, L.; Yuan, H. Identification of Two Myrosinases from a Leclercia adecarboxylata Strain and Investigation of Its Tolerance Mechanism to Glucosinolate Hydrolysate. J. Agric. Food Chem. 2021, 69, 14151–14164.
- Rakariyatham, N.; Butrindr, B.; Niamsup, H.; Shank, L. Screening of filamentous fungi for production of myrosinase. Braz. J. Microbiol. 2005, 36, 242–245.
- Xu, X.; Bi, S.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Induced changes in bioactive compounds of broccoli juices after fermented by animal- and plant-derived Pediococcus pentosaceus. Food Chem. 2021, 357, 144.
- Rakariyatham, N.; Sakorn, P. Biodegradation of glucosinolates in brown mustard seed meal (Brassica juncea) by Aspergillus sp. NR-4201 in liquid and solid-state cultures. Biodegradation 2002, 13, 395–399.
- Llanos Palop, M.; Smiths, J.P.; Brink, B.T. Degradation of sinigrin by Lactobacillus agilis strain R16. Int. J. Food Microbiol. 1995, 26, 219.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
735
Revisions:
2 times
(View History)
Update Date:
06 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




