Sulforaphane (1-isothiocyanate-4-(methylsulfonyl) butane, SFN) is produced by the degradation of glucoraphanin under the action of myrosinase (β-D-thioglucosidase) [
1,
2,
3]. SFN and its precursor (glucoraphanin) are important safeguard substances used by plants to defend themselves against insects, pathogens, and herbivores [
4]. Recently, researchers have found that SFN possesses antioxidant, anti-inflammatory, and antibacterial functions [
5,
6]. In addition, SFN could prevent and cure cancer [
7,
8,
9], improve the nervous system [
10,
11,
12], and prevent coronavirus disease 2019 [
13]. Therefore, SFN has attracted extensive attention from researchers around the world.
Meanwhile, studies have reported that in the microbial conversion of glucoraphanin, probiotics, which are a class of active microorganisms that colonize the human intestinal environment, could improve the composition of human intestinal flora and enhance intestinal digestion [
30,
31]. Probiotics have also shown preventive and therapeutic effects on colorectal cancer, mental disorders, diabetes, and other diseases [
32,
33]. Therefore, probiotics are widely used in health products, dietary supplements, and prebiotics [
34,
35,
36]. In contrast, studies have shown that lactic acid bacteria (
LAB) can convert glucoraphanin from broccoli into SFN under suitable conditions, and high SFN concentrations were found in fermented broccoli puree [
37,
38]. The
LAB fermentation results also indicated that, in addition to the mammalian gut flora, microorganisms from other sources could also degrade glucoraphanin and produce SFN.
2. Intestinal Microorganisms Can Enhance the Utilization of SFN
2.1. Structure and Biochemical Characteristics of SFN, Glucoraphanin, and Myrosinase
SFN (1-isothiocyanate-4-(methylsulfonyl) is an isothiocyanates (a general formula R-N=C=S). Due to the presence of an active electrophilic carbon atom in the SFN group (-N=C=S), SFN is easily reversible with thiols under physiological conditions, resulting in pH-sensitive dithiocarbamates reacting with amines and forming thiourea [
1,
39,
40]. This is the reason why SFN is sensitive to temperature and other conditions. SFN is converted by its precursor glucoraphanin under the enzymatic hydrolysis reaction of myrosinase. Meanwhile, glucoraphanin (4-methylsulfinyl butyl glucosinolate) is a methionine-derived aliphatic glucosinolate. Glucoraphanin is widely found in cruciferous plants, especially
broccoli. Glucoraphanin is a water-soluble sulfur-containing anionic secondary metabolite consisting of a β-glucosinolate N-hydroxysulfate with a side chain center and a β-D-glucopyranose residue [
41,
42,
43]. Glucoraphanin demonstrates no physiological activity. Therefore, the best form of utilization for SFN is to preserve.
Myrosinase, also known as β-glucosidase, is a ubiquitous enzyme in cruciferous plants that can efficiently degrade glucosinolates. The essence of myrosinase is a glycoprotein, and currently found in plants, aphids, and other myrosinase of the glycoside hydrolase family 1 [
44,
45]. The differences in myrosinase from different sources are mainly reflected in molecular weight, subunit number, and side chain sugar content, which leads to the different ability of myrosinase to degrade glucoraphanin.
2.2. Increasing the Intestinal SFN Production Rate Is a Scientific Approach to Enhance SFN Utilization
Although SFN has strong anticancer properties, it is very unstable and loses its biological activity under certain conditions, such as the presence of oxygen, which could reduce the utilization of SFN [
46,
47,
48]. To improve SFN utilization, researchers considered the possibility of directly producing SFN in vivo and conducted in vitro simulation studies [
49]. Xu et al. found that glucoraphanin could be converted into SFN under a simulated gastrointestinal environment in vitro, and the maximum conversion rate could reach 46.2%. Moreover, when glucoraphanin was directly fed to germ-free and human-microbiota-associated mice, SFN degradation products were found in the urine of the mice, which indicated that SFN could be produced in the intestinal environment and that SFN could be utilized [
50].
Lai et al. used male F344 rats as an animal model and demonstrated that the cecum can also degrade glucoraphanin and produce SFN [
27]. Once glucoraphanin was directly gavaged into male F344 rats, SFN was detected in the plasma of the rats after 120 min, and the level of SFN in the plasma remained constant for 1 h. In addition, a study indicated that the intestinal environment not only is a limiting factor for SFN utilization, but also has a promoting effect on SFN utilization [
29]. However, humans can consume glucoraphanin-rich
Brassica vegetables directly instead of consuming some glucoraphanin. Raw
broccoli is used for studies on humans, and the results indicate that glucosinolates in
broccoli are degraded in the human body and the degradation products of glucosinolates are detected in the blood and urine of volunteers [
51]. Therefore, the studies on humans suggest that SFN can be produced in the human intestinal environment.
Meanwhile, the bioavailability of SFN in raw
broccoli could reach 37%, which is significantly higher than that in cooked
broccoli, and the consumption of cooked
broccoli would delay the absorption of SFN [
52]. Egner et al. found that the bioavailability of SFN was far superior to glucoraphanin in the human body [
53]. Another study showed that the main reason for the higher bioavailability of SFN than sulforaphane is that glucoraphanin must be hydrolyzed to be absorbed [
54].
However, because humans consume more cooked than raw vegetables, researchers have used cooked white
cabbage to simulate the in vivo degradation of glucoraphanin in a rat duodenal model [
55]. The results show that 82% of glucoraphanin is released from white
cabbage seeds after 10 min, but no degradation of glucoraphanin is detected. However, the in vitro simulation results for the rat duodenum are different from the results from the study on male F344 rats. Therefore, Wu et al. explored the possibility of SFN production by the gut microbiota [
28,
56]. Research using a male C57BL/6 mouse shows that glucoraphanin could be degraded to SFN in the intestine, and the production of SFN is related to the intestinal flora. Hwang et al. show that 13 pmol/g fresh weight of SFN is produced in the gut after 120 min, and approximately 29% of the SFN is taken up and utilized by cells, indicating that the intestinal environment has a promoting effect on the production and utilization of SFN [
57].
Meanwhile, Sangkret et al. found that the main elements affecting SFN production were myrosinase activity, temperature, pH, and reaction time [
14]. Recently, researchers obtained a new myrosinase-producing bacterium from marine sediment (Marine Bacterium
Shewanella baltica Myr-37) [
58]. Once the reaction temperature is 40 °C and pH = 7.0, myrosinase can efficiently degrade sulforaphane to SFN in 25 min, the yield is 0.57 mg/mL, and the corresponding SFN conversion efficiency is 89%. However, intestinal myrosinase is also affected by epithiospecifier protein (EP) and sulfur–selenium interaction(S–Se) in the process of SFN formation [
48,
59,
60]. EP interferes with the production of SFN, while S–Se induces the expression of myrosinase gene to produce more myrosinase.
The intestinal environment can degrade glucoraphanin to produce SFN without the action of plant-derived myrosinase, and the main factor for SFN production is the effect of the intestinal flora. However, the mechanisms by which the gut microbiota degrades glucoraphanin to produce SFN, and the gut microbes involved, are not clear. Therefore, researchers investigated the mechanisms of the microbial transformation of glucoraphanin.
2.3. Microorganisms Converted Glucoraphanin into SFN Using Myrosinase Synthesis
Myrosinase is a beta-thioglucosidase glucohydrolase that was originally discovered in cruciferous plants; it can resist in vitro damage and degrade glucoraphanin [
61,
62,
63]. Studies have found that some microorganisms can also synthesize myrosinase (
Table 1) [
64,
65,
66]. Naoki Tani et al. first isolated a species of
Enterobacter cloacae, which could synthesize myrosinase, but its molecular weight was smaller than the endogenous myrosinase of the plant. Meanwhile,
Bacteroides thetaiotaomicron (another dominant species derived from the human colon) could convert glucosinolates into allyl isothiocya-nate [
67]. With the further development of research on SFN production by intestinal flora, it was discovered that a variety of intestinal strains can degrade glucoraphanin to produce SFN [
68].
Table 1. Microorganisms with myrosinase synthesis function.
With the development of research on human intestinal enzyme-producing flora, researchers successfully isolated
Enterococcus gallinarum HG001 and
Escherichia coli HG002, which could synthesize myrosinase from the intestines of C57BL/6 mice [
69]. However, the mechanisms by which intestinal myrosinase produces SFN remain unclear. Watanabe et al., studying
LAB as research objects, found that intestinal myrosinase may be involved in the metabolism of glucoraphanin through the β-glucoside-specific IIB, IIC, and IIA phosphotransferase system components (
Figure 1) [
72]. With the intestinal myrosinase synthesis mechanisms becoming clear, researchers further investigated the myrosinase synthesis capabilities of microorganisms to identify the types of microorganisms that synthesize myrosinase.
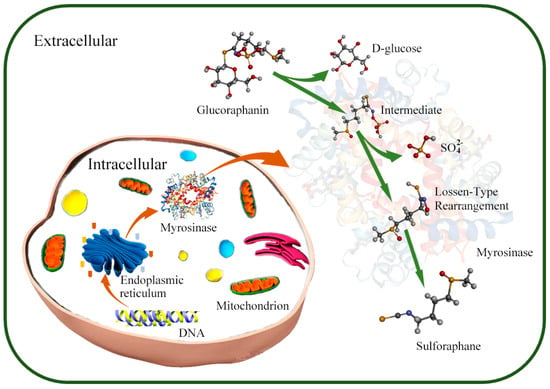
Figure 1. Mechanism of SFN production by microorganisms. In microorganisms, myrosinase is synthesized and secreted to environment through ribosome and endoplasmic reticulum under the myrosinase gene regulation; the glucoraphanin is converted into sulforaphane under the action of myrosinase in extracellular environment.
Researchers turned to fungal microbes that are ubiquitous. Rakariyatham et al. investigated the ability of
Aspergillus sp. NR-4201 to synthesize myrosinase [
77].
Aspergillus sp. NR-4201 converted all glucosinolates into allyl cyanide within 32 h, indicating it can synthesize myrosinase. Subsequently, Nuansri et al. also explored whether
Aspergillus sp. NR46F13 could synthesize myrosinase [
75], and their results show that 3.19 U mL–1 myrosinase was isolated from the medium of this strain after 48 h of culture. With the continuous exploration of microorganisms, researchers isolated
Leclercia adecarboxylata and
Citrobacter Wye1, which could synthesize myrosinase, from the soil [
73,
74].
Palop et al. investigated the potential of 42
Lactobacillus species to degrade glucosinolates, and the results indicate that strain R16 shows a strong ability to degrade glucosinolates [
78]. In addition, Mullaney et al. explored the different abilities of
L. plantarum KW30,
Lactococcus lactis subsp. lactis KF147, and
E. coli Nissle 1917, while
E. cloacae was used to degrade glucoraphanin [
70]. The comparative research of myrosinase obtained from different microbial sources shows that the myrosinase of the intestinal flora is more capable of degrading glucoraphanin than those of plant-derived microorganisms. Some researchers investigated the feasibility of using
LAB fermentation as an alternative method to maintain myrosinase activity and enhance the bioconversion of glucosides into SFN [
37]. The fermentation results show that the fermentation of
LAB could achieve stable SFN production.
Some researchers further explored the effect of high-temperature sterilization or preheating
broccoli puree on the fermentation of
LAB to produce SFN [
38,
71]. The results show that high-temperature sterilization and preheating
broccoli puree enhances the yields of SFN produced by
LAB fermentation, and the SFN yield from
broccoli puree that was preheated in advance is 16 times higher than that of non-preheated
broccoli puree. Xu et al. show that using
Pediococcus pentosaceus for fermentation in
broccoli juice also produces more SFN [
76]. Exogenous myrosinase could stably produce SFN under the condition of sufficient raw materials, which provides a new route for SFN production. In addition, the results indicate that microbial-derived myrosinase has the same efficacy as plant-derived myrosinase, but microbial-derived myrosinase is easier to obtain in vitro.
Microorganisms that could synthesize myrosinase have also been isolated from soil and broccoli, in addition to those isolated from the intestinal environment. However, the myrosinase-synthesizing abilities of the microorganisms were restricted by the culture conditions and their gene sequences; therefore, it was necessary to further investigate the factors that restrict the synthesis of high-yield myrosinase enzymes.