Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paolo Spinnato | -- | 3046 | 2022-12-06 08:06:04 | | | |
| 2 | Conner Chen | Meta information modification | 3046 | 2022-12-06 08:57:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Spinnato, P.; Patel, D.B.; Carlo, M.D.; Bartoloni, A.; Cevolani, L.; Matcuk, G.R.; Crombé, A. Imaging of Soft-Tissue infections. Encyclopedia. Available online: https://encyclopedia.pub/entry/38098 (accessed on 08 February 2026).
Spinnato P, Patel DB, Carlo MD, Bartoloni A, Cevolani L, Matcuk GR, et al. Imaging of Soft-Tissue infections. Encyclopedia. Available at: https://encyclopedia.pub/entry/38098. Accessed February 08, 2026.
Spinnato, Paolo, Dakshesh B. Patel, Maddalena Di Carlo, Alessandra Bartoloni, Luca Cevolani, George R. Matcuk, Amandine Crombé. "Imaging of Soft-Tissue infections" Encyclopedia, https://encyclopedia.pub/entry/38098 (accessed February 08, 2026).
Spinnato, P., Patel, D.B., Carlo, M.D., Bartoloni, A., Cevolani, L., Matcuk, G.R., & Crombé, A. (2022, December 06). Imaging of Soft-Tissue infections. In Encyclopedia. https://encyclopedia.pub/entry/38098
Spinnato, Paolo, et al. "Imaging of Soft-Tissue infections." Encyclopedia. Web. 06 December, 2022.
Copy Citation
Musculoskeletal soft-tissue infections include a wide range of clinical conditions that are commonly encountered in both emergency departments and non-emergency clinical settings. Since clinical signs, symptoms, and even laboratory tests can be unremarkable or non-specific, imaging plays a key role in many cases.
magnetic resonance imaging
ultrasonography
interventional
radiography
1. Introduction
Musculoskeletal soft-tissue infections include a wide range of clinical conditions that are commonly encountered in both emergency departments and non-emergency clinical settings. These infections may occur in all age groups with a predilection for the elderly [1][2][3]. There are several well-known predisposing conditions, such as extremes of age, immunosuppression/systemic diseases, illicit drug abuse, alcoholism, peripheral vascular disease, trauma, burns, surgery, obesity, and malnutrition [1][4][5][6]. Moreover, some soft-tissue infections are limited to specific geographical areas with warm and humid climates.
A wide range of microorganisms responsible for these infections are usually found in polymicrobial combinations. Both aerobic and anaerobic bacteria can be present at the same time. In several cases, soft-tissue infections can be caused by a single microorganism (e.g., hemolytic Streptococcus spp, Staphylococcus spp, and Clostridium perfringens). The most common polymicrobial infections are caused by combinations of Staphylococcus, Streptococcus, Enterococcus, Enterobacteriaceae, and Bacteroides species [1][7]. Fungi and other atypical organisms can cause these infections too. The most frequent of these latter organisms is candida spp [7].
The most frequent soft-tissue infection subtypes include infectious cellulitis, fasciitis, infective tenosynovitis/bursitis, pyomyositis, and infected myonecrosis [1].
The most common clinical signs of soft-tissue infections are fever, malaise, local pain, oedema, erythema, palpable mass, and sometimes crepitus due to the presence of soft tissue gas [8]. Anyway, these clinical signs are usually scarce or even not present.
Since clinical signs, symptoms, and even laboratory tests (e.g., leukocytosis, C-reactive protein, and erythrocyte sedimentation rate) of musculoskeletal soft-tissue infections can be unremarkable or non-specific, imaging plays a key role in many cases [1][2].
2. Imaging of Soft-Tissue infections: General Considerations
Imaging is a key element in characterizing soft-tissue infections, with the aim of confirming clinical suspicion and identifying the location and extent of involvement. Although it is rarely diagnostic, conventional radiography (CR) is often the initial modality in suspected soft-tissue infections [2]. CR can detect or confirm a local thickening of soft-tissue, and identify or exclude the presence of foreign bodies (Figure 1).
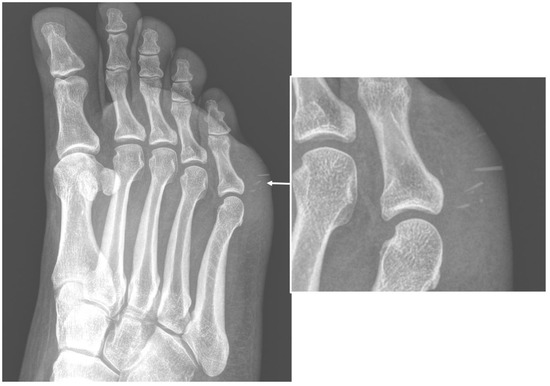
Figure 1. Conventional radiography (anterior–posterior oblique projection) of the right foot in a 44-year-old woman, who were presented to the emergency department with complaints about worsening pain and swelling in the plantar external region. The examinations confirmed local swelling of soft tissue and revealed the presence of thin foreign bodies, sea urchin quills (arrows and in enlargement), which was confirmed after surgical excision and anamnestic confirmation.
Magnetic resonance imaging (MRI) is the most sensitive imaging tool for the assessment of soft-tissue infections due to its resolution, optimal inherent contrast, and great anatomical details that permit the accurate evaluation of the extent of tissue involvement [2]. Computed tomography (CT) may be useful for the assessment of soft-tissue infections in several conditions including infections with gas components (e.g., necrotizing infections) and for the detection of foreign bodies. Ultrasound (US) can be particularly useful for non-invasive diagnosis of soft-tissue infections and collections (e.g., abscesses and joint effusions). Moreover, US is widely used as a guidance for percutaneous needle aspiration of soft-tissue collection (Figure 2), offering the possibility to perform microbiological analyses on the specimens [2].
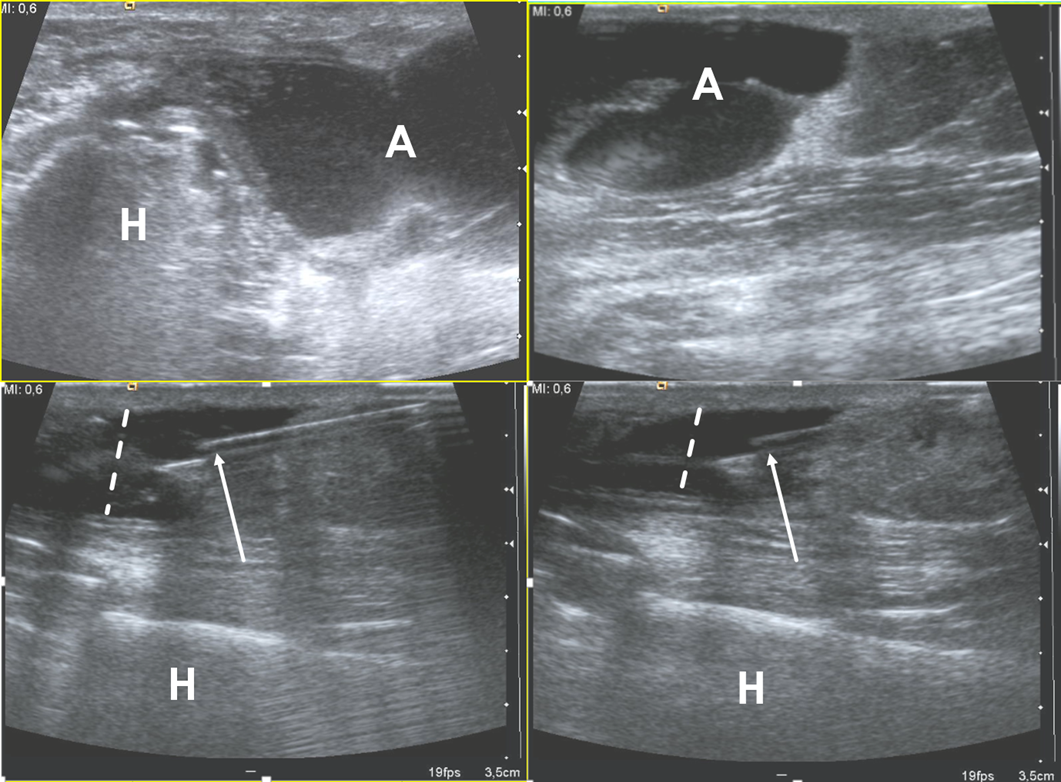
Figure 2. Ultrasound examination of a soft-tissue abscess of lateral aspect of the left harm in a patient who were recently operated for humeral fracture. The needle’s tip is placed inside the abscess (arrows) under ultrasound guidance (in-plane approach); during the fluid aspiration, the reduction of the abscess can be appreciated (dotted lines). H = humerus, A = abscess.
2.1. Cellulitis
Cellulitis is an acute infection of the skin and the subcutaneous fat and may present without or with systemic signs of infection. It can involve the superficial fascia but does not involve the deep fascia. Infection follows a visible or invisible break in the skin surface, with Streptococci and Staphylococcus aureus being the most common causative organisms [9][10]. The risk factors include cutaneous lesions and wounds, diabetes, immunocompromised states, vascular insufficiency, and foreign bodies [9][11]. Skin erythema, swelling, warmth, tenderness, and lymphadenopathy are common in clinical presentation [10]. It is a clinical diagnosis, but imaging may be performed when there is rapid progression, systemic symptoms, or concern for deeper involvement [9]. The aim is to exclude deeper extension and abscess formation [12]. Imaging can also show foreign bodies (see later). Uncomplicated cellulitis is managed medically with antibiotics.
Imaging is usually not performed, but plain radiographs are often obtained. The findings are nonspecific, showing diffuse soft tissue swelling and increased density of the subcutaneous fat with a loss of fat planes [13]. It can detect soft tissue gas which is seen in necrotizing infections.
Ultrasound (US) has commonly been used as a front-line examination and shows subcutaneous soft tissue edema, increased echogenicity of the subcutaneous tissues, hypoechoic fluid insinuating, and separation of fat lobules that gives a cobblestone pattern [2][13]. With color or power doppler, increased vascularity may be seen. US can identify abscess, which is seen as an anechoic or hypoechoic collection with echogenic rim and increased peripheral vascularity, but not internal vascularity [13]. US is also often used to exclude deep venous thrombosis which can clinically mimic cellulitis.
Computed tomography (CT) with intravenous iodinated contrast has also been used to evaluate cellulitis and shows skin thickening, underlying fat stranding or subcutaneous edema, and thickening of underlying fascia [13][14]. Enhancement of the septations and asymmetric distribution differentiate it from bland edema as can be seen with cardiac or renal failure. Abscess, if present, is seen as a ring-enhancing fluid collection.
Magnetic resonance imaging (MRI) shows a similar appearance with skin thickening and increased signal in subcutaneous fat on fluid-sensitive sequences [2][15]. The increased signal may be streaky (fat stranding) or diffuse, and there is a corresponding low signal on T1 weighted sequence [1][12]. Edema-like signal can be seen extending along the fascia [2]. Following the administration of intravenous gadolinium-based contrast material, there is diffuse enhancement [13] that is similar to CT and helps differentiate cellulitis from noninfectious causes of edema, such as cardiac or renal insufficiency [11][14]. An abscess, when present, will be seen as intermediate to low T1 and high fluid-sensitive signal collection with peripheral enhancement after the intravenous contrast administration [16].
2.2. Necrotizing and Non-Necrotizing Fascitiis
Infective fasciitis is an infection of the fascia and can be non-necrotizing (NNF) or necrotizing. Necrotizing fasciitis (NF), as a part of necrotizing soft-tissue infections (NSTI), is a rare but rapidly progressive infection of the deep soft tissue and fascia. If not treated promptly, it can lead to high morbidity and mortality [10][17]. Early surgical intervention is an important prognostic factor [10].
This infection is commonly presented as type 1, which is polymicrobial with common organisms that include aerobes and anaerobes. It commonly affects the perineum and trunk and is common in patients of advanced ages who have decreased immunity from preexisting diseases. Type 2 disease is monomicobial with Group A Streptococcus (most common) or staphylococcus aureus (including methicillin-resistant Staphylococcus aureus) being the common organisms. Patients are usually younger with trauma being a common method of inoculation. Vibrio and Clostridium species (type 3) and fungi (type 4) are additional causative organisms [10][18]. Predisposing factors include diabetes, obesity, immunosuppressed states, trauma (which can be minor, such as insect bites), intravenous drug use, venous insufficiency, and peripheral vascular disease.
Common sites of infection include the extremities, perineum, trunk, and head and neck. NF has been given specific names when affecting certain regions—Ludwig angina when it affects the submandibular region and Fournier gangrene when it affects the perineum [19].
This infection commonly follows a penetrating injury or surgery, but can also occur without a history of trauma [17][19]. Following inoculation, there is a rapid growth of bacteria with production of exotoxins and activation of cytokines. Blood vessels are thrombosed and damaged with resultant ischemia and necrosis. The infection spreads along the fascia, far and wide, and can affect all tissues from skin to muscles [2][10][19]. Necrotizing soft-tissue infection (NSTI) is an advocated terminology to encompass all necrotizing infections deep to the skin, including the fascia [2][11][20].
2.3. Soft-Tissue Foreign Body
Each year, more than 11 million patients present to emergency departments with traumatic wounds and lacerations, with approximately 10% of these cases having a retained foreign body [21]. These retained foreign bodies are most commonly found in the extremities, with nearly one third involving the wrist, hand, or fingers [22]. The most common retained foreign bodies include wood, glass, and metal [23]. Soft tissue foreign bodies may also be the result of self-inflicted penetrating trauma, also known as self-embedding behavior [24]. Foreign bodies may also result from medical procedures, due to retained sponges or instruments or broken fragments of drains, implants, or surgical devices [25]. Metallic foreign bodies are common after gun-related injuries. Most foreign bodies are recognized at the time of injury, but more than one third may be missed at initial physical evaluation and can remain asymptomatic [26]. Although most foreign bodies can be removed by the injured patient without a problem, some cannot and are presented to the care of a physician. One series of foreign bodies in the hand reported a wide range of time anywhere from the day of injury to 20 years later for the removal of the retained foreign body (mean of seven months), with 43% being removed within one week and 11% being retained for more than one year [26].
Retained foreign bodies can result in a granulomatous immune response as the surrounding tissues attempt to wall off the foreign body from the patient, also known as foreign body reaction [27]. This starts with an aggregation of macrophages, lymphocytes, plasma cells, and fibroblasts that surround the foreign body. As most foreign bodies are too large to phagocytose, the macrophages undergo epithelioid transformation and fuse to form multinucleated giant cells and produce cytokines that stimulate fibroblasts to encapsulate the foreign body in a collagen matrix [28]. For organic foreign bodies, such as wood or animal parts, the foreign body reaction will continue until degradation; however, for nondegradable foreign bodies, the reaction continues until a well-formed capsule separates the foreign body from the immune system and may become asymptomatic for years or until trauma disrupts this capsule [29].
The first-line imaging modality for suspected foreign body should be conventional radiography, which is capable of detecting 80% of all foreign bodies [30][31]. This mostly depends on the atomic number and density of the foreign body; lower-density objects are radiolucent, but up to 98% of higher-density radiopaque objects are also depicted on conventional radiographs [23][31]. Metal, glass, stone, graphite, and calcified biologics (e.g., sea urchin spines) are all radiopaque, whereas wood, plastic, acrylics, and non-calcified biologics (e.g., larvae) are radiolucent [22][23].
Ultrasound is the best imaging modality for the evaluation of suspected radiolucent foreign bodies, with all foreign bodies demonstrating hyperechogenicity on ultrasound with posterior acoustic shadowing; foreign bodies with an irregular or curved surface demonstrate a “clean” shadow, and those with a flat or smooth surface, such as metal, glass, or plastic, demonstrate a “dirty” shadow with posterior reverberation (comet tail artifact) (Figure 3) [32][33][34].
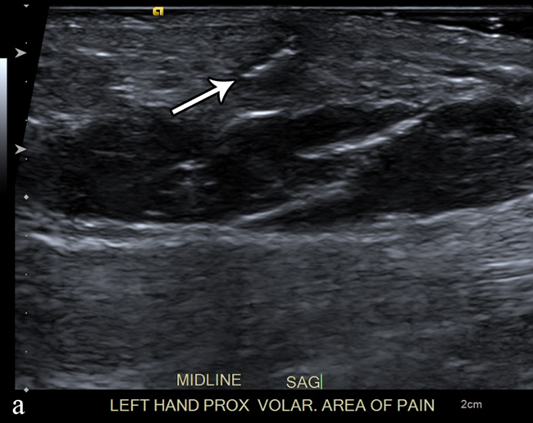
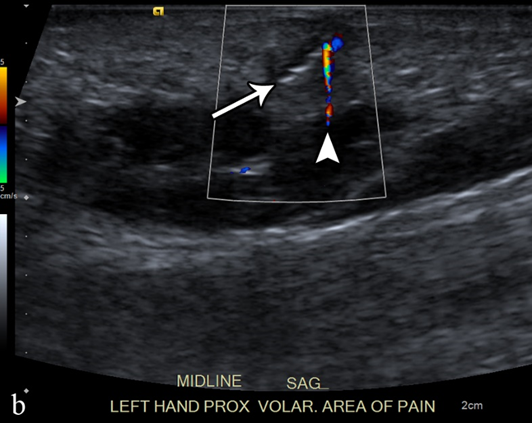
Figure 3. A 50-year-old man with left volar mid-palm pain after recent injury by glass. Grayscale US (a) demonstrates a 4 mm linear echogenic retained foreign body (arrows), with a surrounding hypoechoic halo (foreign body reaction/granuloma) in the subcutaneous tissues, and a color comet-tail artifact (arrowhead) on color Doppler (b).
2.4. Abscess
According to the Society of Skeletal Radiology (SSR), a soft-tissue abscess (STA) corresponds to a localized collection of pus resulting from the invasion of a pathogen, with a peripheral capsule created by macrophage, fibrin, and granulation tissue generally following an untreated phlegmon [35]. STAs can be acute or chronic, and deep-seated or superficial. When located intra-muscularly, STAs have also been named pyomyositis.
The origins of STAs can be direct inoculations due to skin-puncture injuries and foreign bodies and, less frequently, hematogenous spreading from a nearby infected site. They are predisposed by immunosuppression, diabetes, systemic diseases, malnutrition, drug abuse, obesity, extreme age, pre-existing cutaneous lesions, trauma, or surgery [1].
The most common pathogens correspond to Gram-positive cocci, especially Staphylococcus aureus (with methicillin resistance in nearly half of the cases) and hemolytic Streptococcus spp., but many germs are encountered, such as Clostridium spp, Enterococcus, Enterobacteriaceae, and Bacteroides spp or Pseudomonas aeruginosa [36][37]. However, most STAs are polymicrobial with a mixture of aerobic and anaerobic bacteria.
Clinically, patients usually demonstrate fever, possibly sepsis, and painful, erythematous, and inflammatory swelling. Biologically, laboratory analysis shows increased leukocytes and C-reactive proteins.
Conventional radiographs are of limited interest. They may show swelling, increased opacity, dystrophic calcification, and bull of gas and air–liquid level depending on the nature and chronicity of the STA. If the STA is close to the bone, a periosteal reaction may be seen [38].
On ultrasonography with Doppler, STAs display a central heterogeneous, hypoechoic, and non-vascularized collection and an irregular and highly vascularized pseudo-capsule, which is surrounded by edematous, hyperechoic, and hyperemic phlegmon tissues. Internal debris may be seen as small, swirling hyperechoic structures inside the collection and move depending on dynamic compression and the patient’s motion. Additionally, gas can be detected as a bright punctate echo with acoustic shadowing in the case of anaerobic bacteria [38][39][40][41]. Peripheral calcifications and marked liquefaction indicate a chronic STA. The location, dimension, septations, uniqueness or multiplicity, as well as the presence of gas and calcifications, should be detailed in the radiological report.
On CT, ideally performed with an intravenous contrast medium injection, STAs present as fluid attenuation, a collection circumscribed by an enhanced, irregular thin wall. Again, enhanced septa, gas, and calcifications can be seen but not systematically. The surrounding tissues can also demonstrate edema, cellulitis, and mild enhancement [42][43].
Conventional MRI with injection of gadolinium chelates provides the highest accuracy in the diagnosis and staging of STAs, with a sensitivity of 97% and a specificity of 77% (Figure 4) [44].
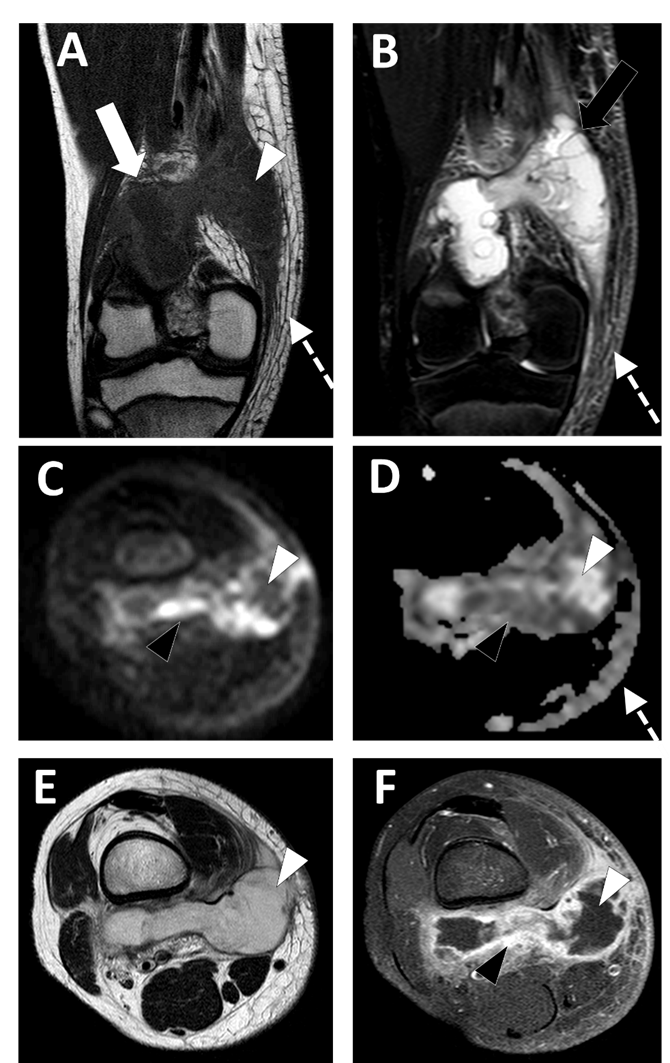
Figure 4. Deep-seated abscess of the right popliteal region in a 13.5-year-old boy presenting with a soft tissue painful swelling, fever, and inflammatory syndrome on blood analysis. The MRI comprises the following sequences: (A) coronal T1-weighted imaging (WI), (B) coronal T2-WI, (C) axial TRACE of diffusion MRI, (D) apparent diffusion coefficient (ADC) map, (E) axial T2, and (F) axial T1 with gadolinium chelate injection and fat suppression. On MRI, the abscess is presented as a fluid-like collection (white arrowhead) with high signal intensity (SI) on T2-WI, low SI on T1-WI, low SI on TRACE, and high ADC value and no contrast enhancement.
2.5. Infectious Myositis
Pyomyositis is a purulent bacterial infection of skeletal muscle that is complicated by abscess formation. Its prevalence is increasing, accounting for up to 1 case in every 3000–4000 annual hospital admissions [45][46].
Some authors distinguish the etiology of pyomyositis: a primary pyomyositis is an infection of the skeletal muscle itself caused by the hematogenous spread of microorganisms, and a secondary pyomyositis is when the infection of the muscle is by contiguity from adjacent structures, such as bone, joint, or soft tissue [45].
Most commonly, infectious pyomyositis is caused by bacteria, predominantly Staphylococcus aureus, whereas tuberculous and nonbacterial pyomyositis caused by virus, fungi, and parasites are rare [47].
Community-acquired Methicillin resistant Staphylococcus aureus (CA-MRSA) is an emergent infectious agent found not only in children, but also in the adult population [48].
The virulence of this pathogen may in part be related to its ability to produce toxins, such as Panton–Valentine leukocidin (PVL) which has a unique ability to kill leukocytes, resulting in bacterial evasion of the bactericidal function of leukocytes [49].
Symptoms such as limping, hip pain, and fever can be subtle in the initial stages, and this can lead to a delayed diagnosis. Pelvic muscles, thighs, and calves are the most common locations [50][51].
Pelvic location is typical in children > 10 years; the initial focus of bone infection is usually subtle, localized in a “metaphyseal-equivalent” region and has a high association with soft tissue abnormalities [52].
Ultrasound can be the first diagnostic step, especially in children, to evaluate other causes of limping, such as septic arthritis, but sensitivity is low in detecting a deep abscess and in the early stages of the infection.
Contrast-enhanced CT can show enlarged muscles, with heterogeneous attenuation and a central fluid collection with rim contrast enhancement, but it is not accurate in the early stages of pyomyositis and in the detection of infectious bone involvement [51].
Contrast-enhanced MRI is the gold standard in evaluating pyomyositis (Figure 5).
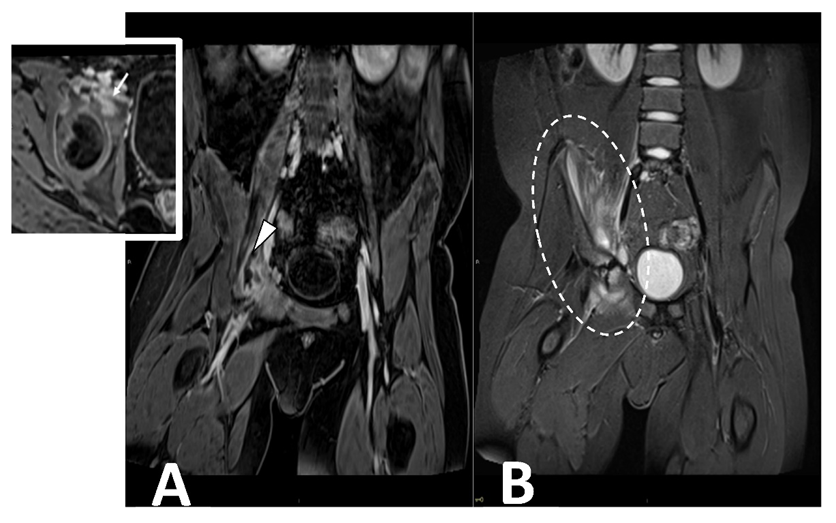
Figure 5. CE MRI in a 10-year-old boy presenting with limping and fever. Coronal ad axial T1 vibe FS post-contrast images (Panel A) demonstrate a bone infectious focus in the acetabulm (arrow) and a large abscess in the iliac and pettineus muscle with a peripheral rim enhancement (arrowhead). Coronal stir (Panel B) shows bone marrow edema in the acetabulum with enlargement and inflammation of surrounding muscular structures (oval dotted line).
2.6. Infectious Tenosynovitis and Bursitis
The term tenosynovitis refers to the inflammation of the tendon sheath, and, if secondary to an infection, it can be an orthopedic emergency. The tendon sheath is a fluid containing a closed compartment that may be predisposed to infectious diseases. Tenosynovitis can be caused by inflammation, trauma, and less frequent infections [2]. Infectious tenosynovitis can result in permanent disability with contracture of the involved muscular structures [53]. Because of this, the correct diagnosis of this entity and the differentiation from non-infectious causes are crucial, but are nonetheless challenging. The diagnosis of infectious tenosynovitis is fundamental to starting proper treatments and avoiding permanent disability.
One of the most common types of infectious tenosynovitis is the involvement of the flexor digit sheaths, usually secondary to a skin trauma that introduces a pathogen microorganism. A common complication of infectious tenosynovitis is the development of an abscess, mainly pyogenic tenosynovitis [2][53]. The microorganisms most commonly involved in infectious tenosynovitis are Staphylococcus aureus above all, followed by other bacteria, such as Pasteurella multocida (usually in cat bites), Neisseria gonorrhoeae (sexually transmitted), and Elkenella corodens (usually in human bites) [54][55].
Mycobacteria tuberculosis can also lead to infectious tenosynovitis (Figure 6), usually with an insidious clinical presentation characterized by gradual swelling and inconsistent supportive exam findings [4][54].
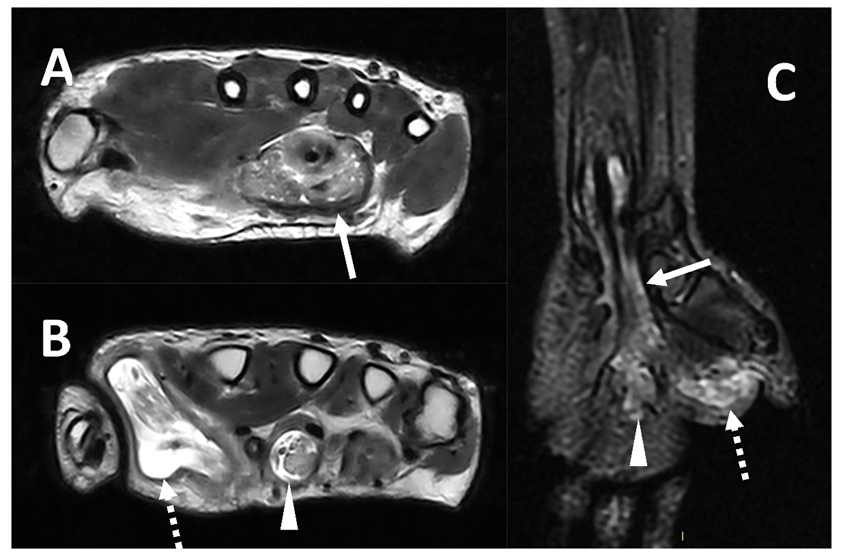
Figure 6. MRI (Panel A and B, T2w axial; Panel C, T2w coronal sequence) of a 39-year-old male complaining of progressively increasing swelling of the right wrist and hand shows a marked tenosynovitis involving the common flexor digitorum sheaths (arrows), as well as the flexor pollicis longus (dotted arrows) and the flexor of the third digit (arrowhead). Both synovial thickening and fluid collection can be detected. The final diagnosis is tenosynovitis related to mycobacterium tuberculosis.
References
- Turecki, M.B.; Taljanovic, M.S.; Stubbs, A.Y.; Graham, A.R.; Holden, D.A.; Hunter, T.B.; Rogers, L.F. Imaging of Musculoskeletal Soft Tissue Infections. Skelet. Radiol. 2010, 39, 957–971.
- Altmayer, S.; Verma, N.; Dicks, E.A.; Oliveira, A. Imaging Musculoskeletal Soft Tissue Infections. Semin. Ultrasound. CT MR 2020, 41, 85–98.
- Headley, A.J. Necrotizing soft tissue infections: A primary care review. Am. Fam. Physician 2003, 68, 323–328.
- McHenry, C.R.; Piotrowski, J.J.; Petrinic, D.; Malangoni, M.A. Determinants of Mortality for Necrotizing Soft-Tissue Infections. Ann. Surg. 1995, 221, 558–563.
- Bosshardt, T.L.; Henderson, V.J.; Organ, C.H. Necrotizing soft-tissue infections. Arch. Surg. 1996, 131, 846–852.
- Chauhan, S.; Jain, S.; Varma, S.; Chauhan, S.S. Tropical pyomyositis (myositis tropicans): Current perspective. Postgrad. Med. J. 2004, 80, 267–270.
- Elliott, D.; Kufera, J.A.; Myers, R.A. The microbiology of necrotizing soft tissue infections. Am. J. Surg. 2000, 179, 361–366.
- Hill, M.K.; Sanders, C.V. Skin and soft tissue infections in critical care. Crit. Care Clin. 1998, 14, 251–262.
- Bystritsky, R.J. Cellulitis. Infect. Dis. Clin. N. Am. 2021, 35, 49–60.
- Stevens, D.L.; Bryant, A.E.; Goldstein, E.J. Necrotizing soft tissue infections. Infect. Dis. Clin. N. Am. 2021, 35, 135–155.
- Hayeri, M.R.; Ziai, P.; Shehata, M.L.; Teytelboym, O.M.; Huang, B.K. Soft-tissue infections and their imaging mimics: From cellulitis to necrotizing fasciitis. Radiographics 2016, 36, 1888–1910.
- Simpfendorfer, C.S. Radiologic approach to musculoskeletal infections. Infect. Dis. Clin. N. Am. 2017, 31, 299–324.
- Chang, C.-D.; Wu, J.S. Imaging of musculoskeletal soft tissue infection. Semin. Roentgenol. 2017, 52, 55–62.
- Yu, J.S.; Habib, P. MR imaging of urgent inflammatory and infectious conditions affecting the soft tissues of the musculoskeletal system. Emerg. Radiol. 2009, 16, 267–276.
- Schmid, M.R.; Kossmann, T.; Duewell, S. Differentiation of necrotizing fasciitis and cellulitis using mr imaging. AJR Am. J. Roentgenol. 1998, 170, 615–620.
- Kim, H.S.; Lee, J.; Yi, S.Y.; Jun, H.J.; Choi, Y.-L.; Ahn, G.H.; Seo, S.W.; Lim, D.H.; Ahn, Y.C.; Park, J.O.; et al. Liposarcoma: Exploration of clinical prognostic factors for risk based stratification of therapy. BMC Cancer 2009, 9, 205.
- Garcia, N.M.; Cai, J. Aggressive soft tissue infections. Surg. Clin. N. Am. 2018, 98, 1097–1108.
- Pelletier, J.; Gottlieb, M.; Long, B.; Perkins, J.C. Necrotizing soft tissue infections (NSTI): Pearls and pitfalls for the emergency clinician. J. Emerg. Med. 2022, 62, 480–491.
- Stevens, D.L.; Bryant, A.E. Necrotizing Soft-Tissue Infections. N. Engl. J. Med. 2017, 377, 2253–2265.
- Paz Maya, S.; Dualde Beltrán, D.; Lemercier, P.; Leiva-Salinas, C. Necrotizing fasciitis: An urgent diagnosis. Skelet. Radiol. 2014, 43, 577–589.
- Potini, V.C.; Francisco, R.; Shamian, B.; Tan, V. Sequelae of foreign bodies in the wrist and hand. Hand 2013, 8, 77–81.
- Carneiro, B.C.; Cruz, I.A.N.; Chemin, R.N.; Rizzetto, T.A.; Guimarães, J.B.; Silva, F.D.; Junior, C.Y.; Pastore, D.; Ormond Filho, A.G.; Nico, M.A.C. Multimodality imaging of foreign bodies: New insights into old challenges. Radiographics 2020, 40, 1965–1986.
- Jarraya, M.; Hayashi, D.; de Villiers, R.V.; Roemer, F.W.; Murakami, A.M.; Cossi, A.; Guermazi, A. Multimodality imaging of foreign bodies of the musculoskeletal system. AJR Am. J. Roentgenol. 2014, 203, W92–W102.
- Brunner, J.; Russel, M.; Herr, K.; Benjamin, E.; Myers, L.; Boyko, O.; Jaffray, P.; Reddy, S. Nonsuicidal self-injury-related foreign bodies in the emergency department. Semin. Ultrasound CT MR 2015, 36, 80–87.
- Del Cura, J.L.; Aza, I.; Zabala, R.M.; Sarabia, M.; Korta, I. US-guided localization and removal of soft-tissue foreign bodies. Radiographics 2020, 40, 1188–1195.
- Anderson, M.A.; Newmeyer, W.L.; Kilgore, E.S. Diagnosis and treatment of retained foreign bodies in the hand. Am. J. Surg. 1982, 144, 63–67.
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 2015, 8, 5671–5701.
- Luttikhuizen, D.T.; Harmsen, M.C.; Van Luyn, M.J.A. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006, 12, 1955–1970.
- Pagán, A.J.; Ramakrishnan, L. The formation and function of granulomas. Annu. Rev. Immunol. 2018, 36, 639–665.
- Ipaktchi, K.; Demars, A.; Park, J.; Ciarallo, C.; Livermore, M.; Banegas, R. Retained palmar foreign body presenting as a late hand infection: Proposed diagnostic algorithm to detect radiolucent objects. Patient Saf. Surg. 2013, 7, 25.
- Manthey, D.E.; Storrow, A.B.; Milbourn, J.M.; Wagner, B.J. Ultrasound versus radiography in the detection of soft-tissue foreign bodies. Ann. Emerg. Med. 1996, 28, 7–9.
- Boyse, T.D.; Fessell, D.P.; Jacobson, J.A.; Lin, J.; van Holsbeeck, M.T.; Hayes, C.W. US of soft-tissue foreign bodies and associated complications with surgical correlation. Radiographics 2001, 21, 1251–1256.
- Bray, P.W.; Mahoney, J.L.; Campbell, J.P. Sensitivity and specificity of ultrasound in the diagnosis of foreign bodies in the hand. J. Hand Surg. Am. 1995, 20, 661–666.
- Rubin, J.M.; Adler, R.S.; Bude, R.O.; Fowlkes, J.B.; Carson, P.L. Clean and Dirty Shadowing at US: A Reappraisal. Radiology 1991, 181, 231–236.
- Alaia, E.F.; Chhabra, A.; Simpfendorfer, C.S.; Cohen, M.; Mintz, D.N.; Vossen, J.A.; Zoga, A.C.; Fritz, J.; Spritzer, C.E.; Armstrong, D.G.; et al. MRI nomenclature for musculoskeletal infection. Skelet. Radiol. 2021, 50, 2319–2347.
- Paydar, K.Z.; Hansen, S.L.; Charlebois, E.D.; Harris, H.W.; Young, D.M. Inappropriate antibiotic use in soft tissue infections. Arch. Surg. 2006, 141, 850–854.
- Frazee, B.W.; Lynn, J.; Charlebois, E.D.; Lambert, L.; Lowery, D.; Perdreau-Remington, F. High prevalence of methicillin-resistant staphylococcus aureus in emergency department skin and soft tissue infections. Ann. Emerg. Med. 2005, 45, 311–320.
- Struk, D.W.; Munk, P.L.; Lee, M.J.; Ho, S.G.; Worsley, D.F. Imaging of soft tissue infections. Radiol. Clin. N. Am. 2001, 39, 277–303.
- Bureau, N.J.; Chhem, R.K.; Cardinal, E. Musculoskeletal infections: US manifestations. Radiographics 1999, 19, 1585–1592.
- Craig, J.G. Infection: Ultrasound-guided procedures. Radiol. Clin. N. Am. 1999, 37, 669–678.
- Loyer, E.M.; Kaur, H.; David, C.L.; DuBrow, R.; Eftekhari, F.M. Importance of dynamic assessment of the soft tissues in the sonographic diagnosis of echogenic superficial abscesses. J. Ultrasound Med. 1995, 14, 669–671.
- Fayad, L.M.; Carrino, J.A.; Fishman, E.K. Musculoskeletal infection: Role Of CT In the emergency department. Radiographics 2007, 27, 1723–1736.
- Ma, L.D.; Frassica, F.J.; Bluemke, D.A.; Fishman, E.K. CT and MRI evaluation of musculoskeletal infection. Crit. Rev. Diagn. Imaging 1997, 38, 535–568.
- Chandnani, V.P.; Beltran, J.; Morris, C.S.; Khalil, S.N.; Mueller, C.F.; Burk, J.M.; Bennett, W.F.; Shaffer, P.B.; Vasila, M.S.; Reese, J. Acute experimental osteomyelitis and abscesses: Detection with MR IMAGING versus CT. Radiology 1990, 174, 233–236.
- Bickels, J.; Ben-Sira, L.; Kessler, A.; Wientroub, S. Primary pyomyositis. J. Bone Joint. Surg. Am. 2002, 84, 2277–2286.
- Gubbay, A.J.; Isaacs, D. Pyomyositis in children. Pediatr. Infect. Dis. J. 2000, 19, 1009–1012; quiz 1013.
- Thammaroj, P.; Panitchote, A.; Muktabhant, C.; Chowchuen, P. Discrimination between tuberculous and bacterial pyomyositis in magnetic resonance features. Eur. J. Radiol. Open 2020, 7, 100214.
- Dhanoa, A.; Singh, V.A.; Mansor, A.; Yusof, M.Y.; Lim, K.-T.; Thong, K.-L. Acute haematogenous community-acquired methicillin-resistant staphylococcus aureus osteomyelitis in an adult: Case report and review of literature. BMC Infect. Dis. 2012, 12, 270.
- DeLeo, F.R.; Otto, M.; Kreiswirth, B.N.; Chambers, H.F. Community-associated meticillin-resistant staphylococcus aureus. Lancet 2010, 375, 1557–1568.
- Hernandez, R.J.; Strouse, P.J.; Craig, C.L.; Farley, F.A. Focal pyomyositis of the perisciatic muscles in children. AJR Am. J. Roentgenol. 2002, 179, 1267–1271.
- Gonzalez Moran, G.; Garcia Duran, C.; Albiñana, J. Imaging on pelvic pyomyositis in children related to pathogenesis. J. Child. Orthop. 2009, 3, 479–484.
- Bartoloni, A.; Aparisi Gómez, M.P.; Cirillo, M.; Allen, G.; Battista, G.; Guglielmi, G.; Tomà, P.; Bazzocchi, A. Imaging of the limping child. Eur. J. Radiol. 2018, 109, 155–170.
- Pollen, A.G. Acute infection of the tendon sheaths. Hand. 1974, 6, 21–25.
- Spann, M.; Talmor, M.; Nolan, W.B. Hand infections: Basic principles and management. Surg. Infect. 2004, 5, 210–220.
- Krieger, L.E.; Schnall, S.B.; Holtom, P.D.; Costigan, W. Acute gonococcal flexor tenosynovitis. Orthopedics 1997, 20, 649–650.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
06 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




