Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chanchao Lorthongpanich | -- | 2768 | 2022-12-06 05:12:55 | | | |
| 2 | Rita Xu | -8 word(s) | 2760 | 2022-12-06 06:25:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Damkham, N.; Issaragrisil, S.; Lorthongpanich, C. Yes-Associated Protein. Encyclopedia. Available online: https://encyclopedia.pub/entry/38087 (accessed on 07 February 2026).
Damkham N, Issaragrisil S, Lorthongpanich C. Yes-Associated Protein. Encyclopedia. Available at: https://encyclopedia.pub/entry/38087. Accessed February 07, 2026.
Damkham, Nattaya, Surapol Issaragrisil, Chanchao Lorthongpanich. "Yes-Associated Protein" Encyclopedia, https://encyclopedia.pub/entry/38087 (accessed February 07, 2026).
Damkham, N., Issaragrisil, S., & Lorthongpanich, C. (2022, December 06). Yes-Associated Protein. In Encyclopedia. https://encyclopedia.pub/entry/38087
Damkham, Nattaya, et al. "Yes-Associated Protein." Encyclopedia. Web. 06 December, 2022.
Copy Citation
Yes-associated protein (YAP) and WW domain-containing transcription regulator protein 1 (WWTR1, also known as TAZ) are transcriptional coactivators in the Hippo signaling pathway. Both are well-known regulators of cell proliferation and organ size control, and they have significant roles in promoting cell proliferation and differentiation. The roles of YAP and TAZ in stem cell pluripotency and differentiation have been extensively studied.
YAP
stem cells
differentiation
hematopoietic stem cells
1. Introduction
Accumulating evidence strongly suggests that cell biological changes are regulated not only by internal soluble factors (cytokines and hormones) but also by physical and mechanical cues. The type of extracellular matrix (ECM) stiffness and adhesiveness are mechanical stimuli currently being studied [1][2][3]. Through mechanotransduction systems, cells translate these stimuli into biochemical signals that regulate multiple aspects of cell changes, such as growth and differentiation. How mechanical impulses are linked to the activity of nuclear transcription factors remains poorly understood.
In addition to the classical role of the Hippo signaling pathway in regulating cell proliferation and apoptosis [4][5][6], the pathway has been demonstrated to be one of the mechanosensing pathways that convey the mechanical signals that modulate cell function. In the mammalian preimplantation embryo, positional sensing ability is crucial for the trophectoderm (TE)-inner cell mass (ICM) fate decision. Through adhesiveness, each embryonic blastomere can sense its positioning within an intact preimplantation embryo. The blastomeres receiving high adhesiveness, i.e., those in the inner cell, can secure their inner cell mass fate, the origin of embryonic stem cells (ESCs). However, the blastomeres in the outer layer of the embryo, i.e., the outer cells, receive fewer adhesive stimuli and become trophectoderm cells [7][8][9] (Figure 1). It has previously been shown that the Hippo component proteins (large tumor suppressor kinases 1/2 (LATS1/2), mammalian STE20-like protein kinase 1/2 (MST1/2), and YAP) are responsible for translating positional information to lineage specification through the cell adhesiveness positional-sensing mechanism [10][11]. Disruption of the Hippo pathway-component gene in early embryos leads to failure of lineage specification and postimplantation development due to the loss of positional sensing information [10][11][12]. In addition to the mammalian preimplantation embryo, the role of the Hippo pathway in mechanotransduction has been implicated in other cells: cancers, mesenchymal stem cells (MSCs), and endothelial cells [13][14].
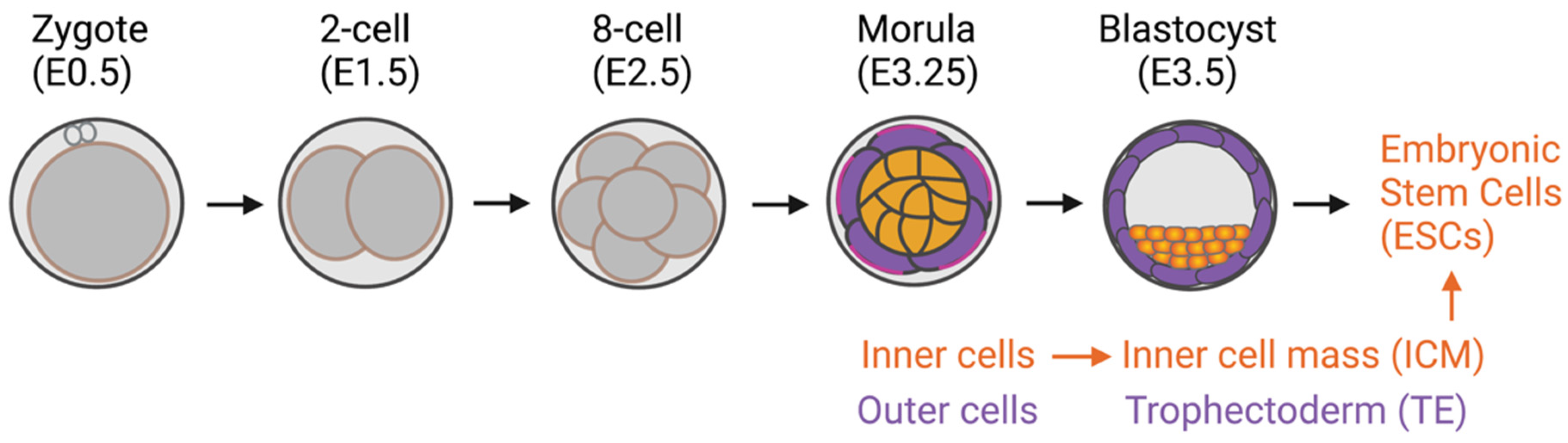
Figure 1. Preimplantation mouse embryo development. Inner cells with high adhesive forces acquire their inner cell mass fate, a source of embryonic stem cells. The outer cells have lower adhesive forces and become trophectoderm cells.
1.1. The Hippo-YAP/TAZ Signaling Pathway
The Hippo signaling pathway was first identified in Drosophila melanogaster through genetic screening [15][16]. Later studies revealed its conserved role in regulating organ size, cell fate, cell growth, and apoptosis in other mammals, including humans [17][18][19][20]. YAP is a critical transcriptional coactivator and a crucial effector protein that regulates downstream target genes involved in cell proliferation and differentiation, namely, Cyclin A, Myc, Ctgf, Cdx2, and Ajuba [5][15][16][18][19][20][21][22][23][24][25][26]. TAZ, a YAP homolog, is another well-recognized Hippo effector protein. However, its role in regulating cell function and whether its function is redundant to YAP is not well understood [27]. Since the known functions of YAP and TAZ are mainly redundant, this research used “YAP/TAZ” to refer to a YAP and TAZ protein complex unless otherwise stated.
In the classical model of the Hippo pathway, YAP/TAZ activity is negatively regulated by Hippo-LATS1/2 core kinases. In the nonactive state of the core kinases, most YAP/TAZ molecules are active and translocate into the nucleus, binding to its transcription factors (TEADs) and driving the target gene expression of the YAP/TAZ-TEAD complex [28][29][30]. Once the core kinases are activated through upstream signals such as cell-cell contacts, the activated core kinases phosphorylate YAP/TAZ, resulting in cytoplasmic retention and inhibition of downstream target gene expression [19][31][32][33][34]. However, whether the response of YAP/TAZ to mechanical stimuli depends on the canonical Hippo-LATS1/2 core kinases has yet to be delineated.
1.2. Stem Cells
Stem cells are cells with the ability to self-renew and differentiate into many cell types in the body [35]. Therefore, stem cells are a holy grail for regenerative medicine [36]. They are classified into four groups by their derivation source: (1) adult stem cells [36], (2) perinatal stem cells [37], (3) ESCs [38], and (4) induced pluripotent stem cells (iPSCs) [39].
Adult stem cells are undifferentiated cells that reside in tissues or organs in the adult body. The primary roles of these cells are to maintain and repair the tissue in which they reside through their self-renewal and differentiation capacity. However, adult stem cells are multipotent or unipotent, meaning they can be differentiated into distinct, but not all, cell types, depending on their tissue of origin. One of the very well-studied adult stem cell types, is MSCs. MSCs are multipotent stem cells that are found in several tissues and can differentiate into at least 3 distinct cell types: osteoblasts, adipocytes, and chondrocytes [40][41]. Gradually increasing information shows the transdifferentiation capacity of MSCs to other cell types, such as neuron-like cells [42][43][44], smooth muscle cells [45][46], and cardiomyocytes [47]. These findings support the wide clinical applications and regenerative capacity of MSCs.
Another well-known, clinically approved adult stem cell type for therapeutic approaches is HSCs. HSCs are responsible for all blood cell production through the process termed hematopoiesis. The classical model of their differentiation hierarchy is that HSCs differentiate into multipotent progenitors (MMPs) that no longer have a self-renewal ability. MPPs differentiate into common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs). In turn, CMPs differentiate into megakaryocyte–erythroid progenitors (MEPs) and granulocyte–macrophage progenitors (GMPs). Both of these progenitors then differentiate into mature cell types, including red blood cells (erythrocytes), megakaryocytes, myeloid cells (monocytes, macrophages, and granulocytes), mass cells, T- and B-lymphocytes, and natural killer cells [48][49][50][51][52][53]. However, several new hematopoietic hierarchy models have recently been proposed [54]. One is an early split model, in which the HSC lineage separates earlier than in the classical model [55][56][57][58]. Another newly described model is a continuous, Waddington-like model [54][59][60][61]. This model suggests that HSCs do not pass through a stable or discrete intermediate form but instead continuously acquire lineage-committed transcription [54][59][60][61].
Perinatal stem cells are stem cells that can be isolated from tissues that are discarded after birth, such as the placenta, umbilical cord, cord blood, and amniotic fluid. Different types of stem and progenitor cells can be isolated from these tissues. The most well-known perinatal stem cells are HSCs isolated from umbilical cord blood and MSCs isolated from umbilical cord blood and perinatal tissues, such as placenta or chorionic tissue [37]. Perinatal stem cells represent an intermediate cell type that combines the qualities of adult stem cells and ESCs and holds broad, multipotent plasticity.
Unlike adult and perinatal stem cells with limited multipotent differentiation capacity, PSCs (ESCs and iPSCs) can self-renew and differentiate into all cell types in the body, including blood cells [62]. ESCs are derived from the inner cell mass of an embryo [63]. Consequently, the related ethical issues are the most challenging aspect of their use. Yamanaka and colleagues successfully generated PSCs by reprogramming the skin fibroblasts to a pluripotent state, called iPSCs [39]. Since then, iPSCs have become the great hope of cell origin to generate personalized cells for regenerative medicine [64][65]. However, the current challenges in generating iPSC-derived target cells are their production efficiency and efficacy [66]. Further research into creating a suitable in vitro niche microenvironment to mimic an in vivo microenvironment could be one way to achieve success [67].
2. Mechanosensing and Forces Regulating YAP/TAZ
“Mechanosensing” is the term used to describe cells’ ability to sense mechanical cues in their microenvironment. “Mechanotransduction” refers to the ability of cells to subsequently translate and respond to mechanical cues by programming cell behaviors [68]. Many mechanical cues modulate the growth and lineage decisions of cells, including ECM stiffness, blood flow, wall or turbulent shear stress, cell shape (geometry), cell density, topographic surfaces, and cytoskeleton tension. However, how cells respond to such cues to generate biological responses is poorly understood. The first evidence of a novel function of YAP as a mechanosensing protein came from the study by Dupont and colleagues on MSCs in 2011 [13]. Their work showed that mechanical forces or cues (ECM stiffness, cell spreading, and cytoskeleton tension) mediate YAP localization and result in the lineage differentiation bias of MSCs [13]. Their study results shed light on the noncanonical role of YAP/TAZ as a mechanosensing molecule in stem cells. Since then, several models have confirmed that YAP can act as a mechanosensor to convey signals that control cell function and biological responses [69][70][71][72][73].
2.1. ECM Stiffness Influences MSC Differentiation via YAP/TAZ
The adipo-osteogenic balance mechanism regulates the ability of MSCs to differentiate into adipocytes or osteoblasts. Dysregulation of this balance has been linked to particular pathophysiological processes: bone loss and obesity. YAP has been reported as a central regulator controlling the balance, given that high YAP expression induces MSCs to differentiate into osteoblasts, whereas low YAP expression induces adipogenesis [74]. Uncovering the relationship between ECM matrix stiffness and YAP/TAZ has led to extensive investigations to determine whether YAP/TAZ acts as a mechanosensing molecule in response to ECM stiffness to control MSC fate differentiation into either osteoblasts or adipocytes.
Many studies have reported that a stiff substrate activates YAP activity, resulting in YAP/TAZ translocation into the nucleus and inducing MSC differentiation into osteoblasts [13][69][75]. In contrast, a soft substrate was reported to inhibit YAP/TAZ activity by restraining YAP/TAZ in the cytoplasm, resulting in MSC differentiation into adipocytes (Figure 2) [13][69][75][76][77]. These results suggest that the activity of YAP is crucial for MSCs to regulate the adipo-osteogenic differentiation balance while undergoing differentiation. In addition, YAP seems to play a role as a negative regulator of MSC differentiation to chondrocytes [78][79][80], while overexpression of TAZ promotes chondrocyte differentiation from MSCs [81]. In contrast, fluid shear stress promotes chondrocyte maturation from the primary chondrocyte progenitor [82]. These findings suggest that both YAP/TAZ and fluid shear stress regulate chondrocyte differentiation. Modulating YAP activity using matrix stiffness or fluid shear stress could direct differentiation into the desired cell type without genetic alternation. This approach could be applied to the production of adipocytes, osteoblasts, or chondrocytes for clinical use, and it may facilitate tissue regeneration [13][69][75][76][77][83].
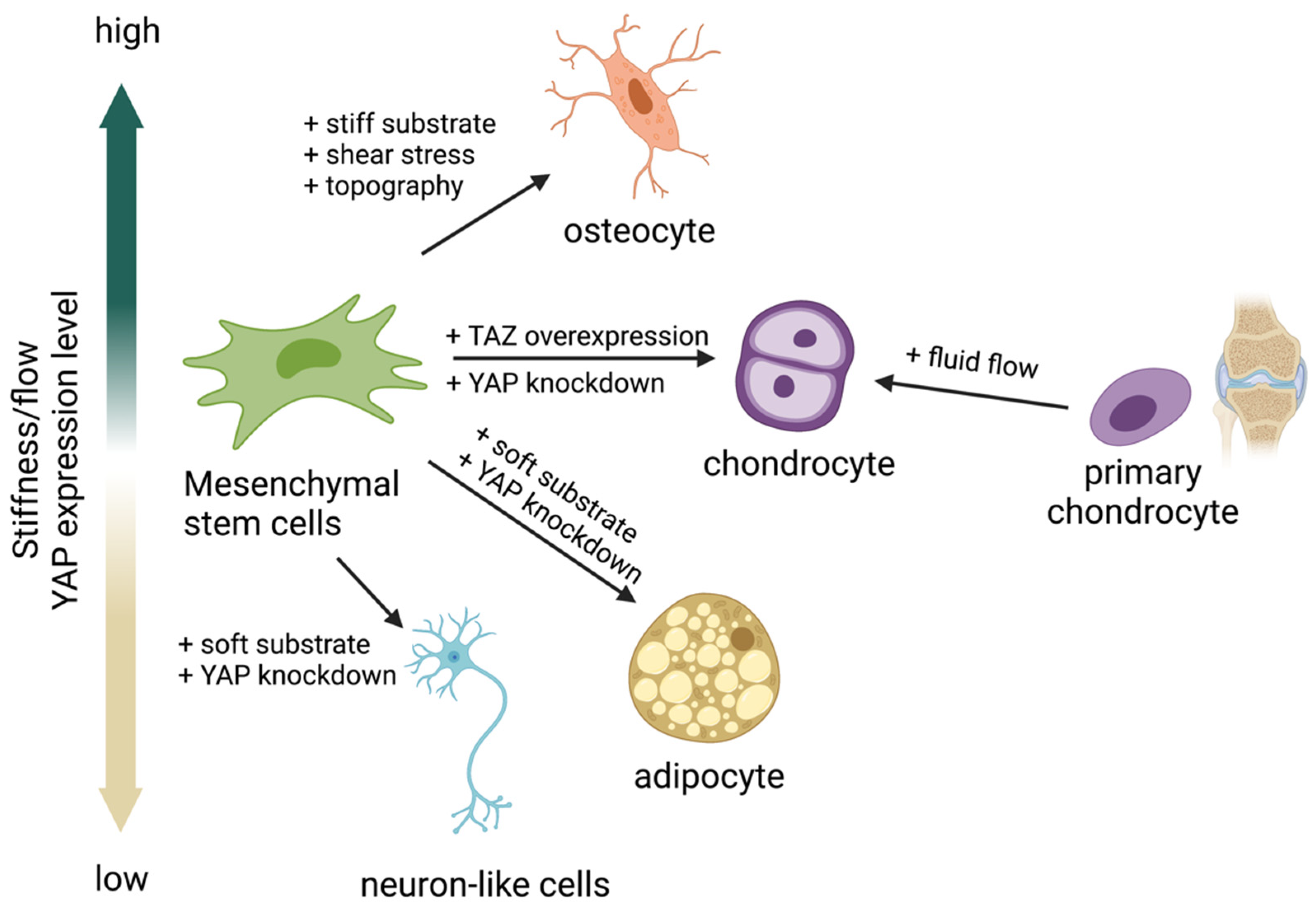
Figure 2. Yes-associated protein (YAP) acts as a mechanosensing molecule in mesenchymal stem cells (MSCs) fate determination.
Several research groups now focus on the transdifferentiation ability of MSCs to cell types other than adipocytes, osteoblasts, and chondrocytes. There have been attempts to differentiate MSCs into neurons [42], corneal epithelial cells [84], keratinocytes [85], and several other cell types. However, success in obtaining fully differentiated cells has been limited. Applying knowledge of creating a biological microenvironment to mimic the in vivo niche and applying a suitable ECM type and stiffness are likely to enhance the degree of differentiation.
2.2. Fluid Shear Stress and Force Modulate YAP/TAZ Activity
Fluid shear stress and force have been found to modulate YAP expression. Several forms can modulate YAP/TAZ activity. They are laminar flow [86][87], disturbed or oscillatory flow [86], circumferential strain [88], fluid shear stress [82], wall shear stress [88], intermittent compressive force (ICF), and continuous compressive force (CCF) [83] (Figure 3). Interestingly, different flow patterns activated YAP nuclear activity to various degrees. It has been shown that applying shear stress to epithelial cells to mimic blood flow induced YAP activity by enhancing nuclear localization in zebrafish endothelial cells [87]. Disturbed flow induced nuclear YAP, while laminar flow or shear stress inhibited YAP in human endothelial cells [86] and human iPSCs [88]. Circumferential strain promoted YAP expression in human iPSCs [88]. ICF increased YAP expression, while CCF reduced YAP expression in the human periodontal ligament and MSC-like cells isolated from tooth connective tissue [83]. There are limited reports on the effects of fluid shear stress on YAP/TAZ activity and cell biological changes relative to the number of studies investigating how ECM works. Further experiments are needed to improve the understanding of the effects of the bloodstream on the differentiation capacity and function of blood cells.
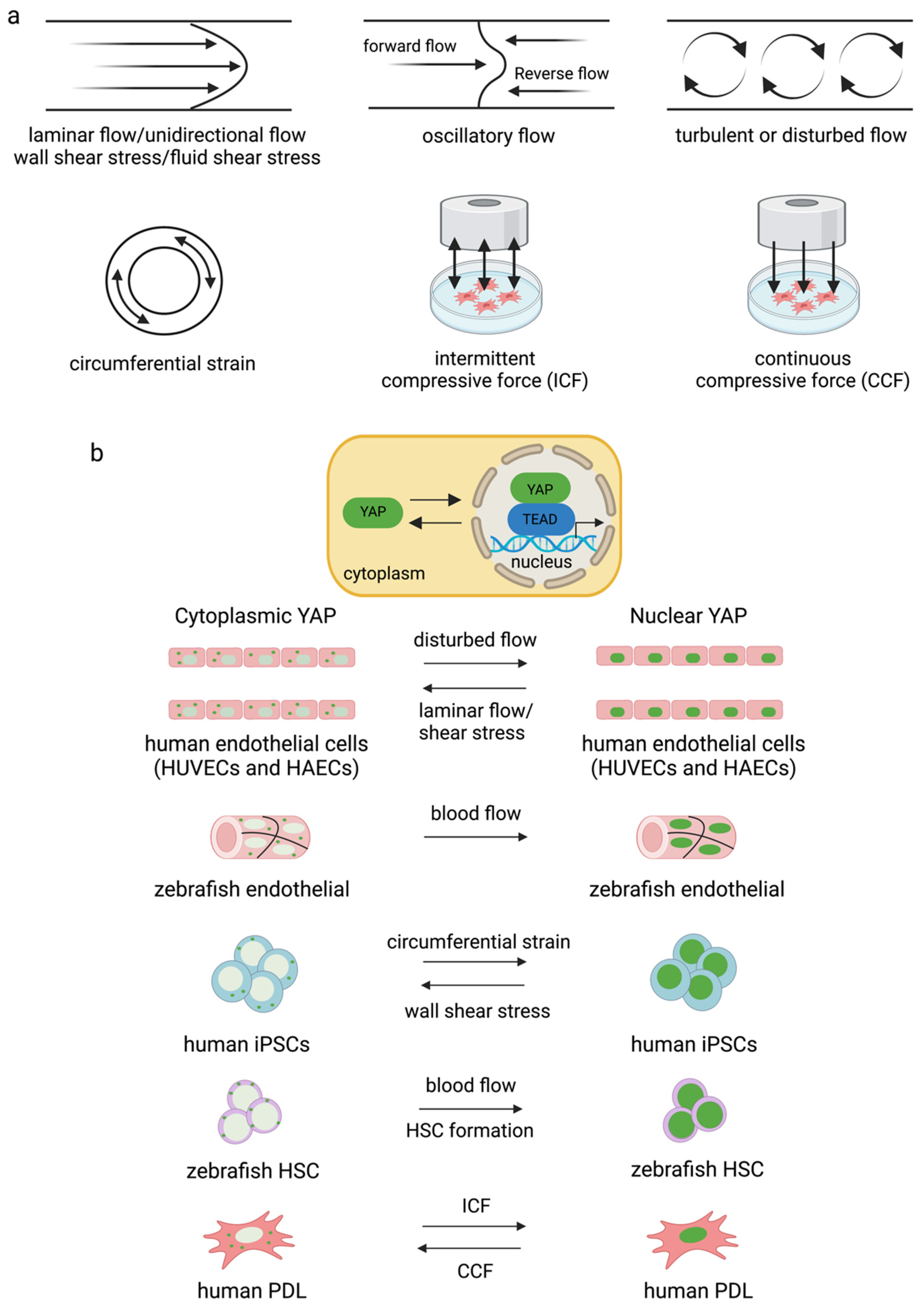
Figure 3. Different types of flow and strain mediate YAP/TAZ activity in different cell types (a,b). Disturbed flow increased YAP activity in endothelial cells [86][89] and blood flow induced nuclear YAP in zebrafish vessels [87]. Circumferential strain induced YAP expression in human iPSCs, and blood flow induced YAP translocated into the nucleus for HSC formation in zebrafish [88]. ICF and CCF mediated YAP expression differently in human PDL [83].
3. Role of YAP during HSC Formation and Blood Cell Production
3.1. Role of YAP during HSC Formation
As mentioned earlier, PSCs can differentiate into all types of cells in the body, including blood cells, via in vitro hematopoiesis. PSC-derived HSCs are one of the most desired blood products, as HSCs are potent starting cells that can be further differentiated into all blood cell types. However, there are still challenges to be overcome regarding production efficiency. The Hippo pathway has been linked to hematopoiesis since the novel role of the pathway in regulating blood cell production was first demonstrated in Drosophila in 2014 [90][91][92]. The pathway was later implicated in mammalian hematopoiesis [93].
Bioinformatic gene regulatory network analysis of mouse ESC differentiation into HSCs and macrophages revealed that YAP/TEAD binds to Tal1 and Fli1 transcription factors during hemangioblast transition to hemogenic endothelial cells [94]. YAP/TEAD is also involved in hematopoietic specification and differentiation in the hemogenic–endothelial transition stage during mESC differentiation into macrophages in vitro [94]. In addition, YAP/TAZ has recently been demonstrated as an essential molecule to regulate HSC fitness, self-renewal, and differentiation fate through interaction with the Scribble protein. The combined loss of Scribble, YAP, and TAZ results in transcriptional upregulation genes involved in HSC fitness in mice [95]. Studies on zebrafish and human iPSC-derived HSCs further confirmed the role of YAP/TAZ in HSC formation [88]. However, YAP seems dispensable for normal and malignant hematopoiesis in mice [96][97]. Recently, the upstream mediators of Lats1/2 and YAP, MST1/2, have been reported to be indispensable molecules in HSC formation. Deleting MST1/2 markedly altered the maturation of HSCs and HSC-derived blood cells [98]. Overall, it can be concluded that the Hippo pathway contributes substantially to HSC production and fate.
3.2. Role of YAP in Myeloid and Lymphoid Lineage Development
3.2.1. Role of YAP in T-Cell Development and Activation
The roles of YAP and TAZ have been determined in Treg and T helper 17 (TH17) cell fate differentiation [99][100]. YAP is required for the generation and function of Treg [99], while TAZ has been shown to promote TH17 cell differentiation from naïve CD4+ T cells [100]. It was demonstrated that the sensing of stiffness by YAP had a critical role in a mouse model during T-cell activation after viral infection. It has been reported that node stiffness increased by approximately 10-fold due to lymphoproliferation. This increased stiffness activated the YAP in T cells, resulting in T-cell activation. Similarly, YAP expression and T-cell activation were elevated when cultured on stiff hydrogels mimicking lymph node stiffness. The YAP sensing of lymph node stiffness appears to mediate the feedback mechanism of T cells during viral infections [101].
3.2.2. Role of YAP in Megakaryocyte Differentiation and Platelet Production
The role of YAP/TAZ in human megakaryocyte differentiation was determined using the MEG-01 cell line and cord-blood-derived megakaryocytes/platelets as a model [93][102]. LATS and YAP have an essential role in megakaryoblast proliferation, maturation, and platelet production, whereas TAZ showed a minor effect [102]. Increasing YAP activity induced megakaryocytic cell proliferation but inhibited maturation, resulting in low platelet production. Conversely, YAP reduction inhibited proliferation but increased platelet production [93]. These results suggest that the dynamic expression of YAP during megakaryopoiesis is essential for megakaryocytic cell growth. Modulating YAP activity using small molecules may present an opportunity to achieve the large-scale in vitro production of platelets for transfusion.
3.2.3. Role of YAP in Red Blood Cell Maturation and Enucleation
The role of YAP in mouse blood cell production has been studied using transgenic mice as a model. YAP1 knockout in mice was created by having YAP deleted in all HSCs, a starting cell in the blood differentiation lineage. Consequently, YAP was deleted from all the subsequent HSC-derived blood cells. However, the results showed that the absence of YAP had no significant effects on overall blood cell production (myeloid, lymphoid, and red blood cells) but showed a minor effect on the anemia phenotype [97]. The overexpression of YAP in hematopoietic cells also did not alter normal hematopoietic stem cell function in mice [96]. However, under stress conditions, YAP was crucial for promoting erythroid progenitor proliferation in mice [103].
Recently, researchers demonstrated that both YAP and TAZ are essential for human erythroid differentiation and maturation from HSCs isolated from umbilical cord blood and mobilized peripheral blood. Depleting either YAP or TAZ during human erythroid differentiation from HSCs significantly impaired erythroblast maturation and resulted in the inhibition of the enucleation of erythrocytes. It is suggested that YAP and TAZ are required in the late stage of human erythropoiesis. However, the transient overexpression of YAP or TAZ in erythroblasts does not have any apparent effect on erythroid maturation and enucleation [104].
References
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519.
- Kothapalli, C.; Mahajan, G.; Farrell, K. Substrate stiffness induced mechanotransduction regulates temporal evolution of human fetal neural progenitor cell phenotype, differentiation, and biomechanics. Biomater. Sci. 2020, 8, 5452–5464.
- Selig, M.; Lauer, J.C.; Hart, M.L. Mechanotransduction and Stiffness-Sensing: Mechanisms and Opportunities to Control Multiple Molecular Aspects of Cell Phenotype as a Design Cornerstone of Cell-Instructive Biomaterials for Articular Cartilage Repair. Int. J. Mol. Sci. 2020, 21, 5399.
- Pan, D. The unfolding of the Hippo signaling pathway. Dev. Biol. 2022, 487, 1–9.
- Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 2010, 19, 491–505.
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434.
- Tarkowski, A.K.; Wroblewska, J. Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J. Embryol. Exp. Morphol. 1967, 18, 155–180.
- Kimber, S.J.; Surani, M.A.; Barton, S.C. Interactions of blastomeres suggest changes in cell surface adhesiveness during the formation of inner cell mass and trophectoderm in the preimplantation mouse embryo. J. Embryol. Exp. Morphol. 1982, 70, 133–152.
- Lorthongpanich, C.; Doris, T.P.; Limviphuvadh, V.; Knowles, B.B.; Solter, D. Developmental fate and lineage commitment of singled mouse blastomeres. Development 2012, 139, 3722–3731.
- Lorthongpanich, C.; Issaragrisil, S. Emerging Role of the Hippo Signaling Pathway in Position Sensing and Lineage Specification in Mammalian Preimplantation Embryos. Biol. Reprod. 2015, 92, 143.
- Lorthongpanich, C.; Messerschmidt, D.M.; Chan, S.W.; Hong, W.; Knowles, B.B.; Solter, D. Temporal reduction of LATS kinases in the early preimplantation embryo prevents ICM lineage differentiation. Genes Dev. 2013, 27, 1441–1446.
- Nishioka, N.; Inoue, K.; Adachi, K.; Kiyonari, H.; Ota, M.; Ralston, A.; Yabuta, N.; Hirahara, S.; Stephenson, R.O.; Ogonuki, N.; et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 2009, 16, 398–410.
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183.
- Chang, Y.C.; Wu, J.W.; Wang, C.W.; Jang, A.C. Hippo Signaling-Mediated Mechanotransduction in Cell Movement and Cancer Metastasis. Front. Mol. Biosci. 2019, 6, 157.
- Xu, T.; Wang, W.; Zhang, S.; Stewart, R.A.; Yu, W. Identifying tumor suppressors in genetic mosaics: The Drosophila lats gene encodes a putative protein kinase. Development 1995, 121, 1053–1063.
- Justice, R.W.; Zilian, O.; Woods, D.F.; Noll, M.; Bryant, P.J. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995, 9, 534–546.
- Wu, S.; Huang, J.; Dong, J.; Pan, D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003, 114, 445–456.
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133.
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761.
- Pan, D.J. Hippo signaling in organ size control. Genes Dev. 2007, 21, 886–897.
- Camargo, F.D.; Gokhale, S.; Johnnidis, J.B.; Fu, D.; Bell, G.W.; Jaenisch, R.; Brummelkamp, T.R. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 2007, 17, 2054–2060.
- Zhao, B.; Li, L.; Lei, Q.; Guan, K.L. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010, 24, 862–874.
- Yagi, R.; Chen, L.F.; Shigesada, K.; Murakami, Y.; Ito, Y.A. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999, 18, 2551–2562.
- Kanai, F.; Marignani, P.A.; Sarbassova, D.; Yagi, R.; Hall, R.A.; Donowitz, M.; Hisaminato, A.; Fujiwara, T.; Ito, Y.T.; Cantley, L.C.; et al. TAZ: A novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000, 19, 6778–6791.
- Yamaguchi, H.; Taouk, G.M. A Potential Role of YAP/TAZ in the Interplay Between Metastasis and Metabolic Alterations. Front. Oncol. 2020, 10, 928.
- Sun, X.; Ren, Z.; Cun, Y.; Zhao, C.; Huang, X.; Zhou, J.; Hu, R.; Su, X.; Ji, L.; Li, P.; et al. Hippo-YAP signaling controls lineage differentiation of mouse embryonic stem cells through modulating the formation of super-enhancers. Nucleic Acids Res. 2020, 48, 7182–7196.
- Plouffe, S.W.; Lin, K.C.; Moore, J.L., 3rd; Tan, F.E.; Ma, S.; Ye, Z.; Qiu, Y.; Ren, B.; Guan, K.L. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J. Biol. Chem. 2018, 293, 11230–11240.
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.Y.; Chinnaiyan, A.M.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971.
- Stein, C.; Bardet, A.F.; Roma, G.; Bergling, S.; Clay, I.; Ruchti, A.; Agarinis, C.; Schmelzle, T.; Bouwmeester, T.; Schübeler, D.; et al. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS Genet. 2015, 11, e1005465.
- Zanconato, F.; Forcato, M.; Battilana, G.; Azzolin, L.; Quaranta, E.; Bodega, B.; Rosato, A.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 2015, 17, 1218–1227.
- Zhang, J.M.; Smolen, G.A.; Haber, D.A. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 2008, 68, 2789–2794.
- Varelas, X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 2014, 141, 1614–1626.
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312.
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68.
- Singh, P.P.; Singh, S. Statins—The Holy Grail for cancer? Ann. Transl. Med. 2013, 1, 1.
- Dvash, T.; Ben-Yosef, D.; Eiges, R. Human embryonic stem cells as a powerful tool for studying human embryogenesis. Pediatr. Res. 2006, 60, 111–117.
- Takahashi, K.; Okita, K.; Nakagawa, M.; Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007, 2, 3081–3089.
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147.
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264.
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689.
- Deng, J.; Petersen, B.E.; Steindler, D.A.; Jorgensen, M.L.; Laywell, E.D. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 2006, 24, 1054–1064.
- Manochantr, S.; Marupanthorn, K.; Tantrawatpan, C.; Kheolamai, P. The expression of neurogenic markers after neuronal induction of chorion-derived mesenchymal stromal cells. Neurol. Res. 2015, 37, 545–552.
- Gu, W.; Hong, X.; Le Bras, A.; Nowak, W.N.; Issa Bhaloo, S.; Deng, J.; Xie, Y.; Hu, Y.; Ruan, X.Z.; Xu, Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J. Biol. Chem. 2018, 293, 8089–8102.
- Gong, Z.; Niklason, L.E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 2008, 22, 1635–1648.
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623.
- Serwold, T.; Ehrlich, L.I.; Weissman, I.L. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood 2009, 113, 807–815.
- Karsunky, H.; Inlay, M.A.; Serwold, T.; Bhattacharya, D.; Weissman, I.L. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood 2008, 111, 5562–5570.
- Kondo, M.; Weissman, I.L.; Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 1997, 91, 661–672.
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000, 404, 193–197.
- Nakorn, T.N.; Miyamoto, T.; Weissman, I.L. Characterization of mouse clonogenic megakaryocyte progenitors. Proc. Natl. Acad. Sci. USA 2003, 100, 205–210.
- Pronk, C.J.; Rossi, D.J.; Månsson, R.; Attema, J.L.; Norddahl, G.L.; Chan, C.K.; Sigvardsson, M.; Weissman, I.L.; Bryder, D. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 2007, 1, 428–442.
- Haas, S.; Trumpp, A.; Milsom, M.D. Causes and Consequences of Hematopoietic Stem Cell Heterogeneity. Cell Stem Cell 2018, 22, 627–638.
- Mercier, F.E.; Scadden, D.T. Not All Created Equal: Lineage Hard-Wiring in the Production of Blood. Cell 2015, 163, 1568–1570.
- Notta, F.; Zandi, S.; Takayama, N.; Dobson, S.; Gan, O.I.; Wilson, G.; Kaufmann, K.B.; McLeod, J.; Laurenti, E.; Dunant, C.F.; et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 2016, 351, aab2116.
- Paul, F.; Arkin, Y.; Giladi, A.; Jaitin, D.A.; Kenigsberg, E.; Keren-Shaul, H.; Winter, D.; Lara-Astiaso, D.; Gury, M.; Weiner, A.; et al. Transcriptional Heterogeneity and Lineage Commitment in Myeloid Progenitors. Cell 2015, 163, 1663–1677.
- Velten, L.; Haas, S.F.; Raffel, S.; Blaszkiewicz, S.; Islam, S.; Hennig, B.P.; Hirche, C.; Lutz, C.; Buss, E.C.; Nowak, D.; et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 2017, 19, 271–281.
- Macaulay, I.C.; Svensson, V.; Labalette, C.; Ferreira, L.; Hamey, F.; Voet, T.; Teichmann, S.A.; Cvejic, A. Single-Cell RNA-Sequencing Reveals a Continuous Spectrum of Differentiation in Hematopoietic Cells. Cell Rep. 2016, 14, 966–977.
- Nestorowa, S.; Hamey, F.K.; Pijuan Sala, B.; Diamanti, E.; Shepherd, M.; Laurenti, E.; Wilson, N.K.; Kent, D.G.; Göttgens, B. A single-cell resolution map of mouse hematopoietic stem and progenitor cell differentiation. Blood 2016, 128, e20–e31.
- Pina, C.; Fugazza, C.; Tipping, A.J.; Brown, J.; Soneji, S.; Teles, J.; Peterson, C.; Enver, T. Inferring rules of lineage commitment in haematopoiesis. Nat. Cell Biol. 2012, 14, 287–294.
- Wattanapanitch, M.; Damkham, N.; Potirat, P.; Trakarnsanga, K.; Janan, M.; U-Pratya, Y.; Kheolamai, P.; Klincumhom, N.; Issaragrisil, S. One-step genetic correction of hemoglobin E/beta-thalassemia patient-derived iPSCs by the CRISPR/Cas9 system. Stem Cell Res. Ther. 2018, 9, 46.
- Laowtammathron, C.; Chingsuwanrote, P.; Choavaratana, R.; Phornwilardsiri, S.; Sitthirit, K.; Kaewjunun, C.; Makemaharn, O.; Terbto, P.; Waeteekul, S.; Lorthongpanich, C.; et al. High-efficiency derivation of human embryonic stem cell lines using a culture system with minimized trophoblast cell proliferation. Stem Cell Res. Ther. 2018, 9, 138.
- Ebert, A.D.; Liang, P.; Wu, J.C. Induced pluripotent stem cells as a disease modeling and drug screening platform. J. Cardiovasc. Pharmacol. 2012, 60, 408–416.
- Jiang, Z.; Han, Y.; Cao, X. Induced pluripotent stem cell (iPSCs) and their application in immunotherapy. Cell Mol. Immunol. 2014, 11, 17–24.
- Doss, M.X.; Sachinidis, A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403.
- Zhang, X.; Zhang, S.; Wang, T. How the mechanical microenvironment of stem cell growth affects their differentiation: A review. Stem Cell Res. Ther. 2022, 13, 415.
- Cobbaut, M.; Karagil, S.; Bruno, L.; Diaz de la Loza, M.D.C.; Mackenzie, F.E.; Stolinski, M.; Elbediwy, A. Dysfunctional Mechanotransduction through the YAP/TAZ/Hippo Pathway as a Feature of Chronic Disease. Cells 2020, 9, 151.
- Hwang, J.H.; Byun, M.R.; Kim, A.R.; Kim, K.M.; Cho, H.J.; Lee, Y.H.; Kim, J.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Extracellular Matrix Stiffness Regulates Osteogenic Differentiation through MAPK Activation. PLoS ONE 2015, 10, e0135519.
- Hsiao, C.; Lampe, M.; Nillasithanukroh, S.; Han, W.; Lian, X.; Palecek, S.P. Human pluripotent stem cell culture density modulates YAP signaling. Biotechnol. J. 2016, 11, 662–675.
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646.
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012, 13, 591–600.
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770.
- Lorthongpanich, C.; Thumanu, K.; Tangkiettrakul, K.; Jiamvoraphong, N.; Laowtammathron, C.; Damkham, N.; U-Pratya, Y.; Issaragrisil, S. YAP as a key regulator of adipo-osteogenic differentiation in human MSCs. Stem Cell Res. Ther. 2019, 10, 402.
- Yuan, H.; Zhou, Y.; Lee, M.S.; Zhang, Y.; Li, W.J. A newly identified mechanism involved in regulation of human mesenchymal stem cells by fibrous substrate stiffness. Acta Biomater. 2016, 42, 247–257.
- Yang, C.; Tibbitt, M.W.; Basta, L.; Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014, 13, 645–652.
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.D.; Schwartz, Z. Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 2017, 12, e0170312.
- Karystinou, A.; Roelofs, A.J.; Neve, A.; Cantatore, F.P.; Wackerhage, H.; De Bari, C. Yes-associated protein (YAP) is a negative regulator of chondrogenesis in mesenchymal stem cells. Arthritis Res. Ther. 2015, 17, 147.
- Deng, Y.; Wu, A.; Li, P.; Li, G.; Qin, L.; Song, H.; Mak, K.K. Yap1 Regulates Multiple Steps of Chondrocyte Differentiation during Skeletal Development and Bone Repair. Cell Rep. 2016, 14, 2224–2237.
- Yang, B.; Sun, H.; Song, F.; Yu, M.; Wu, Y.; Wang, J. YAP1 negatively regulates chondrocyte differentiation partly by activating the beta-catenin signaling pathway. Int. J. Biochem. Cell Biol. 2017, 87, 104–113.
- Li, Y.; Yang, S.; Qin, L.; Yang, S. TAZ is required for chondrogenesis and skeletal development. Cell Discov. 2021, 7, 26.
- Zhong, W.; Tian, K.; Zheng, X.; Li, L.; Zhang, W.; Wang, S.; Qin, J. Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by Yes-associated protein. Stem Cells Dev. 2013, 22, 2083–2093.
- Klincumhom, N.; Lorthongpanich, C.; Thumanu, K.; Septham, P.; Phomyu, W.; Issaragrisil, S.; Pavasant, P. Intermittent compressive force regulates human periodontal ligament cell behavior via yes-associated protein. Heliyon 2022, 8, e10845.
- Nieto-Miguel, T.; Galindo, S.; Reinoso, R.; Corell, A.; Martino, M.; Pérez-Simón, J.A.; Calonge, M. In vitro simulation of corneal epithelium microenvironment induces a corneal epithelial-like cell phenotype from human adipose tissue mesenchymal stem cells. Curr. Eye Res. 2013, 38, 933–944.
- Chavez-Munoz, C.; Nguyen, K.T.; Xu, W.; Hong, S.J.; Mustoe, T.A.; Galiano, R.D. Transdifferentiation of adipose-derived stem cells into keratinocyte-like cells: Engineering a stratified epidermis. PLoS ONE 2013, 8, e80587.
- Wang, K.C.; Yeh, Y.T.; Nguyen, P.; Limqueco, E.; Lopez, J.; Thorossian, S.; Guan, K.L.; Li, Y.J.; Chien, S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 11525–11530.
- Nakajima, H.; Yamamoto, K.; Agarwala, S.; Terai, K.; Fukui, H.; Fukuhara, S.; Ando, K.; Miyazaki, T.; Yokota, Y.; Schmelzer, E.; et al. Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Dev. Cell 2017, 40, 523–536.
- Lundin, V.; Sugden, W.W.; Theodore, L.N.; Sousa, P.M.; Han, A.; Chou, S.; Wrighton, P.J.; Cox, A.G.; Ingber, D.E.; Goessling, W.; et al. YAP Regulates Hematopoietic Stem Cell Formation in Response to the Biomechanical Forces of Blood Flow. Dev. Cell 2020, 52, 446–460.
- Wang, L.; Luo, J.Y.; Li, B.; Tian, X.Y.; Chen, L.J.; Huang, Y.; Liu, J.; Deng, D.; Lau, C.W.; Wan, S.; et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 2016, 540, 579–582.
- Gumbiner, B.M. and N.G. Kim, The Hippo-YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 2014, 127 Pt 4, 709–717.
- Ferguson, G.B.; Martinez-Agosto, J.A. Kicking it up a Notch for the best in show: Scalloped leads Yorkie into the haematopoietic arena. Fly 2014, 8, 206–217.
- Ferguson, G.B.; Martinez-Agosto, J.A. Yorkie and Scalloped signaling regulates Notch-dependent lineage specification during Drosophila hematopoiesis. Curr. Biol. 2014, 24, 2665–2672.
- Milton, C.C.; Grusche, F.A.; Degoutin, J.L.; Yu, E.; Dai, Q.; Lai, E.C.; Harvey, K.F. The Hippo pathway regulates hematopoiesis in Drosophila melanogaster. Curr. Biol. 2014, 24, 2673–2680.
- Varelas, X.; Sakuma, R.; Samavarchi-Tehrani, P.; Peerani, R.; Rao, B.M.; Dembowy, J.; Yaffe, M.B.; Zandstra, P.W.; Wrana, J.L. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 2008, 10, 837–848.
- Goode, D.K.; Obier, N.; Vijayabaskar, M.S.; Lie-A-Ling, M.; Lilly, A.J.; Hannah, R.; Lichtinger, M.; Batta, K.; Florkowska, M.; Patel, R.; et al. Dynamic Gene Regulatory Networks Drive Hematopoietic Specification and Differentiation. Dev. Cell 2016, 36, 572–587.
- Althoff, M.J.; Nayak, R.C.; Hegde, S.; Wellendorf, A.M.; Bohan, B.; Filippi, M.D.; Xin, M.; Lu, Q.R.; Geiger, H.; Zheng, Y.; et al. Yap1-Scribble polarization is required for hematopoietic stem cell division and fate. Blood 2020, 136, 1824–1836.
- Jansson, L.; Larsson, J. Normal Hematopoietic Stem Cell Function in Mice with Enforced Expression of the Hippo Signaling Effector YAP1. PLoS ONE 2012, 7, e32013.
- Donato, E.; Biagioni, F.; Bisso, A.; Caganova, M.; Amati, B.; Campaner, S. YAP and TAZ are dispensable for physiological and malignant haematopoiesis. Leukemia 2018, 32, 2037–2040.
- Lee, D.H.; Kim, T.S.; Lee, D.; Lim, D.S. Mammalian sterile 20 kinase 1 and 2 are important regulators of hematopoietic stem cells in stress condition. Sci. Rep. 2018, 8, 942.
- Ni, X.H.; Tao, J.; Barbi, J.; Chen, Q.; Park, B.V.; Li, Z.; Zhang, N.; Lebid, A.; Ramaswamy, A.; Wei, P.; et al. YAP Is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2018, 8, 1026–1043.
- Geng, J.; Yu, S.; Zhao, H.; Sun, X.; Li, X.; Wang, P.; Xiong, X.; Hong, L.; Xie, C.; Gao, J.; et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of T(H)17 cells and T-reg cells. Nat. Immunol. 2017, 18, 800–812.
- Meng, K.P.; Majedi, F.S.; Thauland, T.J.; Butte, M.J. Mechanosensing through YAP controls T cell activation and metabolism. J. Exp. Med. 2020, 217, e20200053.
- Lorthongpanich, C.; Jiamvoraphong, N.; Supraditaporn, K.; Klaihmon, P.; U-Pratya, Y.; Issaragrisil, S. The Hippo pathway regulates human megakaryocytic differentiation. Thromb. Haemost. 2017, 117, 116–126.
- Hao, S.; Matsui, Y.; Lai, Z.C.; Paulson, R.F. Yap1 promotes proliferation of transiently amplifying stress erythroid progenitors during erythroid regeneration. Exp. Hematol. 2019, 80, 42–54.e4.
- Damkham, N.; Lorthongpanich, C.; Klaihmon, P.; Lueangamornnara, U.; Kheolamai, P.; Trakarnsanga, K.; Issaragrisil, S. YAP and TAZ play a crucial role in human erythrocyte maturation and enucleation. Stem Cell Res. Ther. 2022, 13, 467.
More
Information
Subjects:
Developmental Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
06 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




