Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shaojuan Huang | -- | 2299 | 2022-12-06 02:08:53 | | | |
| 2 | Jessie Wu | + 1 word(s) | 2300 | 2022-12-06 05:46:17 | | | | |
| 3 | Jessie Wu | Meta information modification | 2300 | 2022-12-06 05:48:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Huang, S.; Dong, M.; Chen, Q. Clinical Applications of Targeting Exosomes. Encyclopedia. Available online: https://encyclopedia.pub/entry/38045 (accessed on 07 February 2026).
Huang S, Dong M, Chen Q. Clinical Applications of Targeting Exosomes. Encyclopedia. Available at: https://encyclopedia.pub/entry/38045. Accessed February 07, 2026.
Huang, Shaojuan, Ming Dong, Qiang Chen. "Clinical Applications of Targeting Exosomes" Encyclopedia, https://encyclopedia.pub/entry/38045 (accessed February 07, 2026).
Huang, S., Dong, M., & Chen, Q. (2022, December 06). Clinical Applications of Targeting Exosomes. In Encyclopedia. https://encyclopedia.pub/entry/38045
Huang, Shaojuan, et al. "Clinical Applications of Targeting Exosomes." Encyclopedia. Web. 06 December, 2022.
Copy Citation
Breast cancer metastasis is a complicated process involving multiple physiological changes, and lung, brain, bone and liver are the main metastatic targets. Exosomes are membrane-bound extracellular vesicles that contain secreted cellular constitutes. The biogenesis and functions of exosomes in cancer have been intensively studied, and mounting studies have indicated that exosomes play a crucial role in cancer metastasis.
breast cancer
exosome
organotropism
1. Introduction
Breast cancer is a common frequently occurring malignancy among women worldwide, and it has surpassed lung cancer to become the most diagnosed cancer all over the world with around 2.3 million new cases, accounting for 11.7% of all cancer cases and 24.5% of female cancers [1]. With recent advances in early diagnosis and therapeutic strategies including neoadjuvant therapy, endocrine therapy, molecular targeted therapy, and immunotherapy [2][3], the prognosis of breast cancer has greatly improved. However, breast cancer patients with distant metastasis have worse outcomes, and the five-year survival rate was less than 30% [4]. With approximately 685,000 deaths in 2020, it remains the first leading cause of cancer death in women [1]. Therefore, there is an urgent need to understand the molecular mechanisms underlying breast cancer metastasis for developing novel therapeutic strategies. Exosomes, as one type of extracellular vesicle (EVs), have been reported to play a crucial role in cancer metastasis, namely, contributing to form pre-metastatic niches, influence the tumor microenvironment, and identify specific organotropic metastasis. Here, researchers endeavor to highlight the role of tumor-derived exosomes in breast cancer metastasis, elucidate the underlying mechanism of metastasis organotropism mediated by exosomes, and prospect the potential application of exosomes in breast cancer therapeutics.
1.1. Breast Cancer Classification
Breast cancer develops from epithelial cells in the terminal duct lobular units and can be classified into two subtypes histologically, including ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) [5][6]. According to the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 labelling index (Ki-67) which reflects the proliferation [7], breast cancer has four primary molecular subtypes, namely luminal A, luminal B, HER2-positive, and triple-negative breast cancer (TNBC) [5][8]. The luminal A subtype is ER/PR-positive, with lower levels of Ki-67 (<14), accounting for about 60–70% of diagnostic breast cancers. The luminal B subtype is ER-positive combined with HER-2 positive or Ki-67 high (≥14), accounting for about 10–20%. Both luminal A and luminal B breast cancer are likely to benefit from endocrine therapy, and patients with luminal A breast cancer have a better prognosis compared to luminal B. HER2-positive subtype is ER/PR-negative and HER2-positive, with a diagnostic rate of approximately 13−15%. This subtype can benefit from treatment targeted to HER2 and chemotherapy with good prognosis. TNBC is characterized by ER, PR, and HER2 negativity in 10−15% of cases. Breast cancer susceptibility gene 1 (BRCA1) is a major breast cancer suppressor gene that encodes a protein critical for maintaining genome stability; its mutation predisposes women to TNBC [9]. As BRCA1 mutation impairs homologous recombination repair, poly (ADP-ribose) polymerase (PARP) inhibitors have been approved as target therapy for metastatic TNBC [10]. However, due to its highly aggressive clinical properties, TNBC still has a poorer prognosis compared to other breast cancer subtypes [2][5][11][12]. Although the five-year survival rate for women diagnosed with breast cancer exceeds 90%, all breast cancer subtypes have the potential to exhibit adverse clinic features, such as high invasiveness and recurrence, mainly caused by metastasis [5].
1.2. Breast Cancer Metastasis
Metastasis occurs frequently and accounts for as much as 90% of cancer-related deaths [13]. Breast cancer metastasis is also frequently diagnosed and exhibits organ tropism to lung, brain, bone and liver, which is highly heterogeneous and affects treatment outcomes and patient prognosis [14]. As shown in Figure 1, breast cancer patients are most prone to bone metastasis, accounting for 50.7−68.8% of all metastatic cases with different molecular subtypes. The lung and liver were similar, with 16.0−23.9% and 13.3−19.7% of breast cancer metastasis occurring in the lung or liver, respectively. The incidence of brain metastasis is approximately 1.9−5.7% of all metastatic cases. Based on molecular subtypes, lung metastasis most commonly occurs in TNBC, accounting for about 32% of patients with metastasis, while the HER2-positive subtype is likely to have liver metastasis [15][16].
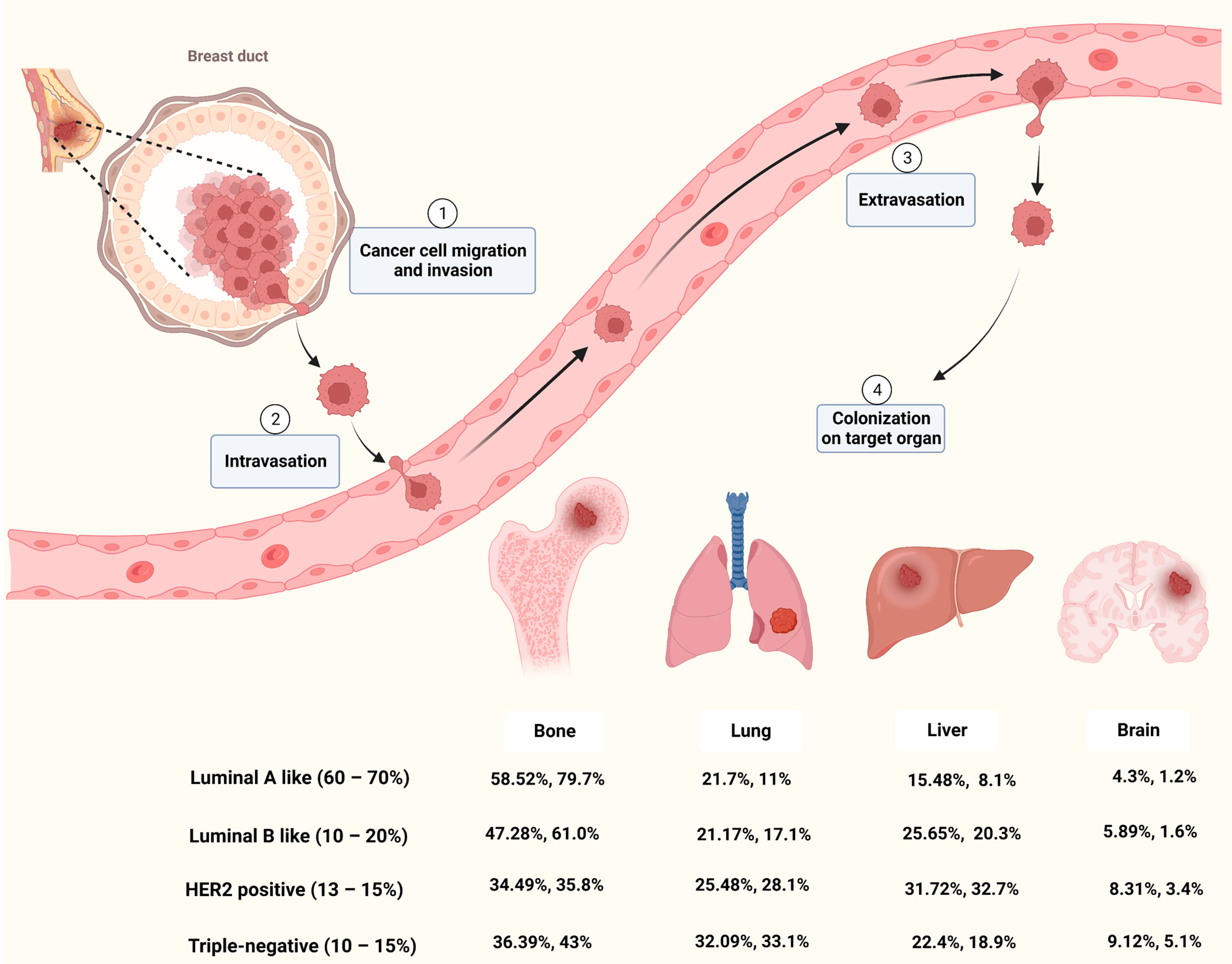
Cancer metastasis is a complicated process that goes through multiple steps such as invasion, intravasation, extravasation and colonization on target organ (Figure 1). Metastasis of tumor cells to distant organs requires not only tumor cell invasiveness, but also a microenvironment that is conducive to tumor survival and proliferation in secondary organs [17]. Although the molecular mechanisms of breast cancer metastasis are not fully understood, mounting studies have shown that primary cancer cells could secrete factors to remodel the microenvironment of the target organ and prime it into a site favorable for cancer cell proliferation, known as pre-metastatic niche (PMN) [18][19][20]. Among these factors, EVs secreted by cancer cells play important roles in microenvironment remodeling and metastatic organotropism [21].
1.3. Extracellular Vesicle and Exosome
EVs are membrane-bound vesicles secreted by various cells which play critical roles in cell-cell communication under physiological and pathological conditions [22]. In general, EVs could be divided into three types based on their morphology: exosomes (30–150 nm), micro-vesicles (150–1000 nm) and apoptotic bodies (500–2000 nm) [23]. Nowadays, EVs are considered non-negligible factors in cellular homeostasis and mediators of cancer metastasis [24]. Exosomes are small EVs with phospholipid bilayers whose heterogeneous “cargoes”, such as protein, lipids, RNA and DNA, vary from different types of cells [25][26]. These cargoes are located inside or on the surface of exosomes and mediate the communication between original cells and recipient cells [26].
Exosomes are generated originally from early endosomes by fusion of endocytic vesicles with plasma membranes. Early endosomes subsequently mature into late endosomes and form the multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs). MVBs could either fuse with plasma membrane for exocytosis of contained ILVs (namely exosomes) into the extracellular space, or with lysosomes or autophagosomes for degradation [27][28]. ILV formation is largely dependent on the endosomal sorting complex required for transport (ESCRT) function. ESCRT is an intricate machinery which is made up of five complexes, which include the ESCRT-0, -Ⅰ, -Ⅱ, -Ⅲ and Vps4-Vta1 complexes [27][29]. Beside the ESCRT-dependent pathway, an alternative pathway could also be involved in exosome biogenesis. Proteolipid proteins (PLP) could be incorporated into ILVs in a sphingolipid ceramide-dependent manner. Then, ceramide induces raft-based microdomains formation and coalescence and promotes the budding of ILVs [30]. Moreover, tetraspanins, such as CD63, CD81, CD9, could regulate ESCRT-independent sorting [27]. Therefore, ESCRT dependent and independent mechanisms co-exist and function synergistically in exosome formation.
In recent years, studies have found that cancer-derived exosome could promote the progression of cancer, including cancer metastasis and drug resistance [31]. Exosomes have been proved to be involved in various processes of cancer metastasis, such as vascular leakiness, epithelial-mesenchymal transition (EMT) induction, immune escape and PMN formation [24][32][33]. Integrins are cell surface adhesion molecules that could mediate cell signaling by interacting with components of the extracellular matrix [34]. It has been demonstrated that integrins on exosomes could guide them into different organs, thereby inducing PMN formation and promoting cancer metastasis. Exosomes with integrins α6β4 and α6β1 tend to accumulate in the lung, while integrin αvβ5 preferably drives exosomes into the liver [35]. In addition, comparative proteomics of exosomes derived from different breast cancer cells demonstrated that their exosomal proteins are heterogenous, which is associated with different cancer cell metastatic properties [36]. These studies suggest that exosomes play a crucial role in causing metastasis organotropism through various mechanisms. In the following section, researchers will summarize the contribution of exosomes to the tropism of breast cancer metastasis to different organs, namely bone, lung, liver and brain, and the metastatic models used in these findings (Table 1), which will help to comprehensively decipher how exosomes are involve in breast cancer metastasis.
Table 1. Breast cancer models used to study the mechanism of exosomes promoting metastasis.
| Metastatic Organs | Exosomal Molecules | Cell Lines | Metastasis Mouse Model | Reference |
|---|---|---|---|---|
| Bone | miR-940 | MDA-MB-231 | Calvaria implantation | [37] |
| miR-218 | MDA-MB-231, MCF-7 | Tail vein injection of EVs | [38] | |
| miR-20a-5p | MDA-MB-231, MCF-7 | In vitro | [39] | |
| miR-21 | MDA-MB-231 | Orthotopic model and Caudal artery injection | [40] | |
| miR-19a, IBSP | MDA-MB-231, MCF7, T47D | Intra-cardiac model, intratibial implantation and orthotopic model | [41] | |
| L-plastin | MDA-MB-231 | Intratibial implantation | [42] | |
| CDH11, ITGA5 | MDA-MB-231, 4T1, MCF7 |
Intra-cardiac model and orthotopic model | [43] | |
| lung | miR-122 | MDA-MB-231, MCF10DCIS.com | Intra-cardiac model and orthotopic model | [44] |
| miR-138-5p | 4T1 | Tail vein injection | [45] | |
| miR-183-5p | 4T1 | Orthotopic model | [46] | |
| miR-200b-3p | 4T1 | Orthotopic model | [47] | |
| Let-7 | 4TO7 | Orthotopic model, tail vein injection and intra-cardiac model | [48] | |
| circPSMA1 | MDA-MB-231 | Orthotopic model | [49] | |
| CCL2 | EO771 | Tail vein injection | [50] | |
| TβRII | MDA-MB-231, 4T1, 4T07 | Intra-cardiac model, tail vein injection and orthotopic model | [51] | |
| Myosin-9 | MDA-MB-231 | Subcutaneous xenograft, orthotopic model | [52] | |
| MMPs | MDA-MB-231 | Tail vein injection, orthotopic model | [53] | |
| MMP-1 | MDA-MB-231 | Tail vein injection | [54] | |
| NDPK | MDA-MB-231 | Tail vein injection | [55] | |
| Annexin II | MDA-MB-231, MDA-MB-831, MDA-MB-4175 | Tail vein injection and intra-cardiac model | [56] | |
| Liver | miR-4443 | MCF-7, MDA-MB-231 | Orthotopic model | [57] |
| miR-197 | MBA-MB-231 or SUM149PT | Subcutaneous injection and tail vein injection | [58] | |
| E-cadherin, p120-catenin | MTPa | Orthotopic model (Rat) | [59] | |
| TGFβ1 | MCF7, MDA-MB-231 | In vitro | [60] | |
| Brain | miR-181c | MDA-MB-231 | Intra-cardiac model | [61] |
| lnc GS1-600G8.5 | MDA-MB-231 | Intra-cardiac model | [62] | |
| CEMIP | MDA-MB-231 | Intra-cardiac model, intracranial injection and orthotopic model | [63] | |
| miR-503 | MCF7, ZR75-1, SKBR3, MDA-MB-231 |
Intra-cardiac model and intracranial injection | [64] | |
| miR-301a-3p | MDA-MB-231 | Retro-orbital injection of EVs | [65] |
2. Clinical Application
2.1. Exosomes as Diagnostic Biomarkers for Breast Cancer
With the convenience of being non-invasive and highly efficient, liquid biopsy brings an opportunity for cancer diagnosis with detection through various body fluids such as blood or urine, rather than invasive methods to remove a piece of cancerous tissue [66]. Liquid biopsy has made some progress in establishing the diagnosis of various cancers by using certain molecules as biomarkers, including CTCs, circulating tumor DNA (ctDNA), tumor-educated platelet (TEP) and exosomes [67][68]. As mentioned above, exosomes contain specific “cargoes” derived from metastatic cancer cells, which not only play an important role in breast cancer progression and metastasis but may also have the potential to be biomarkers for diagnosing metastasis. To discover exosome-based biomarkers, Wang et al. established a comprehensive database—ExoBCD—by combining four high-throughput datasets, transcriptome of 1191 TCGA cases and manual mining of 950 studies. The database identified 306 valuable exosomal molecules, including 49 potential biomarkers and 257 biologically interesting molecules [69]. Since traditional detection methods, such as real-time PCR and Western Blotting analysis, are time-consuming and laborious and require exosome enrichment, which make them unsuitable for exosome-based diagnosis, it is necessary to develop alternative methods. A rapid, sensitive, and low-cost thermophoretic aptasensor (TAS) was developed for the analysis of cancer-associated protein profiles of plasma EVs. Based on this analysis, the EV protein signature was established and used to accurately monitor and predict metastatic breast cancer [70]. Kwizera et al. developed an inexpensive and highly efficient device based on the surface-enhanced Raman scattering (SERS) to detect exosomes and exosomal protein profiles. Using this device, they identified exosomal HER2 and EpCAM as biomarkers in the plasma of HER2-positive breast cancer patients [71]. Then, Lee et al. established another SERS-based platform to detect and quantify exosomal miRNAs in serum for breast cancer diagnosis [72]. In addition, a nano-sized fluorescent oligonucleotide probe-molecular beacon has also been developed for the measurement of miRNAs in blood exosomes with high sensitivity and specificity, such as miR-21 [73][74], miR-27a, miR-375 [74], and miR-1246 [75]. Recently, a microfluidic chip-based exosomal mRNA sensor was developed to directly detect exosomal ERBB2 in blood for the diagnosis of HER2-positive breast cancer [76]. Therefore, technological advances will enable exosomes to be used as biomarkers for breast cancer diagnosis in the future.
2.2. Engineered Exosomes for Therapeutics of Breast Cancer
The natural characteristics of exosomes, such as low toxicity, low immunogenicity, high-flexibility engineering, and inherent targeting and interaction with recipient cells, make them ideal drug carriers for breast cancer therapy [77][78][79][80]. First, exosomes can serve as delivery vesicles for chemotherapeutic drugs such as doxorubicin, with some engineering modification on their surface to improve their targeting efficiency and reduce side effects [81]. Hydrophobic drugs, such as Aspirin, could also be loaded into exosomes to increase their solubility and enhance their cytotoxicity against cancer cells [82]. In addition, to enhance the efficacy of PARP inhibitors, exosomes isolated from TNBC cells were loaded with Olaparib (PARP inhibitor) and superparamagnetic iron oxide (SPIO) nanoparticles, which could be tracked by magnetic particle imaging (MPI) and also effectively inhibited tumor growth [83]. Second, functional small RNAs, such as siRNA and miRNA, can be packaged into exosomes and delivered to cancer cells to downregulate target genes, thereby inhibiting cancer progression [84][85][86][87]. Third, engineered exosomes can be used as vaccines to stimulate an immune response against tumor cells. A novel exosome-like nanoparticles was developed from fibroblast activation protein-α (FAP) engineered cancer cells as a tumor vaccine, which induced robust and specific cytotoxic T lymphocyte immunity against tumor cells and reprogrammed the immunosuppressive microenvironment [88]. Exosomes from α-lactalbumin overexpressing breast cancer cells were packaged together with immunogenic cell death inducers—human neutrophil elastase (ELANE) and Hiltonol (a TLR3 agonist) to construct a vaccine, thereby priming dendritic cells in situ and improving subsequent tumor-reactive CD8+ T cell responses [89]. Exosome can also be engineered to display both anti-CD3 and anti-HER2 antibodies to mediate cytotoxic T cells that directly target HER2-positive breast cancer and improve immunotherapy [90]. Fourth, exosomes can be utilized as nanocarriers to provide cancer-targeted sonosensitizers for sonodynamic therapy (SDT), which employs reactive oxygen species (ROS) generated by ultrasonic excitation to kill cancer cells [91]. Indocyanine green-loaded exosomes were surface-modified with cancer-binding ligand to increase their target specificity, resulting in greater SDT against cancer cells [92]. Sinoporphyrin sodium (DVDMs) could also be loaded into tumor-derived exosomes to increase its efficiency in SDT [93]. Exosomes can be used not only for primary tumor therapy, but also be engineered to treat breast cancer metastasis. As exosomes derived from metastatic breast cancers have natural organotropism to lung [35] and brain [94], therapeutic drugs can be encapsulated with exosomes to target the relevant metastatic foci. Gold nanorods, a nanomaterial for photothermal therapy, were loaded into lung metastatic cancer cells-derived exosomes, exhibiting better therapeutic effects on lung metastases [95]. Finally, more engineered exosomes will be developed to improve precision therapy for breast cancers, especially with regard to metastasis.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- McDonald, E.S.; Clark, A.S.; Tchou, J.; Zhang, P.; Freedman, G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. 1), 9s–16s.
- Emens, L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 511–520.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66.
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31.
- Zhang, A.; Wang, X.; Fan, C.; Mao, X. The Role of Ki67 in Evaluating Neoadjuvant Endocrine Therapy of Hormone Receptor-Positive Breast Cancer. Front. Endocrinol. 2021, 12, 687244.
- Cheang, M.C.; Martin, M.; Nielsen, T.O.; Prat, A.; Voduc, D.; Rodriguez-Lescure, A.; Ruiz, A.; Chia, S.; Shepherd, L.; Ruiz-Borrego, M.; et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist 2015, 20, 474–482.
- Ye, F.; He, M.; Huang, L.; Lang, G.; Hu, X.; Shao, Z.; Di, G.; Cao, A. Insights Into the Impacts of BRCA Mutations on Clinicopathology and Management of Early-Onset Triple-Negative Breast Cancer. Front. Oncol. 2020, 10, 574813.
- Won, K.A.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261.
- Gao, J.J.; Swain, S.M. Luminal A Breast Cancer and Molecular Assays: A Review. Oncologist 2018, 23, 556–565.
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61.
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564.
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2020, 60, 14–27.
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990–27996.
- Gong, Y.; Liu, Y.R.; Ji, P.; Hu, X.; Shao, Z.M. Impact of molecular subtypes on metastatic breast cancer patients: A SEER population-based study. Sci. Rep. 2017, 7, 45411.
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101.
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695.
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal. Transduct. Target. Ther. 2020, 5, 28.
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016, 30, 668–681.
- Wen, S.W.; Sceneay, J.; Lima, L.G.; Wong, C.S.; Becker, M.; Krumeich, S.; Lobb, R.J.; Castillo, V.; Wong, K.N.; Ellis, S.; et al. The Biodistribution and Immune Suppressive Effects of Breast Cancer-Derived Exosomes. Cancer Res. 2016, 76, 6816–6827.
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228.
- Dong, M.; Liu, Q.; Xu, Y.; Zhang, Q. Extracellular Vesicles: The Landscape in the Progression, Diagnosis, and Treatment of Triple-Negative Breast Cancer. Front. Cell. Dev. Biol. 2022, 10, 842898.
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848.
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514.
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics 2010, 73, 1907–1920.
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell. Commun. Signal. 2021, 19, 47.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367.
- Gatta, A.T.; Carlton, J.G. The ESCRT-machinery: Closing holes and expanding roles. Curr. Opin. Cell. Biol. 2019, 59, 121–132.
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247.
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75.
- Steinbichler, T.B.; Dudás, J.; Riechelmann, H.; Skvortsova, I.I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181.
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.P.; Goh, B.C. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617.
- Radisky, D.; Muschler, J.; Bissell, M.J. Order and disorder: The role of extracellular matrix in epithelial cancer. Cancer Investig 2002, 20, 139–153.
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335.
- Gangoda, L.; Liem, M.; Ang, C.S.; Keerthikumar, S.; Adda, C.G.; Parker, B.S.; Mathivanan, S. Proteomic Profiling of Exosomes Secreted by Breast Cancer Cells with Varying Metastatic Potential. Proteomics 2017, 17.
- Hashimoto, K.; Ochi, H.; Sunamura, S.; Kosaka, N.; Mabuchi, Y.; Fukuda, T.; Yao, K.; Kanda, H.; Ae, K.; Okawa, A.; et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. USA 2018, 115, 2204–2209.
- Liu, X.; Cao, M.; Palomares, M.; Wu, X.; Li, A.; Yan, W.; Fong, M.Y.; Chan, W.C.; Wang, S.E. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res. 2018, 20, 127.
- Guo, L.; Zhu, Y.; Li, L.; Zhou, S.; Yin, G.; Yu, G.; Cui, H. Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer Med. 2019, 8, 5687–5701.
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429–1445.
- Wu, K.; Feng, J.; Lyu, F.; Xing, F.; Sharma, S.; Liu, Y.; Wu, S.Y.; Zhao, D.; Tyagi, A.; Deshpande, R.P.; et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat. Commun. 2021, 12, 5196.
- Tiedemann, K.; Sadvakassova, G.; Mikolajewicz, N.; Juhas, M.; Sabirova, Z.; Tabariès, S.; Gettemans, J.; Siegel, P.M.; Komarova, S.V. Exosomal Release of L-Plastin by Breast Cancer Cells Facilitates Metastatic Bone Osteolysis. Transl. Oncol. 2019, 12, 462–474.
- Li, X.Q.; Zhang, R.; Lu, H.; Yue, X.M.; Huang, Y.F. Extracellular Vesicle-Packaged CDH11 and ITGA5 Induce the Premetastatic Niche for Bone Colonization of Breast Cancer Cells. Cancer Res. 2022, 82, 1560–1574.
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.; Li, S.; Chin, A.R.; et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194.
- Xun, J.; Du, L.; Gao, R.; Shen, L.; Wang, D.; Kang, L.; Chen, C.; Zhang, Z.; Zhang, Y.; Yue, S.; et al. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics 2021, 11, 6847–6859.
- Guo, J.; Duan, Z.; Zhang, C.; Wang, W.; He, H.; Liu, Y.; Wu, P.; Wang, S.; Song, M.; Chen, H.; et al. Mouse 4T1 Breast Cancer Cell-Derived Exosomes Induce Proinflammatory Cytokine Production in Macrophages via miR-183. J. Immunol. 2020, 205, 2916–2925.
- Gu, P.; Sun, M.; Li, L.; Yang, Y.; Jiang, Z.; Ge, Y.; Wang, W.; Mu, W.; Wang, H. Breast Tumor-Derived Exosomal MicroRNA-200b-3p Promotes Specific Organ Metastasis Through Regulating CCL2 Expression in Lung Epithelial Cells. Front. Cell Dev. Biol. 2021, 9, 657158.
- Qi, M.; Xia, Y.; Wu, Y.; Zhang, Z.; Wang, X.; Lu, L.; Dai, C.; Song, Y.; Xu, K.; Ji, W.; et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat. Commun. 2022, 13, 897.
- Yang, S.J.; Wang, D.D.; Zhong, S.L.; Chen, W.Q.; Wang, F.L.; Zhang, J.; Xu, W.X.; Xu, D.; Zhang, Q.; Li, J.; et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/β-catenin (cyclin D1) axis. Cell Death Dis. 2021, 12, 420.
- Lima, L.G.; Ham, S.; Shin, H.; Chai, E.P.Z.; Lek, E.S.H.; Lobb, R.J.; Müller, A.F.; Mathivanan, S.; Yeo, B.; Choi, Y.; et al. Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution. Nat. Commun. 2021, 12, 3543.
- Xie, F.; Zhou, X.; Su, P.; Li, H.; Tu, Y.; Du, J.; Pan, C.; Wei, X.; Zheng, M.; Jin, K.; et al. Breast cancer cell-derived extracellular vesicles promote CD8(+) T cell exhaustion via TGF-β type II receptor signaling. Nat. Commun. 2022, 13, 4461.
- Feng, L.; Weng, J.; Yao, C.; Wang, R.; Wang, N.; Zhang, Y.; Tanaka, Y.; Su, L. Extracellular Vesicles Derived from SIPA1(high) Breast Cancer Cells Enhance Macrophage Infiltration and Cancer Metastasis through Myosin-9. Biology 2022, 11, 543.
- Lin, Q.; Chen, X.; Meng, F.; Ogawa, K.; Li, M.; Song, R.; Zhang, S.; Zhang, Z.; Kong, X.; Xu, Q.; et al. ASPH-notch Axis guided Exosomal delivery of Prometastatic Secretome renders breast Cancer multi-organ metastasis. Mol. Cancer 2019, 18, 156.
- Zhu, Y.; Tao, Z.; Chen, Y.; Lin, S.; Zhu, M.; Ji, W.; Liu, X.; Li, T.; Hu, X. Exosomal MMP-1 transfers metastasis potential in triple-negative breast cancer through PAR1-mediated EMT. Breast Cancer Res. Treat. 2022, 193, 65–81.
- Duan, S.; Nordmeier, S.; Byrnes, A.E.; Buxton, I.L.O. Extracellular Vesicle-Mediated Purinergic Signaling Contributes to Host Microenvironment Plasticity and Metastasis in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 597.
- Maji, S.; Chaudhary, P.; Akopova, I.; Nguyen, P.M.; Hare, R.J.; Gryczynski, I.; Vishwanatha, J.K. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol. Cancer Res. 2017, 15, 93–105.
- Wang, J.; Zhang, Q.; Wang, D.; Yang, S.; Zhou, S.; Xu, H.; Zhang, H.; Zhong, S.; Feng, J. Microenvironment-induced TIMP2 loss by cancer-secreted exosomal miR-4443 promotes liver metastasis of breast cancer. J. Cell Physiol. 2020, 235, 5722–5735.
- Li, L.; Xiong, Y.; Wang, N.; Zhu, M.; Gu, Y. Breast Cancer Stem Cells-derived Extracellular Vesicles Affect PPARG Expression by Delivering MicroRNA-197 in Breast Cancer Cells. Clin. Breast Cancer 2022, 22, 478–490.
- Voglstaetter, M.; Thomsen, A.R.; Nouvel, J.; Koch, A.; Jank, P.; Navarro, E.G.; Gainey-Schleicher, T.; Khanduri, R.; Groß, A.; Rossner, F.; et al. Tspan8 is expressed in breast cancer and regulates E-cadherin/catenin signalling and metastasis accompanied by increased circulating extracellular vesicles. J. Pathol. 2019, 248, 421–437.
- Kim, J.; Lee, C.; Kim, I.; Ro, J.; Kim, J.; Min, Y.; Park, J.; Sunkara, V.; Park, Y.S.; Michael, I.; et al. Three-Dimensional Human Liver-Chip Emulating Premetastatic Niche Formation by Breast Cancer-Derived Extracellular Vesicles. ACS Nano 2020, 14, 14971–14988.
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015, 6, 6716.
- Lu, Y.; Chen, L.; Li, L.; Cao, Y. Exosomes Derived from Brain Metastatic Breast Cancer Cells Destroy the Blood-Brain Barrier by Carrying lncRNA GS1-600G8.5. Biomed Res. Int. 2020, 2020, 7461727.
- Rodrigues, G.; Hoshino, A.; Kenific, C.M.; Matei, I.R.; Steiner, L.; Freitas, D.; Kim, H.S.; Oxley, P.R.; Scandariato, I.; Casanova-Salas, I.; et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat. Cell Biol. 2019, 21, 1403–1412.
- Xing, F.; Liu, Y.; Wu, S.Y.; Wu, K.; Sharma, S.; Mo, Y.Y.; Feng, J.; Sanders, S.; Jin, G.; Singh, R.; et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res. 2018, 78, 4316–4330.
- Morad, G.; Daisy, C.C.; Otu, H.H.; Libermann, T.A.; Dillon, S.T.; Moses, M.A. Cdc42-Dependent Transfer of mir301 from Breast Cancer-Derived Extracellular Vesicles Regulates the Matrix Modulating Ability of Astrocytes at the Blood-Brain Barrier. Int. J. Mol. Sci. 2020, 21, 3851.
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455.
- Ye, Q.; Ling, S.; Zheng, S.; Xu, X. Liquid biopsy in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA. Mol. Cancer 2019, 18, 114.
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y.; et al. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer 2022, 21, 86.
- Wang, X.; Chai, Z.; Pan, G.; Hao, Y.; Li, B.; Ye, T.; Li, Y.; Long, F.; Xia, L.; Liu, M. ExoBCD: A comprehensive database for exosomal biomarker discovery in breast cancer. Brief Bioinform. 2021, 22, bbaa088.
- Tian, F.; Zhang, S.; Liu, C.; Han, Z.; Liu, Y.; Deng, J.; Li, Y.; Wu, X.; Cai, L.; Qin, L.; et al. Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat. Commun. 2021, 12, 2536.
- Kwizera, E.A.; O’Connor, R.; Vinduska, V.; Williams, M.; Butch, E.R.; Snyder, S.E.; Chen, X.; Huang, X. Molecular Detection and Analysis of Exosomes Using Surface-Enhanced Raman Scattering Gold Nanorods and a Miniaturized Device. Theranostics 2018, 8, 2722–2738.
- Lee, J.U.; Kim, W.H.; Lee, H.S.; Park, K.H.; Sim, S.J. Quantitative and Specific Detection of Exosomal miRNAs for Accurate Diagnosis of Breast Cancer Using a Surface-Enhanced Raman Scattering Sensor Based on Plasmonic Head-Flocked Gold Nanopillars. Small 2019, 15, e1804968.
- Lee, J.H.; Kim, J.A.; Kwon, M.H.; Kang, J.Y.; Rhee, W.J. In situ single step detection of exosome microRNA using molecular beacon. Biomaterials 2015, 54, 116–125.
- Wang, H.; He, D.; Wan, K.; Sheng, X.; Cheng, H.; Huang, J.; Zhou, X.; He, X.; Wang, K. In situ multiplex detection of serum exosomal microRNAs using an all-in-one biosensor for breast cancer diagnosis. Analyst 2020, 145, 3289–3296.
- Chen, Y.; Zhai, L.Y.; Zhang, L.M.; Ma, X.S.; Liu, Z.; Li, M.M.; Chen, J.X.; Duan, W.J. Breast cancer plasma biopsy by in situ determination of exosomal microRNA-1246 with a molecular beacon. Analyst 2021, 146, 2264–2276.
- Lim, J.; Kang, B.; Son, H.Y.; Mun, B.; Huh, Y.M.; Rho, H.W.; Kang, T.; Moon, J.; Lee, J.J.; Seo, S.B.; et al. Microfluidic device for one-step detection of breast cancer-derived exosomal mRNA in blood using signal-amplifiable 3D nanostructure. Biosens. Bioelectron. 2022, 197, 113753.
- Liu, Q.; Zhang, X.; Zhang, J. Exosome-Based Nanoplatforms: The Emerging Tools for Breast Cancer Therapy. Front. Oncol. 2022, 12, 898605.
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control Release 2015, 219, 396–405.
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195.
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316.
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390.
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Barua, H.T.; Zhang, Y.; Midge, S.B.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int. J. Pharm. 2019, 566, 697–707.
- Jung, K.O.; Jo, H.; Yu, J.H.; Gambhir, S.S.; Pratx, G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials 2018, 177, 139–148.
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364.
- Naseri, Z.; Oskuee, R.K.; Jaafari, M.R.; Forouzandeh Moghadam, M. Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int. J. Nanomed. 2018, 13, 7727–7747.
- Sheykhhasan, M.; Kalhor, N.; Sheikholeslami, A.; Dolati, M.; Amini, E.; Fazaeli, H. Exosomes of Mesenchymal Stem Cells as a Proper Vehicle for Transfecting miR-145 into the Breast Cancer Cell Line and Its Effect on Metastasis. Biomed Res. Int. 2021, 2021, 5516078.
- O’Brien, K.P.; Khan, S.; Gilligan, K.E.; Zafar, H.; Lalor, P.; Glynn, C.; O’Flatharta, C.; Ingoldsby, H.; Dockery, P.; De Bhulbh, A.; et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene 2018, 37, 2137–2149.
- Hu, S.; Ma, J.; Su, C.; Chen, Y.; Shu, Y.; Qi, Z.; Zhang, B.; Shi, G.; Zhang, Y.; Zhang, Y.; et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021, 135, 567–581.
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45.
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.E.; Epstein, A.L.; Lenz, H.J.; Zhang, Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020, 28, 536–547.
- Pan, X.; Wang, H.; Wang, S.; Sun, X.; Wang, L.; Wang, W.; Shen, H.; Liu, H. Sonodynamic therapy (SDT): A novel strategy for cancer nanotheranostics. Sci. China Life Sci. 2018, 61, 415–426.
- Nguyen Cao, T.G.; Kang, J.H.; You, J.Y.; Kang, H.C.; Rhee, W.J.; Ko, Y.T.; Shim, M.S. Safe and Targeted Sonodynamic Cancer Therapy Using Biocompatible Exosome-Based Nanosonosensitizers. ACS Appl. Mater. Interfaces 2021, 13, 25575–25588.
- Liu, Y.; Bai, L.; Guo, K.; Jia, Y.; Zhang, K.; Liu, Q.; Wang, P.; Wang, X. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics 2019, 9, 5261–5281.
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865.
- Huang, H.; Shao, L.; Chen, Y.; Tang, L.; Liu, T.; Li, J.; Zhu, H. Synergistic strategy with hyperthermia therapy based immunotherapy and engineered exosomes-liposomes targeted chemotherapy prevents tumor recurrence and metastasis in advanced breast cancer. Bioeng. Transl. Med. 2022, 7, e10284.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
692
Revisions:
3 times
(View History)
Update Date:
06 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




