Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Breast cancer metastasis is a complicated process involving multiple physiological changes, and lung, brain, bone and liver are the main metastatic targets. Exosomes are membrane-bound extracellular vesicles that contain secreted cellular constitutes. The biogenesis and functions of exosomes in cancer have been intensively studied, and mounting studies have indicated that exosomes play a crucial role in cancer metastasis.
- breast cancer
- exosome
- organotropism
1. Introduction
Breast cancer is a common frequently occurring malignancy among women worldwide, and it has surpassed lung cancer to become the most diagnosed cancer all over the world with around 2.3 million new cases, accounting for 11.7% of all cancer cases and 24.5% of female cancers [1]. With recent advances in early diagnosis and therapeutic strategies including neoadjuvant therapy, endocrine therapy, molecular targeted therapy, and immunotherapy [2,3], the prognosis of breast cancer has greatly improved. However, breast cancer patients with distant metastasis have worse outcomes, and the five-year survival rate was less than 30% [4]. With approximately 685,000 deaths in 2020, it remains the first leading cause of cancer death in women [1]. Therefore, there is an urgent need to understand the molecular mechanisms underlying breast cancer metastasis for developing novel therapeutic strategies. Exosomes, as one type of extracellular vesicle (EVs), have been reported to play a crucial role in cancer metastasis, namely, contributing to form pre-metastatic niches, influence the tumor microenvironment, and identify specific organotropic metastasis. Here, we endeavor to highlight the role of tumor-derived exosomes in breast cancer metastasis, elucidate the underlying mechanism of metastasis organotropism mediated by exosomes, and prospect the potential application of exosomes in breast cancer therapeutics.
1.1. Breast Cancer Classification
Breast cancer develops from epithelial cells in the terminal duct lobular units and can be classified into two subtypes histologically, including ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) [5,6]. According to the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 labelling index (Ki-67) which reflects the proliferation [7], breast cancer has four primary molecular subtypes, namely luminal A, luminal B, HER2-positive, and triple-negative breast cancer (TNBC) [5,8]. The luminal A subtype is ER/PR-positive, with lower levels of Ki-67 (<14), accounting for about 60–70% of diagnostic breast cancers. The luminal B subtype is ER-positive combined with HER-2 positive or Ki-67 high (≥14), accounting for about 10–20%. Both luminal A and luminal B breast cancer are likely to benefit from endocrine therapy, and patients with luminal A breast cancer have a better prognosis compared to luminal B. HER2-positive subtype is ER/PR-negative and HER2-positive, with a diagnostic rate of approximately 13−15%. This subtype can benefit from treatment targeted to HER2 and chemotherapy with good prognosis. TNBC is characterized by ER, PR, and HER2 negativity in 10−15% of cases. Breast cancer susceptibility gene 1 (BRCA1) is a major breast cancer suppressor gene that encodes a protein critical for maintaining genome stability; its mutation predisposes women to TNBC [9]. As BRCA1 mutation impairs homologous recombination repair, poly (ADP-ribose) polymerase (PARP) inhibitors have been approved as target therapy for metastatic TNBC [10]. However, due to its highly aggressive clinical properties, TNBC still has a poorer prognosis compared to other breast cancer subtypes [2,5,11,12]. Although the five-year survival rate for women diagnosed with breast cancer exceeds 90%, all breast cancer subtypes have the potential to exhibit adverse clinic features, such as high invasiveness and recurrence, mainly caused by metastasis [5].
1.2. Breast Cancer Metastasis
Metastasis occurs frequently and accounts for as much as 90% of cancer-related deaths [13]. Breast cancer metastasis is also frequently diagnosed and exhibits organ tropism to lung, brain, bone and liver, which is highly heterogeneous and affects treatment outcomes and patient prognosis [14]. As shown in Figure 1, breast cancer patients are most prone to bone metastasis, accounting for 50.7−68.8% of all metastatic cases with different molecular subtypes. The lung and liver were similar, with 16.0−23.9% and 13.3−19.7% of breast cancer metastasis occurring in the lung or liver, respectively. The incidence of brain metastasis is approximately 1.9−5.7% of all metastatic cases. Based on molecular subtypes, lung metastasis most commonly occurs in TNBC, accounting for about 32% of patients with metastasis, while the HER2-positive subtype is likely to have liver metastasis [15,16].
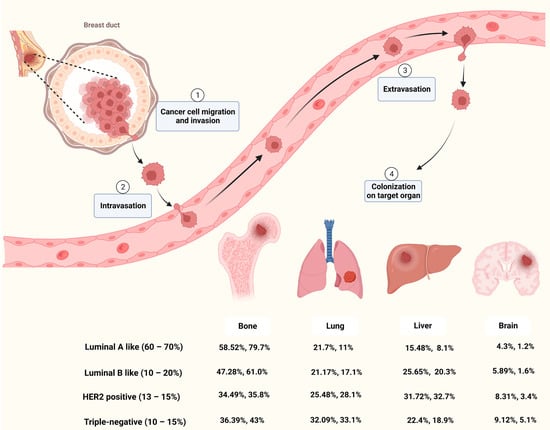
Cancer metastasis is a complicated process that goes through multiple steps such as invasion, intravasation, extravasation and colonization on target organ (Figure 1). Metastasis of tumor cells to distant organs requires not only tumor cell invasiveness, but also a microenvironment that is conducive to tumor survival and proliferation in secondary organs [17]. Although the molecular mechanisms of breast cancer metastasis are not fully understood, mounting studies have shown that primary cancer cells could secrete factors to remodel the microenvironment of the target organ and prime it into a site favorable for cancer cell proliferation, known as pre-metastatic niche (PMN) [18,19,20]. Among these factors, EVs secreted by cancer cells play important roles in microenvironment remodeling and metastatic organotropism [21].
1.3. EVs and Exosome
EVs are membrane-bound vesicles secreted by various cells which play critical roles in cell-cell communication under physiological and pathological conditions [22]. In general, EVs could be divided into three types based on their morphology: exosomes (30–150 nm), micro-vesicles (150–1000 nm) and apoptotic bodies (500–2000 nm) [23]. Nowadays, EVs are considered non-negligible factors in cellular homeostasis and mediators of cancer metastasis [24]. Exosomes are small EVs with phospholipid bilayers whose heterogeneous “cargoes”, such as protein, lipids, RNA and DNA, vary from different types of cells [25,26]. These cargoes are located inside or on the surface of exosomes and mediate the communication between original cells and recipient cells [26].
Exosomes are generated originally from early endosomes by fusion of endocytic vesicles with plasma membranes. Early endosomes subsequently mature into late endosomes and form the multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs). MVBs could either fuse with plasma membrane for exocytosis of contained ILVs (namely exosomes) into the extracellular space, or with lysosomes or autophagosomes for degradation [27,28]. ILV formation is largely dependent on the endosomal sorting complex required for transport (ESCRT) function. ESCRT is an intricate machinery which is made up of five complexes, which include the ESCRT-0, -Ⅰ, -Ⅱ, -Ⅲ and Vps4-Vta1 complexes [27,29]. Beside the ESCRT-dependent pathway, an alternative pathway could also be involved in exosome biogenesis. Proteolipid proteins (PLP) could be incorporated into ILVs in a sphingolipid ceramide-dependent manner. Then, ceramide induces raft-based microdomains formation and coalescence and promotes the budding of ILVs [30]. Moreover, tetraspanins, such as CD63, CD81, CD9, could regulate ESCRT-independent sorting [27]. Therefore, ESCRT dependent and independent mechanisms co-exist and function synergistically in exosome formation.
In recent years, studies have found that cancer-derived exosome could promote the progression of cancer, including cancer metastasis and drug resistance [31]. Exosomes have been proved to be involved in various processes of cancer metastasis, such as vascular leakiness, epithelial-mesenchymal transition (EMT) induction, immune escape and PMN formation [24,32,33]. Integrins are cell surface adhesion molecules that could mediate cell signaling by interacting with components of the extracellular matrix [34]. It has been demonstrated that integrins on exosomes could guide them into different organs, thereby inducing PMN formation and promoting cancer metastasis. Exosomes with integrins α6β4 and α6β1 tend to accumulate in the lung, while integrin αvβ5 preferably drives exosomes into the liver [35]. In addition, comparative proteomics of exosomes derived from different breast cancer cells demonstrated that their exosomal proteins are heterogenous, which is associated with different cancer cell metastatic properties [36]. These studies suggest that exosomes play a crucial role in causing metastasis organotropism through various mechanisms. In the following section, we will summarize the contribution of exosomes to the tropism of breast cancer metastasis to different organs, namely bone, lung, liver and brain, and the metastatic models used in these findings (Table 1), which will help to comprehensively decipher how exosomes are involve in breast cancer metastasis.
Table 1. Breast cancer models used to study the mechanism of exosomes promoting metastasis.
| Metastatic Organs | Exosomal Molecules | Cell Lines | Metastasis Mouse Model | Reference |
|---|---|---|---|---|
| Bone | miR-940 | MDA-MB-231 | Calvaria implantation | [37] |
| miR-218 | MDA-MB-231, MCF-7 | Tail vein injection of EVs | [38] | |
| miR-20a-5p | MDA-MB-231, MCF-7 | In vitro | [39] | |
| miR-21 | MDA-MB-231 | Orthotopic model and Caudal artery injection | [40] | |
| miR-19a, IBSP | MDA-MB-231, MCF7, T47D | Intra-cardiac model, intratibial implantation and orthotopic model | [41] | |
| L-plastin | MDA-MB-231 | Intratibial implantation | [42] | |
| CDH11, ITGA5 | MDA-MB-231, 4T1, MCF7 |
Intra-cardiac model and orthotopic model | [43] | |
| lung | miR-122 | MDA-MB-231, MCF10DCIS.com | Intra-cardiac model and orthotopic model | [44] |
| miR-138-5p | 4T1 | Tail vein injection | [45] | |
| miR-183-5p | 4T1 | Orthotopic model | [46] | |
| miR-200b-3p | 4T1 | Orthotopic model | [47] | |
| Let-7 | 4TO7 | Orthotopic model, tail vein injection and intra-cardiac model | [48] | |
| circPSMA1 | MDA-MB-231 | Orthotopic model | [49] | |
| CCL2 | EO771 | Tail vein injection | [50] | |
| TβRII | MDA-MB-231, 4T1, 4T07 | Intra-cardiac model, tail vein injection and orthotopic model | [51] | |
| Myosin-9 | MDA-MB-231 | Subcutaneous xenograft, orthotopic model | [52] | |
| MMPs | MDA-MB-231 | Tail vein injection, orthotopic model | [53] | |
| MMP-1 | MDA-MB-231 | Tail vein injection | [54] | |
| NDPK | MDA-MB-231 | Tail vein injection | [55] | |
| Annexin II | MDA-MB-231, MDA-MB-831, MDA-MB-4175 | Tail vein injection and intra-cardiac model | [56] | |
| Liver | miR-4443 | MCF-7, MDA-MB-231 | Orthotopic model | [57] |
| miR-197 | MBA-MB-231 or SUM149PT | Subcutaneous injection and tail vein injection | [58] | |
| E-cadherin, p120-catenin | MTPa | Orthotopic model (Rat) | [59] | |
| TGFβ1 | MCF7, MDA-MB-231 | In vitro | [60] | |
| Brain | miR-181c | MDA-MB-231 | Intra-cardiac model | [61] |
| lnc GS1-600G8.5 | MDA-MB-231 | Intra-cardiac model | [62] | |
| CEMIP | MDA-MB-231 | Intra-cardiac model, intracranial injection and orthotopic model | [63] | |
| miR-503 | MCF7, ZR75-1, SKBR3, MDA-MB-231 |
Intra-cardiac model and intracranial injection | [64] | |
| miR-301a-3p | MDA-MB-231 | Retro-orbital injection of EVs | [65] |
2. Clinical Application
2.1. Exosomes as Diagnostic Biomarkers for Breast Cancer
With the convenience of being non-invasive and highly efficient, liquid biopsy brings an opportunity for cancer diagnosis with detection through various body fluids such as blood or urine, rather than invasive methods to remove a piece of cancerous tissue [88]. Liquid biopsy has made some progress in establishing the diagnosis of various cancers by using certain molecules as biomarkers, including CTCs, circulating tumor DNA (ctDNA), tumor-educated platelet (TEP) and exosomes [89,90]. As mentioned above, exosomes contain specific “cargoes” derived from metastatic cancer cells, which not only play an important role in breast cancer progression and metastasis but may also have the potential to be biomarkers for diagnosing metastasis. To discover exosome-based biomarkers, Wang et al. established a comprehensive database—ExoBCD—by combining four high-throughput datasets, transcriptome of 1191 TCGA cases and manual mining of 950 studies. The database identified 306 valuable exosomal molecules, including 49 potential biomarkers and 257 biologically interesting molecules [91]. Since traditional detection methods, such as real-time PCR and Western Blotting analysis, are time-consuming and laborious and require exosome enrichment, which make them unsuitable for exosome-based diagnosis, it is necessary to develop alternative methods. A rapid, sensitive, and low-cost thermophoretic aptasensor (TAS) was developed for the analysis of cancer-associated protein profiles of plasma EVs. Based on this analysis, the EV protein signature was established and used to accurately monitor and predict metastatic breast cancer [92]. Kwizera et al. developed an inexpensive and highly efficient device based on the surface-enhanced Raman scattering (SERS) to detect exosomes and exosomal protein profiles. Using this device, they identified exosomal HER2 and EpCAM as biomarkers in the plasma of HER2-positive breast cancer patients [93]. Then, Lee et al. established another SERS-based platform to detect and quantify exosomal miRNAs in serum for breast cancer diagnosis [94]. In addition, a nano-sized fluorescent oligonucleotide probe-molecular beacon has also been developed for the measurement of miRNAs in blood exosomes with high sensitivity and specificity, such as miR-21 [95,96], miR-27a, miR-375 [96], and miR-1246 [97]. Recently, a microfluidic chip-based exosomal mRNA sensor was developed to directly detect exosomal ERBB2 in blood for the diagnosis of HER2-positive breast cancer [98]. Therefore, technological advances will enable exosomes to be used as biomarkers for breast cancer diagnosis in the future.
2.2. Engineered Exosomes for Therapeutics of Breast Cancer
The natural characteristics of exosomes, such as low toxicity, low immunogenicity, high-flexibility engineering, and inherent targeting and interaction with recipient cells, make them ideal drug carriers for breast cancer therapy [99,100,101,102]. First, exosomes can serve as delivery vesicles for chemotherapeutic drugs such as doxorubicin, with some engineering modification on their surface to improve their targeting efficiency and reduce side effects [103]. Hydrophobic drugs, such as Aspirin, could also be loaded into exosomes to increase their solubility and enhance their cytotoxicity against cancer cells [104]. In addition, to enhance the efficacy of PARP inhibitors, exosomes isolated from TNBC cells were loaded with Olaparib (PARP inhibitor) and superparamagnetic iron oxide (SPIO) nanoparticles, which could be tracked by magnetic particle imaging (MPI) and also effectively inhibited tumor growth [105]. Second, functional small RNAs, such as siRNA and miRNA, can be packaged into exosomes and delivered to cancer cells to downregulate target genes, thereby inhibiting cancer progression [106,107,108,109]. Third, engineered exosomes can be used as vaccines to stimulate an immune response against tumor cells. A novel exosome-like nanoparticles was developed from fibroblast activation protein-α (FAP) engineered cancer cells as a tumor vaccine, which induced robust and specific cytotoxic T lymphocyte immunity against tumor cells and reprogrammed the immunosuppressive microenvironment [110]. Exosomes from α-lactalbumin overexpressing breast cancer cells were packaged together with immunogenic cell death inducers—human neutrophil elastase (ELANE) and Hiltonol (a TLR3 agonist) to construct a vaccine, thereby priming dendritic cells in situ and improving subsequent tumor-reactive CD8+ T cell responses [111]. Exosome can also be engineered to display both anti-CD3 and anti-HER2 antibodies to mediate cytotoxic T cells that directly target HER2-positive breast cancer and improve immunotherapy [112]. Fourth, exosomes can be utilized as nanocarriers to provide cancer-targeted sonosensitizers for sonodynamic therapy (SDT), which employs reactive oxygen species (ROS) generated by ultrasonic excitation to kill cancer cells [113]. Indocyanine green-loaded exosomes were surface-modified with cancer-binding ligand to increase their target specificity, resulting in greater SDT against cancer cells [114]. Sinoporphyrin sodium (DVDMs) could also be loaded into tumor-derived exosomes to increase its efficiency in SDT [115]. Exosomes can be used not only for primary tumor therapy, but also be engineered to treat breast cancer metastasis. As exosomes derived from metastatic breast cancers have natural organotropism to lung [35] and brain [86], therapeutic drugs can be encapsulated with exosomes to target the relevant metastatic foci. Gold nanorods, a nanomaterial for photothermal therapy, were loaded into lung metastatic cancer cells-derived exosomes, exhibiting better therapeutic effects on lung metastases [116]. Finally, more engineered exosomes will be developed to improve precision therapy for breast cancers, especially with regard to metastasis.
This entry is adapted from the peer-reviewed paper 10.3390/ijms232213993
This entry is offline, you can click here to edit this entry!
