Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefano Guerriero | -- | 2842 | 2022-12-05 11:48:56 | | | |
| 2 | Conner Chen | + 7 word(s) | 2849 | 2022-12-06 07:24:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Guerriero, S.; Ajossa, S.; Pagliuca, M.; Borzacchelli, A.; Deiala, F.; Springer, S.; Pilloni, M.; Taccori, V.; Pascual, M.A.; Graupera, B.; et al. Transvaginal Ultrasound as Technique for Diagnosis of Endometriosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/38026 (accessed on 08 February 2026).
Guerriero S, Ajossa S, Pagliuca M, Borzacchelli A, Deiala F, Springer S, et al. Transvaginal Ultrasound as Technique for Diagnosis of Endometriosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/38026. Accessed February 08, 2026.
Guerriero, Stefano, Silvia Ajossa, Mariachiara Pagliuca, Antonietta Borzacchelli, Fabio Deiala, Serena Springer, Monica Pilloni, Valeria Taccori, Maria Angela Pascual, Betlem Graupera, et al. "Transvaginal Ultrasound as Technique for Diagnosis of Endometriosis" Encyclopedia, https://encyclopedia.pub/entry/38026 (accessed February 08, 2026).
Guerriero, S., Ajossa, S., Pagliuca, M., Borzacchelli, A., Deiala, F., Springer, S., Pilloni, M., Taccori, V., Pascual, M.A., Graupera, B., Saba, L., & Alcazar, J.L. (2022, December 05). Transvaginal Ultrasound as Technique for Diagnosis of Endometriosis. In Encyclopedia. https://encyclopedia.pub/entry/38026
Guerriero, Stefano, et al. "Transvaginal Ultrasound as Technique for Diagnosis of Endometriosis." Encyclopedia. Web. 05 December, 2022.
Copy Citation
Transvaginal ultrasound (TVUS) is surely the first-line technique for the diagnosis of endometriosis. The possibility to perform a dynamic ultrasonographic examination can provide additional information that is not easily obtained with other imaging modalities.
endometriosis
transvaginal ultrasound
1. Introduction
Endometriosis is a common chronic disease affecting 5–10% of women of reproductive age globally [1]. Histologically, this disease is defined by the presence of endometrium-like tissue (epithelium and/or stroma) outside the endometrium and myometrium, usually with an associated inflammatory process [1] and several different clinical presentations that are mainly based on the presence of pain but also infertility [2]. According to the International Working Group of the AAGL (American Association of Gynecologic Laparoscopists), ESGE (European Society of Gynecologic Endoscopy), ESHRE (European Society of Human Reproduction and Embryology) and WES (World Endometriosis Society), superficial endometriosis is defined by the presence of endometrium-like tissue involving the peritoneal surface. The lesions can have different appearances and colors e.g., clear and black [2]. In contrast, the same group defines deep endometriosis (DE) as endometrium-like tissue lesions in the abdomen that extend on or under the peritoneal surface. They are usually nodular, able to invade adjacent structures, and associated with fibrosis and the disruption of normal anatomy [2]. Ovarian endometriosis is defined as endometrium-like tissue in the form of ovarian cysts [2]. Endometriomas may be either invagination cysts or true cysts, with the cyst wall also containing endometrial-like tissue and dark blood-stained fluid [2].
The ovary is a common site affected by endometriosis [3]. The intra-pelvic localizations of endometriosis include involvement of the uterosacral ligaments (USLs), vagina, rectum, rectovaginal space, or bladder (frequently at the posterior bladder dome) [4]. Endometriosis can also affect extra-pelvic organs, such as the abdominal wall, the diaphragm and nerves (for example sciatic nerves) [5], but these topics were covered in a previous review [6].
2. Transvaginal Ultrasound
Transvaginal ultrasound (TVUS) is surely the first-line technique for the diagnosis of endometriosis [7]. The possibility to perform a dynamic ultrasonographic examination can provide additional information that is not easily obtained with other imaging modalities [7].
2.1. Ovarian Endometriosis
The ultrasound features of ovarian endometriosis vary in pre- and postmenopausal patients [8][9]. The most common ultrasound appearance of ovarian endometriosis is the typical “unilocular cyst with ground glass echogenicity of the cyst fluid” [9]. This appearance became less common with increasing age, in particular after 35 years old [8]. Although the mean diameter remains the same in the different ages of premenopausal women, endometriomas with papillations are more common in older women, with 3% in younger women compared with 14% of women ≥ 45 years old [8]. In a Cochrane review, Nisenblat et al. [10] found that the sensitivity and specificity for a diagnosis of endometrioma using TVUS are 93% and 96%, respectively. The age of the patients seems to also interfere with the diagnostic accuracy [8]. As a matter of fact, subjective impressions of the presence of endometrioma showed a sensitivity of around 90% in younger patients that decrease to 70% after 45 years of age. In contrast, the specificity remains the same (97–98%).
2.2. Superficial Endometriosis
Although some authors suggest that some findings (presence of thickened pericolic fat) correlate with the presence of superficial endometriosis, no reproducibility study has been published [11]. Robinson et al. [12] found a poor sensitivity (51%) and specificity (55%) of transvaginal ultrasound markers in the diagnosis of superficial endometriosis of the USL. Reid et al. found that, in the absence of ovarian endometriosis and DE nodules, ovarian immobility was associated with superficial endometriosis of the pelvic side wall [13]. Leonardi et al. described a novel technique, the so-called “sonoPODgraphy”, that apparently allows for better detection of superficial endometriosis [14].
2.3. Deep Endometriosis
Regarding DE, the consensus from the International Deep Endometriosis Analysis (IDEA) group [15] proposed four basic sonographic steps when examining women with suspected or known endometriosis: evaluation of the uterus and adnexa (signs of adenomyosis and endometriomas), evaluation of “soft markers”, assessment of the status of the pouch of Douglas (POD) using the “sliding sign”, and assessment of DE nodules in the anterior and posterior compartments (bladder, vaginal vault, USLs, bowel, rectum, rectosigmoid junction and sigmoid colon).
The identification of “soft markers” is an easy-to-learn assessment that can even be performed by less experienced operators [16]. “Soft markers” were designed to identify patients who would most likely benefit from a more detailed scan by an expert examiner [16]. Markers that showed a correlation with the presence of DE are the absence of the “sliding sign” (applying gentle pressure to the cervix to mobilize the uterus to determine whether the anterior rectum glides freely or not over the posterior vagina, cervix and uterus) and/or the presence of “kissing ovaries” (both ovaries located in close proximity or touching each other in the POD) in patients with clinical suspicion of endometriosis [16]. In a recent systematic review and meta-analysis, Alcazar et al. [17] found that the “sliding sign” has good diagnostic performance for predicting POD obliteration and bowel involvement in women with suspected endometriosis. In addition, ovarian immobility in TVUS is significantly associated with ipsilateral pelvic pain, USL and pelvic sidewall superficial endometriosis, endometrioma, posterior compartment DE and POD obliteration [13]. Another very rare localization is the presence of tubal endometriosis. The only study present in the literature [18] found that hydrosalpinx as an ultrasonographic sign used alone is characterized by a high specificity but low sensitivity for the detection of tubal endometriosis, which can be improved by the addition of other ultrasonographic markers previously described.
According to the IDEA consensus [15], the location of DE nodule localizations should be divided into two different pelvic compartments: the anterior and the posterior. The anterior compartment includes the urinary bladder, uterovesical region and ureters, while the posterior compartment includes the posterior vaginal fornix, anterior rectum/anterior rectosigmoid junction, retrocervical space and sigmoid colon [15].
Recent studies evaluated the prevalence, diagnosis and clinical features of DE involving the parametrium, considering it as a third compartment in addition to the anterior and posterior ones. According to the #Enzian Classification for endometriosis [19], the mediolateral/dorsolateral compartments include the USL, cardinal ligament and pelvic sidewall. From the anatomical point of view, the lateral compartment includes the parametrium, paracolpium, pelvic floor, the parametrial segment of the ureters, nerves (inferior hypogastric plexus and obturatory nerve), ovarian fossae and pelvic sidewalls [20][21]. Generally, TVUS should be performed with a scarcely filled bladder. However, if bladder endometriosis is suspected, patients should be asked not to empty their bladder before the ultrasound examination [15]. The image orientation (up or down) depends on the choice of the operator [22].
2.3.1. Anterior Endometriosis
The DE in the anterior compartment can include hypoechoic linear or spherical lesions, with or without regular contours, cystic spaces, hyperechoic foci and/or regular contours involving the muscularis (most commonly), (sub)mucosa of the bladder (lesions that involve only the serosa represent superficial endometriosis) or the uterovesical space [15] (Figure 1). Albeit not formally assessed in prospective studies, the obliteration of the uterovesical space can be evaluated using the sliding of these structures. If the posterior bladder slides freely over the anterior uterine wall, the uterovesical space is considered non-obliterated. On the other hand, if the bladder does not slide freely, the uterovesical space can be considered obliterated [15]. In a meta-analysis, Guerriero et al. [23] found that for the detection of bladder endometriosis, the sensitivity and specificity were 62% and 100%, respectively. Similar results were reported in a more recent meta-analysis [24].

Figure 1. TVUS imaging method: a bladder endometriotic nodule with 2D TVUS (black and white images) and with 3D TVUS (color image).
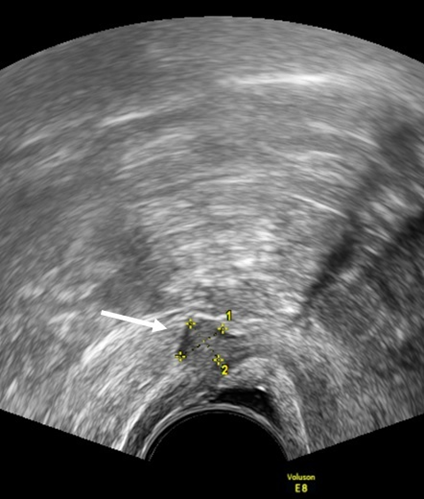
Figure 2. TVUS imaging method: the arrow and calipers show a nodule of the rectovaginal space in the sagittal plane.
Experienced sonographers can reliably identify silent ureteral involvement due to endometriosis with TVUS [23]. The most common location of ureteral stenosis is 3–4 cm above the vesicoureteral junction and, in about half of the cases, ureteral dilatation involvement is not associated with hydronephrosis on abdominal ultrasound [25]. In a prospective observational study that enrolled 848 patients with chronic pelvic pain, Pateman et al. [26] found that the diagnosis of ureteral endometriosis had a sensitivity and specificity of 92% and 100%, respectively. Although a key point for surgical planning includes the close relationship with the ureteral path [27], the measurement of the distance between the nodule of the ovarian fossae and the ureteral path is not described in the literature.
2.3.2. Posterior and Lateral Endometriosis
In the rectovaginal space (RVS) the DE nodules are described as lesions below a horizontal plane that passes along the lower margin of the posterior lip of the cervix under the peritoneum [15]. (Figure 2). Guerriero et al. [23] found that in the detection of endometriosis in the RVS, the sensitivity and specificity were 49% and 98%, respectively.
As for the USL, the lesions appear as nodules with regular or irregular margins and often with hyperechoic points, or as a linear hypoechoic thickening with regular or irregular margins [15] (Figure 3). In the detection of USL endometriosis, the sensitivity and specificity are 53% and 93%, respectively [23]. Vaginal lesions should be suspected when the posterior vaginal fornix is thickened with or without surrounding cystic anechoic areas or if there is a hypoechoic nodule, homogeneous or inhomogeneous, with or without large cystic areas [23]. (Figure 4). In the detection of vaginal endometriosis, the sensitivity and specificity are 58% and 96%, respectively [23]. A recent meta-analysis reported similar data with a sensitivity of 52% and a specificity of 98% [24].
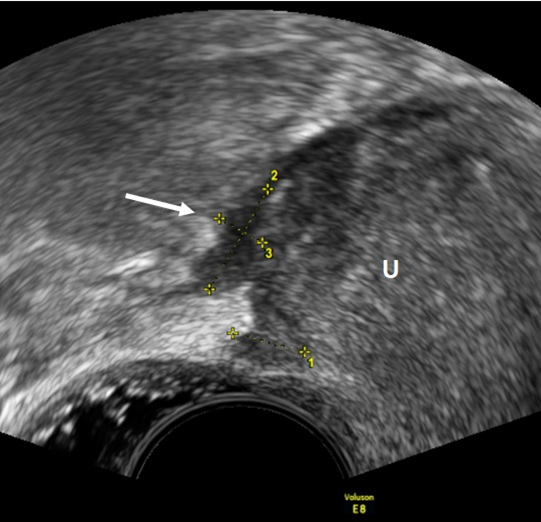
Figure 3. TVUS imaging method: USL lesion indicated by an arrow and calipers in the sagittal plane. U: uterus.

Figure 4. TVUS imaging method: the arrow and the calipers show a posterior vaginal fornix nodule in the sagittal plane. U: uterus.
A nodule on the rectosigmoid colon can appear as an irregular hypoechoic nodule in the anterior wall of the rectosigmoid colon with or without hypoechoic or, rarely, a hyperechoic focus with visible retraction and adhesions [15] (Figure 5). Sensitivity and specificity in the detection of endometriotic lesions in the rectosigmoid colon are 91% and 97%, respectively [28]. In addition, TVUS enables the accurate assessment of a rectosigmoid DE lesion size with moderate-to-good reliability and correlation for lesion thickness measurements, which may be essential for diagnosis, surgical risk assessment and planning of surgical treatment [29]. TVUS is also reliable at measuring the lesion-to-anal-verge distance of rectosigmoid DE, thereby estimating the height of the bowel anastomosis in patients undergoing a-full-thickness resection either by discoid resections or segmental resections [30][31]. A recent prospective study performed by the IDEA group found a higher TVUS detection rate of DE overall than that reported by the most recent meta-analysis on the topic (sensitivity of 79%), but with a lower specificity [32].
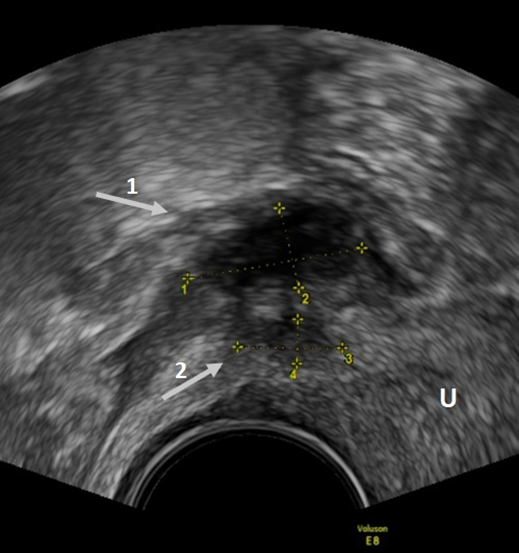
Figure 5. TVUS imaging method: arrow 1 shows a rectosigmoid nodule, while arrow 2 shows a USL nodule in the sagittal plane.
According to the study of Exacoustos et al. [33], parametrial involvement is suggested by infiltrating, irregular and hypoechogenic tissue extending laterally to the cervix or vagina. Parametrial endometriosis involvement occurs more frequently in patients with severe endometriosis, possibly with hypogastric/sacral plexus or sciatic nerve involvement [34][35]. In a recent meta-analysis, Guerriero et al. [36] found that in the detection of endometriosis in the parametrium, the sensitivity and specificity are 31% and 98%, respectively. This low sensitivity can be explained by the fact that specific training is needed to properly visualize this anatomical area [36]. Assessing the USL might suggest a thorough evaluation of the parametrium [37].
2.3.3. Additional Ultrasonographic Techniques and TVUS Classification Systems
To further increase the diagnostic accuracy of TVUS, there are some additional techniques that can be performed. The simplest additional technique is the tenderness approach [38]. It is a modality of TVUS that is performed by increasing the amount of ultrasound gel inside the probe cover by introducing 12 mL of ultrasound gel instead of the usual 4 mL. The gel lid creates a stand-off that allows for the visualization of adjacent tissues to the probe. It is also useful to evaluate painful sites evocated by the gentle pressure of the probe, even without abdominal palpation. No data are present on the level of expertise needed. This technique known as “tenderness-guided” TVUS [39] shows high specificity and sensitivity in the detection of vaginal and rectovaginal space endometriosis. Good specificity associated with a lower sensitivity was obtained in the diagnosis of DE of USL, rectosigmoid involvement or anterior DE [38]. This technique was meant to reproduce physical examination that shows sensitivities in endometriosis diagnosis of 41% for the ovary, 50% for the USL, 76% for the POD, 73% for the vagina, 78% for the RVS, 25% for the urinary bladder and 39% for the rectosigmoid [40].
Regarding the need for bowel preparation before a TVUS examination, Ros et al. [41] concluded that it was well-tolerated and useful to aid in the detection and more precise description of rectosigmoid nodules. Ferrero et al. [42], on the other hand, found that bowel preparation does not improve the diagnostic performance of TVUS in detecting rectosigmoid endometriosis and in assessing the characteristics of endometriotic nodules.
In addition, office gel sonovaginography (SVG) appears to be an effective outpatient imaging technique for the prediction of bowel DE, with higher accuracy for the prediction of rectosigmoid compared with anterior rectal DE [43]. SVG can improve the detection of DE involving the rectovaginal space by creating an acoustic window between the transvaginal probe and the surrounding vaginal structures [44]. A further technique that is used is rectal water-contrast transvaginal ultrasonography (RWC-TVUS). Although RWC-TVUS and SVG have similar accuracies in the diagnosis of DE, RWC-TVUS has a higher performance when assessing the characteristics of rectosigmoid endometriosis. Like conventional TVUS, a limitation of enhanced TVUS techniques is that they cannot diagnose endometriotic nodules located above the rectosigmoid, as they are beyond the field of view of ultrasonography [45]. Additional evaluation through the abdominal wall using a high-resolution linear transducer might be needed in some cases, for example, to assess cecal or appendicular endometriosis. Some authors [46] suggested using a laparoscopic intraoperative 12.4 MHz ultrasonographic transducer placed on the surface of the rectosigmoid nodules to study the appearances and to perform measurements. Due to the few cases evaluated, it is probably too early to introduce this practice in daily management.
Based on the high diagnostic accuracy reported in the literature, of all classification and staging systems for endometriosis, many are based on ultrasound. Table 1 summarizes the most recent (of the last 10 years) endometriosis classification/staging system based on ultrasound.
Table 1. The different and more recent endometriosis classification/staging system based on ultrasound.
| Classification/Staging System | Pub. Year | Based on | Based Only on Ultrasound | Aim of Classification |
|---|---|---|---|---|
| #Enzian classification [19] | 2021 | Surgical observation/magnetic resonance imaging (MRI)/ultrasound | No | Description of endometriosis |
| Endometriosis Fertility Index (EFI) [47] | 2021 | Surgical observation/ultrasound/clinical examination | No | Probability of pregnancy after endometriosis surgery |
| Adhesion scoring system [48] | 2020 | Ultrasound | Yes | Prediction of the pelvic adhesions |
| ENDORECT * [49] | 2019 | MRI/ultrasound/clinical examination | No | Prediction of rectosigmoid involvement |
| Preoperative ultrasound-based endometriosis staging system (UBESS) [50][51][52] | 2016 | Ultrasound | Yes | Prediction of the level of complexity of laparoscopic surgery |
* Based on UBESS 3.
In order to achieve the quality of care of a tertiary center obtained by expert examination in regular practices, in particular in the USA, Young et al. [53] suggested accessing the endometriotic specific sites and training sonographers, as well as radiologic and gynecologic sonologists, in the sonographic features of deep endometriosis and other associated signs. In addition, Deslandes et al. [54] suggested that doubling the scan time should be considered due to a demonstrated additional average time of 5.4 min in endometriosis sonographic assessment in comparison with routine transvaginal ultrasound.
Three-dimensional TVUS (3D TVUS) can also be used for the evaluation of DE. This technique allows for the possibility of obtaining volumes that can be reconstructed manyfold after a single sweep with an infinite number of viewing planes [55]. In particular, it is possible to obtain the coronal plane of the lesion, which is a plane that is practically impossible to obtain with the use of 2D TVUS [56]. In addition, 3D TVUS rendering can allow for a better analysis of a nodule because 3D reconstruction might make the irregular shapes and borders more evident [57], as well as the use of volume contrast imaging (VCI) with 2–4 mm slices. The possibility to re-evaluate stored 3D volumes, the so-called “virtual navigation”, can be used as an educational tool to improve the learning curve of less expert operators. In fact, Guerriero et al. [57] found that the combined use of real-time TVUS and offline 3D volume virtual navigation was helpful to improve training in a short time (2 weeks) for the ultrasound assessment of DE. Regarding the diagnoses superficial endometriosis but also endometriosis of ureters and parametrium, there are presently no studies in the literature that describe the accuracy of 3D TVUS. Barra et al. [58] found that 3D reconstructions do not improve the performance of 2D TVUS in diagnosing the presence and characteristics of bladder endometriosis. However, the use of virtual organ computer-aided analysis (VOCAL) allows for a significantly better volume assessment. Guerriero et al. [59] found that for 3D TVUS, the sensitivity and specificity in the diagnosis of rectosigmoid colon endometriosis were 91% and 97%, respectively, similar to 2D, while in the diagnosis of endometriosis of the USL, rectovaginal space and vaginal fornix overall, the sensitivity and specificity, thanks to the available coronal plane, are 87% and 94%, respectively. In another study, retrocervical and rectosigmoid endometriotic nodules displayed significantly different three-dimensional sonographic mean gray values, which allowed for a quantitative evaluation with extremely good reproducibility [60]. In addition, introital 3D ultrasonography seems to be an effective method for the diagnosis of endometriosis of the rectovaginal septum [61]. In a recent study, Guerriero et al. [62] found that the typical ultrasonographic sign of rectosigmoid endometriosis is reasonably recognizable to observers with different levels of expertise when assessed in stored 3D volumes.
References
- Taylor, H.S.; Kotlyar, A.M.; A Flores, V. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852.
- Tomassetti, C.; Johnson, N.P.; Petrozza, J. An International Terminology for Endometriosis, 2021. J. Minim. Invasive Gynecol. 2021, 28, 1849–1859.
- Exacoustos, C.; De Felice, G.; Pizzo, A.; Morosetti, G.; Lazzeri, L.; Centini, G.; Piccione, E.; Zupi, E. Isolated Ovarian Endometrioma: A History Between Myth and Reality. J. Minim. Invasive Gynecol. 2018, 25, 884–891.
- Menni, K.; Facchetti, L.; Cabassa, P. Extragenital endometriosis: Assessment with MR imaging. A pictorial review. Br. J. Radiol. 2016, 89, 20150672.
- Andres, M.P.; Arcoverde, F.; Souza, C.C.; Fernandes, L.F.C.; Abrão, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2019, 27, 373–389.
- Guerriero, S.; Conway, F.; Pascual, M.A. Ultrasonography and Atypical Sites of Endometriosis. Diagnostics 2020, 10, 345.
- Collins, B.G.; Ankola, A.; Gola, S.; McGillen, K.L. Transvaginal US of Endometriosis: Looking Beyond the Endometrioma with a Dedicated Protocol. RadioGraphics 2019, 39, 1549–1568.
- Guerriero, S.; Van Calster, B.; Somigliana, E.; Ajossa, S.; Froyman, W.; De Cock, B.; Coosemans, A.; Fischerová, D.; Van Holsbeke, C.; Alcazar, J.L.; et al. Age-related differences in the sonographic characteristics of endometriomas. Hum. Reprod. 2016, 31, 1723–1731.
- Van Holsbeke, C.; Van Calster, B.; Guerriero, S. Endometriomas: Their ultrasound characteristics. Ultrasound Obstet. Gynecol. 2010, 35, 730–740.
- Nisenblat, V.; Bossuyt, P.M.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endome-triosis. Cochrane Database Syst. Rev. 2016, 2016, CD009591.
- Chowdary, P.; Stone, K.; Ma, T. Multicentre retrospective study to assess diagnostic accuracy of ultrasound for superficial endometriosis—Are we any closer? Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 279–284.
- Robinson, A.J.; Rombauts, L.; Ades, A.; Leong, K.; Paul, E.; Piessens, S. Poor sensitivity of transvaginal ultrasound markers in diagnosis of superficial endometriosis of the uterosacral ligaments. J. Endometr. Pelvic Pain Disord. 2018, 10, 10–17.
- Reid, S.; Leonardi, M.; Lu, C.; Condous, G. The association between ultrasound-based ‘soft markers’ and endometriosis type/location: A prospective observational study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 171–178.
- Leonardi, M.; Robledo, K.; Espada, M.; Vanza, K.; Condous, G. SonoPODography: A new diagnostic technique for visualizing superficial endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 124–131.
- Guerriero, S.; Condous, G.; van den Bosch, T.; Valentin, L.; Leone, F.P.G.; Van Schoubroeck, D.; Exacoustos, C.; Installé, A.J.F.; Martins, W.P.; Abrao, M.S.; et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: A consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet. Gynecol. 2016, 48, 318–332.
- Guerriero, S.; Ajossa, S.; Pascual, M.A.; Rodriguez, I.; Piras, A.; Perniciano, M.; Saba, L.; Paoletti, A.M.; Mais, V.; Alcazar, J.L. Ultrasonographic soft markers for detection of rectosigmoid deep endometriosis. Ultrasound Obstet. Gynecol. 2019, 55, 269–273.
- Alcázar, J.L.; Eguez, P.M.; Forcada, P.; Ternero, E.; Martínez, C.; Pascual, M.; Guerriero, S. Diagnostic accuracy of sliding sign for detecting pouch of Douglas obliteration and bowel involvement in women with suspected endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2022, 60, 477–486.
- Stepniewska, A.K.; Clarizia, R.; De Mitri, P. Role of ultrasonographic parameters for predicting tubal involvement in infertile patients affected by endometriosis: A ret-rospective cohort study. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102208.
- Keckstein, J.; Saridogan, E.; Ulrich, U.A.; Sillem, M.; Oppelt, P.; Schweppe, K.W.; Krentel, H.; Janschek, E.; Exacoustos, C.; Malzoni, M.; et al. The #Enzian classification: A comprehensive non-invasive and surgical description system for endometriosis. Acta Obstet. Gynecol. Scand. 2021, 100, 1165–1175.
- Mariani, L.L.; Mancarella, M.; Novara, L.; Biglia, N. Sonographic features of endometriosis infiltrating the lateral parametrium. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102116.
- Bazot, M.; Delaveau, M.-C.; Daraï, E.; Bendifallah, S. Value of sonography in assessing parametrial endometriotic involvement: Preliminary results. J. Endometr. Pelvic Pain Disord. 2021, 13, 58–65.
- Taksøe-Vester, C.; Dreisler, E.; Andreasen, L.A.; Dyre, L.; Ringsted, C.; Tabor, A.; Tolsgaard, M.G. Up or down? A randomized trial comparing image orientations during transvaginal ultrasound training. Acta Obstet. Gynecol. Scand. 2018, 97, 1455–1462.
- Guerriero, S.; Ajossa, S.; Minguez, J.A.; Jurado, M.; Mais, V.; Melis, G.B.; Alcazar, J.L. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2015, 46, 534–545.
- Gerges, B.; Li, W.; Leonardi, M.; Mol, B.; Condous, G. Meta-analysis and systematic review to determine the optimal imaging modality for the detection of bladder deep endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 261, 124–133.
- Carfagna, P.; De Cicco Nardone, C.; De Cicco Nardone, A. Role of transvaginal ultrasound in evaluation of ureteral in-volvement in deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2018, 51, 550–555.
- Pateman, K.; Holland, T.K.; Knez, J. Should a detailed ultrasound examination of the complete urinary tract be routinely performed in women with suspected pelvic endometriosis? Hum. Reprod. 2015, 30, 2802–2807.
- Chamié, L.P. Ultrasound evaluation of deeply infiltrative endometriosis: Technique and interpretation. Abdom. Radiol. 2019, 45, 1648–1658.
- Guerriero, S.; Ajossa, S.; Orozco, R. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in the rec-tosigmoid: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2016, 47, 281–289.
- Aas-Eng, M.K.; Lieng, M.; Dauser, B.; Diep, L.M.; Leonardi, M.; Condous, G.; Hudelist, G. Transvaginal sonography determines accurately extent of infiltration of rectosigmoid deep endometriosis. Ultrasound Obstet. Gynecol. 2021, 58, 933–939.
- Aas-Eng, M.K.; Dauser, B.; Lieng, M.; Diep, L.M.; Leonardi, M.; Condous, G.; Hudelist, G. Transvaginal sonography accurately measures lesion-to-anal-verge distance in women with deep endometriosis of the rectosigmoid. Ultrasound Obstet. Gynecol. 2020, 56, 766–772.
- Di Giovanni, A.; Casarella, L.; Coppola, M.; Iuzzolino, D.; Rasile, M.; Malzoni, M. Combined Transvaginal/Transabdominal Pelvic Ultrasonography Accurately Predicts the 3 Dimensions of Deep Infiltrating Bowel Endometriosis Measured after Surgery: A Prospective Study in a Specialized Center. J. Minim. Invasive Gynecol. 2018, 25, 1231–1240.
- Leonardi, M.; Uzuner, C.; Mestdagh, W.; Lu, C.; Guerriero, S.; Zajicek, M.; Dueckelmann, A.; Filippi, F.; Buonomo, F.; Pascual, M.A.; et al. Diagnostic accuracy of transvaginal ultrasound for detection of endometriosis using International Deep Endometriosis Analysis (IDEA) approach: Prospective international pilot study. Ultrasound Obstet. Gynecol. 2022, 60, 404–413.
- Exacoustos, C.; Manganaro, L.; Zupi, E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pr. Res. Clin. Obstet. Gynaecol. 2014, 28, 655–681.
- Mabrouk, M.; Raimondo, D.; Arena, A.; Iodice, R.; Altieri, M.; Sutherland, N.; Salucci, P.; Moro, E.; Seracchioli, R. Parametrial Endometriosis: The Occult Condition that Makes the Hard Harder. J. Minim. Invasive Gynecol. 2018, 26, 871–876.
- Chiantera, V.; Petrillo, M.; Abesadze, E.; Sozzi, G.; Dessole, M.; Di Donna, M.C.; Scambia, G.; Sehouli, J.; Mechsner, S. Laparoscopic Neuronavigation for Deep Lateral Pelvic Endometriosis: Clinical and Surgical Implications. J. Minim. Invasive Gynecol. 2018, 25, 1217–1223.
- Guerriero, S.; Martinez, L.; Gomez, I.; Pascual, M.A.; Ajossa, S.; Pagliuca, M.; Alcázar, J.L. Diagnostic accuracy of transvaginal sonography for detecting parametrial involvement in women with deep endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2021, 58, 669–676.
- Leonardi, M.; Martins, W.P.; Espada, M.; Arianayagam, M.; Condous, G. Proposed technique to visualize and classify uterosacral ligament deep endometriosis with and without infiltration into parametrium or torus uterinus. Ultrasound Obstet. Gynecol. 2019, 55, 137–139.
- Guerriero, S.; Ajossa, S.; Gerada, M.; D’Aquila, M.; Piras, B.; Melis, G.B. “Tenderness-guided” transvaginal ultrasonography: A new method for the detection of deep endometriosis in patients with chronic pelvic pain. Fertil. Steril. 2007, 88, 1293–1297.
- Guerriero, S.; Ajossa, S.; Gerada, M.; Virgilio, B.; Angioni, S.; Melis, G.B. Diagnostic value of transvaginal “tenderness-guided” ul-trasonography for the prediction of location of deep endometriosis. Hum. Reprod. 2008, 23, 2452–2457.
- Hudelist, G.; Ballard, K.; English, J.; Wright, J.; Banerjee, S.; Mastoroudes, H.; Thomas, A.; Singer, C.F.; Keckstein, J. Transvaginal sonography vs. clinical examination in the preoperative diagnosis of deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2011, 37, 480–487.
- Ros, C.; Rius, M.; Abrao, M.S.; Deguirior, C.; Martínez-Zamora, M.; Gracia, M.; Carmona, F. Bowel preparation prior to transvaginal ultrasound improves detection of rectosigmoid deep infiltrating endometriosis and is well tolerated: Prospective study of women with suspected endometriosis without surgical criteria. Ultrasound Obstet. Gynecol. 2020, 57, 335–341.
- Ferrero, S.; Scala, C.; Stabilini, C.; Vellone, V.G.; Barra, F.; Maggiore, U.L.R. Transvaginal sonography with vs without bowel preparation in diagnosis of rectosigmoid endometriosis: Prospective study. Ultrasound Obstet. Gynecol. 2019, 53, 402–409.
- Reid, S.; Lu, C.; Hardy, N.; Casikar, I.; Reid, G.; Cario, G.; Chou, D.; Almashat, D.; Condous, G. Office gel sonovaginography for the prediction of posterior deep infiltrating endometriosis: A multicenter prospective observational study. Ultrasound Obstet. Gynecol. 2014, 44, 710–718.
- Reid, S.; Winder, S.; Condous, G. Sonovaginography: Redefining the concept of a “normal pelvis” on transvaginal ultrasound pre-laparoscopic intervention for suspected endometriosis. Australas. J. Ultrasound Med. 2011, 14, 21–24.
- Barra, F.; Maggiore, U.L.R.; Evangelisti, G.; Scala, C.; Alessandri, F.; Vellone, V.G.; Stabilini, C.; Ferrero, S. A prospective study comparing rectal water contrast-transvaginal ultrasonography with sonovaginography for the diagnosis of deep posterior endometriosis. Acta Obstet. et Gynecol. Scand. 2021, 100, 1700–1711.
- Puppo, A.; Olearo, E.; Gattolin, A.; Rimonda, R.; Novelli, A.; Ceccaroni, M. Intraoperative Ultrasound for Bowel Deep Infiltrating Endometriosis: A Preliminary Report. J. Ultrasound Med. 2021, 40, 1417–1425.
- Tomassetti, C.; Bafort, C.; Vanhie, A.; Meuleman, C.; Fieuws, S.; Welkenhuysen, M.; Timmerman, D.; Van Schoubroeck, D.; D’Hooghe, T. Estimation of the Endometriosis Fertility Index prior to operative laparoscopy. Hum. Reprod. 2020, 36, 636–646.
- Ichikawa, M.; Akira, S.; Kaseki, H.; Watanabe, K.; Ono, S.; Takeshita, T. Accuracy and clinical value of an adhesion scoring system: A preoperative diagnostic method using transvaginal ultrasonography for endometriotic adhesion. J. Obstet. Gynaecol. Res. 2020, 46, 466–478.
- Chattot, C.; Huchon, C.; Paternostre, A.; Du Cheyron, J.; Chouillard, E.; Fauconnier, A. Endorect: A preoperative score to accu-rately predict rectosigmoid involvement in patients with endometriosis. Hum. Reprod. Open 2019, 2019, 1–9.
- Menakaya, U.; Reid, S.; Lü, C.; Bassem, G.; Infante, F.; Condous, G. Performance of ultrasound-based endometriosis staging system (UBESS) for predicting level of complexity of laparoscopic surgery for endometriosis. Ultrasound Obstet. Gynecol. 2016, 48, 786–795.
- Tompsett, J.; Leonardi, M.; Gerges, B. Ultrasound-Based Endometriosis Staging System: Validation Study to Predict Com-plexity of Laparoscopic Surgery. J. Minim. Invasive Gynecol. 2019, 26, 477–483.
- Espada, M.; Leonardi, M.; Aas-Eng, K. A Multicenter International Temporal and External Validation Study of the Ultra-sound-based Endometriosis Staging System. J. Minim. Invasive Gynecol. 2021, 28, 57–62.
- Young, S.W.; Groszmann, Y.; Dahiya, N.; Caserta, M.; Yi, J.; Wasson, M.; Patel, M.D. Sonographer-acquired ultrasound protocol for deep endometriosis. Abdom. Radiol. 2019, 45, 1659–1669.
- Deslandes, A.; Parange, N.; Childs, J.T. How long does a transvaginal ultrasound examination for endometriosis take in comparison to a routine transvaginal ultrasound examination? Australas J. Ultrasound Med. 2022, 25, 20–27.
- Downey, D.B.; Fenster, A.; Williams, J.C. Clinical Utility of Three-dimensional US. RadioGraphics 2000, 20, 559–571.
- Raine-Fenning, N.; Jayaprakasan, K.; Deb, S. Three-dimensional ultrasonographic characteristics of endometriomata. Ultrasound Obstet. Gynecol. 2008, 31, 718–724.
- Guerriero, S.; Alcázar, J.L.; Ajossa, S.; Pilloni, M.; Melis, G.B. Three-Dimensional Sonographic Characteristics of Deep Endometriosis. J. Ultrasound Med. 2009, 28, 1061–1066.
- Barra, F.; Alessandri, F.; Scala, C.; Ferrero, S. Ultrasonographic 3D Evaluation in the Diagnosis of Bladder Endometriosis: A Pro-spective Comparative Diagnostic Accuracy Study. Gynecol. Obstet. Investig. 2021, 86, 299–306.
- Guerriero, S.; Saba, L.; Ajossa, S.; Peddes, C.; Angiolucci, M.; Perniciano, M.; Melis, G.B.; Alcázar, J.L. Three-dimensional ultrasonography in the diagnosis of deep endometriosis. Hum. Reprod. 2014, 29, 1189–1198.
- Guerriero, S.; Pilloni, M.; Alcazar, J.L. Tissue characterization using mean gray value analysis in deep infiltrating endome-triosis. Ultrasound Obstet Gynecol. 2013, 41, 459–464.
- Pascual, M.A.; Guerriero, S.; Hereter, L.; Barri-Soldevila, P.; Ajossa, S.; Graupera, B.; Rodriguez, I. Diagnosis of endometriosis of the rectovaginal septum using introital three-dimensional ultrasonography. Fertil. Steril. 2010, 94, 2761–2765.
- Guerriero, S.; Pascual, M.A.; Ajossa, S. The reproducibility of ultrasonographic findings of rectosigmoid endometriosis among examiners with different level of expertise. J. Ultrasound Med. 2022, 41, 403–408.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
06 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




