
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thomas Bintsis | -- | 3296 | 2022-12-02 16:17:22 | | | |
| 2 | Catherine Yang | Meta information modification | 3296 | 2022-12-05 04:56:38 | | |
Video Upload Options
The manufacture of fermented milk products has a long history, and these products were initially produced either from spontaneous fermentation or using a batch of previously produced product, that is, back-slopping. Milk of different mammal species has traditionally been used for the manufacture of fermented milk products. Cow’s milk is the basis for most dairy fermented products around the world. Milk from other mammals, including sheep, goat, camel, mare, buffalo, and yak may have been historically more important and remain so in certain regions. The milks from different species have differences in chemical composition and in certain, vital for the fermentation, components. The diversity of fermented milk products is further influenced by the wide variety of manufacturing practices. A great number of fermented dairy products have been traditionally produced worldwide, and many of them are still produced either following the same traditional process or manufactured industrially, using standardized processes under controlled conditions with specified starter cultures.
1. The Expansion of Fermented Milk Products
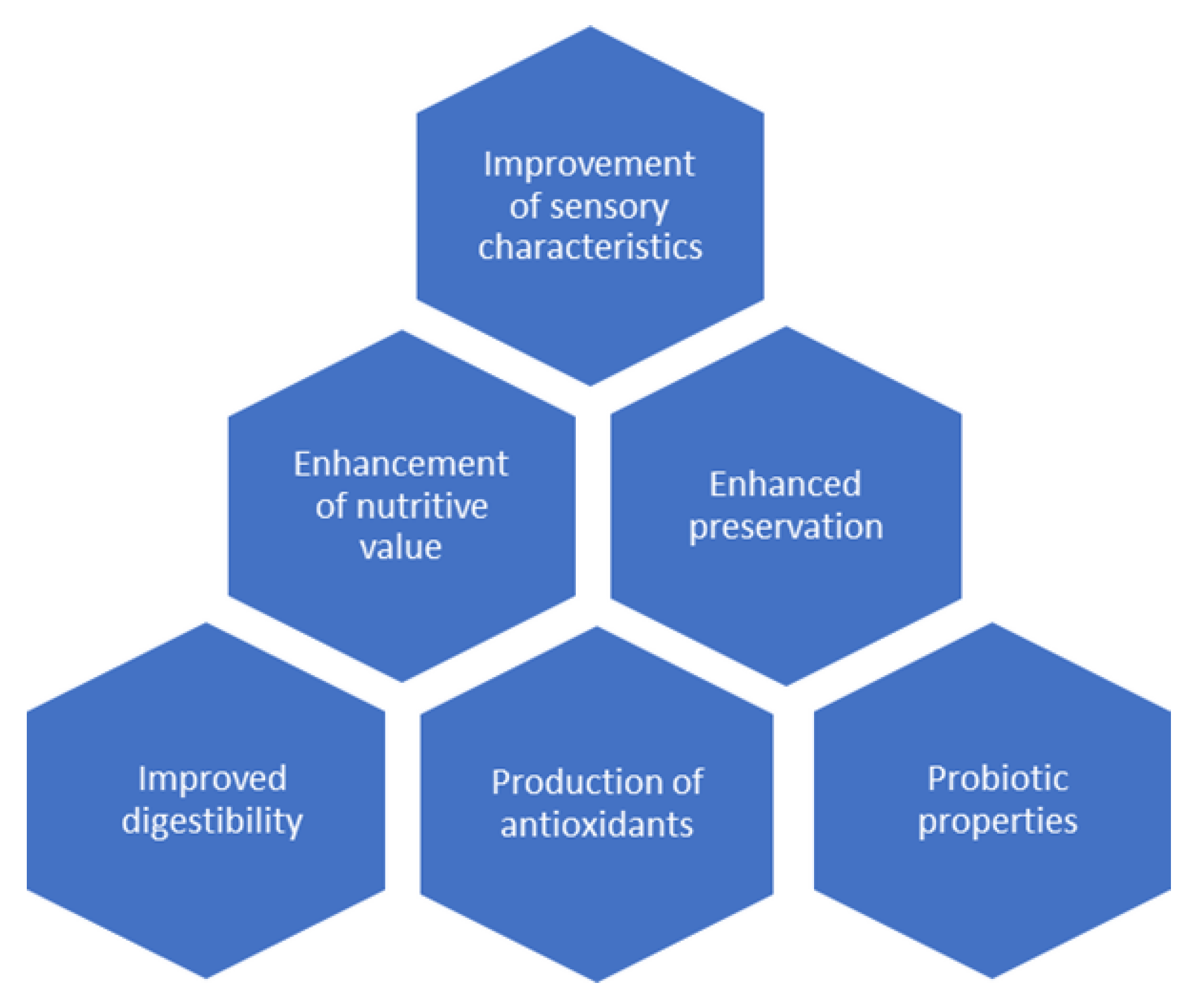
2. Microbiology of Fermented Milk Products
References
- Mannaa, M.; Han, G.; Seo, Y.-S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota gut microbiome. Foods 2021, 10, 2861.
- Robinson, R.K.; Tamime, A.Y. Types of Fermented Milks. In Fermented Milks; Tamime, A.Y., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 1–10.
- Yakulk’s Beginnings. Available online: https://www.yakult.co.jp/english/inbound/history/ (accessed on 30 October 2022).
- Robinson, R.K.; Itsaranuwat, P. Properties of Yoghurt and their Appraisal. In Fermented Milks; Tamime, A.Y., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 76–94.
- Robinson, R.K.; Tamime, A.Y.; Wszolek, M. Microbiology of Fermented Milks. In Dairy Microbiology, 3rd ed.; Robinson, R.K., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2002; pp. 367–430.
- Nejati, F.; Junne, S.; Neubauer, P. A big world in small grain: A review of natural milk Kefir starters. Microorganisms 2020, 8, 192.
- Tamime, A.Y.; Robinson, R.K. Tamime and Robison’s Yoghurt Science and Technology, 3rd ed.; Woodhead Publishing: Cambridge, UK, 2007.
- De Oliveira Leite, A.M.; Miguel, M.A.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M.I. Microbiological, technological and therapeutic properties of kefir: A natural probiotic beverage. Braz. J. Microbiol. 2013, 44, 341–349.
- Gebreselassie, N.; Abrahamsen, R.K.; Beyene, F.; Narvhus, J.A. A survey on spontaneously fermented buttermilk in Northern Ethiopia. Afr. J. Food Sci. Tech. 2012, 3, 78–89.
- Dimassi, O.; Iskandarani, Y.; Afram, M.; Akiki, R.; Rached, M. Production and physicochemical properties of labneh anbaris, a traditional fermented cheese like product, in Lebanon. Int. J. Envir. Agric. Biotech. 2020, 5, 509–516. Available online: https://ijeab.com/ (accessed on 3 November 2022).
- Odunfa, S.A. African fermented foods: From art to science. MIRCEN J. Appl. Microbiol. Biotechnol. 1988, 4, 259–273.
- Şanal, H.; Güler, Z. Changes in Non-essential Element Concentrations during Torba Yoghurt Production. Akad. Gida 2010, 8, 6–12.
- Nielsen, B.; Gűrakan, G.G.; Unlű, G. Kefir: A multifaceted fermented dairy product. Probiot. Antimicrob. Proteins 2014, 6, 123–135.
- Danova, S.; Petrov, K.; Pavlov, P.; Petrova, P. Isolation and characterization of Lactobacillus strains involved in koumiss fermentation. Int. J. Dairy Technol. 2005, 58, 100–105.
- O’Callaghan, Y.C.; Shevade, A.V.; Guinee, T.P.; O’Connor, T.P.; O’Brien, N.M. Comparison of the nutritional composition of experimental fermented milk: Wheat bulgur blends and commercially available kishk and tarhana products. Food Chem. 2019, 278, 110–118.
- Maleke, M.S.; Adefisoye, M.A.; Doorsamy, W.; Adebo, O.A. Processing, nutritional composition and microbiology of amasi: A Southern African fermented milk product. Sci. Afr. 2021, 12, e00795.
- Wang, J.; Chen, X.; Liu, W.; Yang, M.; Zhang, H. Identification of Lactobacillus from koumiss by conventional and molecular methods. Eur. Food Res. Technol. 2008, 227, 1555–1561.
- Lore, T.A.; Mbugua, S.K.; Wangoh, J. Enumeration and identification of microflora in suusac, a Kenyan traditional fermented camel milk product. LWT Food Sci. Tech. 2005, 38, 125–130.
- Carr, F.J.; Chill, D.; Maida, N. The lactic acid bacteria: A literature survey. Crit. Rev. Microbiol. 2002, 28, 281–370.
- Bintsis, T. Lactic Acid Bacteria: Their Applications in Foods. J. Bacteriol. Mycol. 2018, 5, 1065–1069.
- Smit, G.; Smit, B.A.; Engels, E.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610.
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594.
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951.
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins–a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105.
- De Vuyst, L.; Leroy, F. Bacteriocins from lactic acid bacteria: Production, purification, and food applications. J. Mol. Microbiol. Biotechnol. 2007, 13, 194–199.
- Tamime, A.Y. Microbiology of Starter Cultures. In Dairy Microbiology, 3rd ed.; Robinson, R.K., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2002; pp. 261–366.
- Tamime, A.Y.; Skriver, A.; Nilsson, L.-E. Starter cultures. In Fermented Milks; Tamime, A.Y., Ed.; Blackwell Publishing: Oxford, UK, 2006; pp. 11–52.
- Parente, E.; Cogan, T.M.; Powell, I.B. Starter Cultures: General Aspects. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; Fox, P.O., Ed.; Elsevier: Oxford, UK, 2017; pp. 201–226.
- Bintsis, T.; Athanasoulas, A. Dairy Starter Cultures. In Dairy Microbiology—A Practical Approach; Papademas, P., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 114–154.
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016, 7, 377.
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217.
- Tamang, J.P.; Shin, D.H.; Jung, S.J.; Chae, S.W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578.
- FAO. The Future of Food and Agriculture. In Trends and Challenges; FAO: Rome, Italy, 2017; Available online: http://www.fao.org/3/a-i6583e.pdf (accessed on 15 November 2022).
- Alexandraki, V.; Tsakalidou, E.; Papadimitriou, K.; Holzapfel, W.H. Commission on Genetic Resources for Food and Agriculture. In Status and Trends of the Conservation and Sustainable Use of Microorganisms in Food Processes; FAO Background Study Paper; FAO: Rome, Italy, 2013; No. 65.
- Shiby, V.K.; Mishra, H.N. Fermented Milks and Milk Products as Functional Foods—A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 482–496.
- Watanabe, K.; Makino, H.; Sasamoto, M.; Kudo, Y.; Fujimoto, J.; Demberel, S. Biidobacterium mongoliense sp. nov., from airag, a traditional fermented mare’s milk product from Mongolia. Int. J. Syst. Evol. Microbiol. 2009, 59, 1535–1540.
- Mohammadi, R.; Sohrabvandi, S.; Mohammad Mortazavian, A. The starter culture characteristics of probiotic microorganisms in fermented milks. Engineer. Life Sci. 2012, 12, 399–409.
- Khorshidian, N.; Yousefi, M.; Mortazavian, A.M. Fermented milk: The Most Popular Probiotic Food Carrier. In Probiotic and Prebiotics in Foods: Challenges, Innovations and Advances; Gomes da Cruz, A., Prudencio, E.S., Esmerino, E.A., Cristina da Silva, M., Eds.; Academic Press: London, UK, 2020; Volume 94, pp. 91–114.
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative foods: Microbiology, biochemistry, potential human health benefits and public health issues. Foods 2021, 10, 69.
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785.
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients 2019, 11, 1189.
- Shah, N.P. Functional cultures and health benefits. Int. Dairy J. 2007, 1, 1262–1277.
- Aiddo, Κ.; Naunt, M.J.R. Functional Yeasts and Molds in Fermented Foods and Beverages. In Fermented Foods and Beverages of the World; Tamang, J.P., Kailasapathy, K., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 127–148.
- Carpino, S.; Rapisarda, T.; Belvedere, G.; Papademas, P.; Neocleus, M.; Scadt, I.; Pasta, C.; Licitra, G. Effect of dehydration by sun or by oven on volatiles and aroma compounds of Trachanas. Dairy Sci. Technol. 2010, 90, 715–727.
- Georgala, A. The Nutritional Value of Two Fermented Milk/Cereal Foods Named ‘Greek Trahanas’ and ‘Turkish Tarhana’: A Review. J. Nutr. Disord. Ther. 2013, S11, 2161-0509.
- Ekinci, R. The effect of fermentation and drying on the water-soluble vitamin content of tarhana, a traditional Turkish cereal food. Food Chem. 2005, 90, 127–132.
- Ozdemir, S.; Gocmen, D.; Kumral, A.Y. A traditional Turkish fermented cereal food: Tarhana. Food Rev. Int. 2007, 23, 107–121.
- Ross, R.P.; Morgan, S.; Hill, C. Preservation and fermentation: Past, present and future. Int. J. Food Microbiol. 2002, 79, 3–16.
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140.
- Powell, J.E.; Witthuhn, R.C.; Todorov, S.D.; Dicks, L.M.T. Characterization of bacteriocin ST8KF produced by a kefir isolate Lactobacillus plantarum ST8KF. Int. Dairy J. 2007, 17, 190–198.
- Todorov, S.D. Bacteriocin production by Lactobacillus plantarum AMA-K isolated from Amasi, a Zimbabwean fermented milk product and study of adsorption of bacteriocin AMA-K to Listeria spp. Braz. J. Microbiol. 2008, 38, 178–187.
- Liu, W.; Zhang, L.; Yi, H.; Shi, J.; Xue, C.; Li, H.; Jiao, Y.; Shigwedha, N.; Du, M.; Han, X. Qualitative detection of class IIa bacteriocinogenic lactic acid bacteria from traditional Chinese fermented food using a YGNGV-motif-based assay. J. Microbiol. Methods 2014, 100, 121–127.
- Takono, T.; Yamamoto, N. Health Effects of Fermented Milks. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: Oxford, UK, 2011; pp. 483–488.
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Donkey milk for the manufacture of novel functional fermented beverages. J. Food Sci. 2015, 80, S1352–S1359.
- Macouzet, M.; Lee, B.H.; Robert, N. Production of conjugated linoleic acid by probiotic Lactobacillus acidophilus La-5. J. Appl. Microbiol. 2009, 106, 1886–1891.
- Manzo, N.; Pizzolongo, F.; Montefusco, I.; Aponte, M.; Blaiotta, G.; Romano, R. The effects of probiotics and prebiotics on the fatty acid profile and conjugated linoleic acid content of fermented cow milk. Int. J. Food Sci. Nutr. 2015, 66, 254–259.
- Moghdam, B.E.; Keivaninahr, F.; Nazemi, A.; Fouladi, M.; Mokarram, R.R.; Benis, K.Z. Optimization of conjugated linoleic acid production by Bifidobacterium animalis subsp. Lactis and its application in fermented milk. LWT 2019, 108, 344–352.
- Toba, T.; Kotani, T.; Adachi, S. Capsular polysaccharide of a slime-forming Lactococcus lactis ssp. cremoris LAPT 3001 isolated from Swedish fermented milk ‘långfil’. Int. J. Food Microbiol. 1991, 12, 167–171.
- Ruas-Madiedo, P.; Gueimonde, M.; Margolles, A.; de los Reyes-Gavilan, C.G.; Salminen, S. Short communication: Effect of exopolysaccharide isolated from “viili” on the adhesion of probiotics and pathogens to intestinal mucus. J. Dairy Sci. 2006, 89, 2355–2358.
- Ryan, P.M.; Ross, R.P.; Fitzgerald, G.F.; Caplice, N.M.; Stanton, C. Sugar-coated: Exopolysaccharide producing lactic acid bacteria for food and human health applications. Food Funct. 2015, 6, 679–693.
- Tarakoli, M.; Najafi, M.B.H.; Mehebbi, M. Effect of the milk fat content and starter culture selection on proteolysis and antioxidant activity of probiotic yogurt. Heliyon 2019, 5, e01204.
- Tamang, J.P. Himalayan Fermented Foods: Microbiology, Nutrition, and Ethnic Values; CRC Press: Boca Raton, FL, USA; Taylor & Francis: New York, NY, USA, 2010.
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527.
- Gorbach, S.L. Probiotics and gastrointestinal health. Am. J. Gastroenterol. 2000, 95, S2–S4.
- Kort, R.; Sybesma, W. Probiotics for every body. Trends Biotech. 2012, 30, 613–615.
- Boyle, R.J.; Tang, M.L. The role of probiotics in the management of allergic disease. Clin. Experim. Allerg. 2006, 36, 568–576.
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806.
- Kaur, H.; Kaur, G.; Ali, S.A. Dairy-Based Probiotic-Fermented Functional Foods: An Update on Their Health-Promoting Properties. Fermentation 2022, 8, 425.
- Limdi, K.J.; O’Neill, C.; McLaughlin, J. Do probiotics have a therapeutic role in gastroenterology? World J. Gastroenterol. 2006, 12, 5447–5457.
- Young, P.; Cash, D.B. Probiotic use in irritable bowel syndrome. Curr. Gastroenterol. Rep. 2006, 8, 321–326.
- Sudha, M.R.; Chauhan, P.; Dixit, K.; Babu, S.; Jamil, K. Probiotics as complementary therapy for hypercholesterolemia. Biol. Med. 2009, 1, 1–31.
- Kankainen, M.; Paulin, L.; Tynkkynen, S.; von Ossowski, I.; Reunanen, J.; Partanen, P.; Satokari, R.; Vesterlund, S.; Hendrickx, A.P.A. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a humanmucus binding protein. Proc. Natl. Acad. Sci. USA 2009, 106, 17193–17198.
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG–host interactions. Microb. Cell Factor. 2014, 13, S7.
- Solis-Pereyra, B.; Lemonnier, D. Induction of human cytokines by bacteria used in dairy foods. Nutr. Res. 1993, 13, 1127–1140.
- Chen, M.; Ye, X.; Shen, D.; Ma, C. Modulatory Effects of Gut Microbiota on Constipation: The Commercial Beverage Yakult Shapes Stool Consistency. J. Neurogastoenterol. Motil. 2019, 25, 475–477.
- Jeong, D.; Kim, D.-H.; Kang, I.-B.; Kim, H.; Song, K.-Y.; Kim, H.-S.; Seo, K.-H. Modulation of gut microbiota and increase in fecal water content in mice induced by administration of Lactobacillus kefiranofaciens DN1. Food Funct. 2017, 8, 680–686.
- Fernandez, M.A.; Marette, A. Novel perspectives on fermented milks and cardiometabolic health with a focus on type 2 diabetes. Nutr. Rev. 2018, 76, 16–28.
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. J. Dairy Sci. 2018, 101, 900–911.
- Park, S.-Y.; Seong, K.-S.; Lim, S.-D. Anti-obesity effect of yogurt fermented by Lactobacillus plantarum Q180 in diet-induced obese rats. Korean J. Food Sci. Anim. Res. 2016, 36, 77.
- Mofid, V.; Izadi, A.; Mojtahedi, S.Y.; Khedmat, L. Therapeutic and nutritional effects of Synbiotic yogurts in children and adults: A clinical review. Probiot. Antimicrob. Prot. 2019, 12, 851–859.
- Tamime, A.Y.; Barclay, M.N.I.; Amarowicz, R.; McNulty, D. Kishk—A dried fermented milk/cereal mixture. 1. Composition of gross components, carbohydrates, organic acids and fatty acids. Lait 1999, 79, 331–339.
- Hsu, Y.-J.; Huang, W.-C.; Lin, J.-S.; Chen, Y.-M.; Ho, S.-T.; Huang, C.-C.; Tung, Y.-T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862.
- Kim, D.H.; Jeong, D.; Kim, H.; Seo, K.H. Modern perspectives on the health benefits of kefir in next generation sequencing era: Improvement of the host gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 1782–1793.
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15.
- Cocolin, L.; Alessandria, V.; Dolci, P.; Gorra, R.; Rantsiou, K. Culture independent methods to assess the diversity and dynamics of microbiota during food fermentation. Int. J. Food Microbiol. 2013, 167, 29–43.
- Tamang, J.P. Biochemical and Modern Identification Techniques—Microfloras of Fermented Foods. In Encyclopaedia of Food Microbiology, 2nd ed.; Batt, C., Tortorello, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 250–258.
- Ramos, C.L.; de Almeida, E.G.; de Melo Pereira, G.V.; Cardoso, P.G.; Dias, E.S.; Schwan, R.F. Determination of dynamic characteristics of microbiota in a fermented beverage produced by Brazilian Amerindians using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2010, 140, 225–231.
- Cocolin, L.; Aggio, D.; Manzano, M.; Cantoni, C.; Comi, G. An application of PCR-DGGE analysis to profile the yeast populations in raw milk. Int. Dairy J. 2002, 12, 407–411.
- Jianzhonga, Z.; Xiaolia, L.; Hanhub, J.; Mingshengb, D. Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 2009, 26, 770–775.
- Geronikou, A.; Srimahaeak, T.; Rantsiou, K.; Triantafillidis, G.; Larsen, N.; Jespersen, L. Occurrence of yeasts in white-brined cheeses: Methodologies for identification, spoilage potential and good manufacturing practices. Front. Microbiol. 2020, 11, 582778.
- Wolfe, B.E.; Dutton, R.J. Fermented foods as experimentally tractable microbial ecosystems. Cell 2015, 161, 49–55.
- Liu, W.J.; Sun, Z.H.; Zhang, Y.B.; Zhang, C.L.; Menghebilige; Yang, M.; Sun, T.S.; Bao, Q.H.; Chen, W.; Zhang, H.P. A survey of the bacterial composition of kurut from Tibet using a culture-independent approach. J. Dairy Sci. 2012, 95, 1064–1072.
- Konuspayeva, G.; Baubekova, A.; Akhmetsadykova, S.; Faye, B. Traditional dairy fermented products in Central Asia. Int. Dairy J. 2022, 137, 105514.
- Nehme, L.; Shalameh, C.; Tabet, E.; Nehme, M.; Hosri, C. Innovative improvement of Shanklish cheese production in Lebanon. Int. Dairy J. 2019, 90, 23–27.
- Beshkova, D.M.; Simova, E.D.; Simov, Z.I.; Frengova, G.I.; Spasov, Z.N. Pure cultures for making kefir. Food Microbiol. 2002, 19, 537–544.
- Yilmaz, B.; Elibol, E.; Shangpliang, H.N.J.; Ozogul, F.; Tamang, J.P. Microbial Communities in Home-Made and Commercial Kefir and Their Hypoglycemic Properties. Fermentation 2022, 8, 590.
- Kalamaki, M.S.; Angelidis, A.S. High-Throughput, Sequence-Based Analysis of the Microbiota of Greek Kefir Grains from Two Geographic Regions. Food Tech. Biotech. 2020, 58, 138–146.
- Rea, M.C.; Lennartsson, T.; Dillon, P.; Drinan, F.D.; Reville, W.J.; Heapes, M.; Cogan, T.M. Irish kefir-like grains: Their structure, microbial composition and fermentation kinetics. J. Appl. Bacteriol. 1996, 81, 83–94.
- Garrote, G.L.; Abraham, A.G.; de Antoni, G.L. Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 2001, 68, 639–652.
- Kalamaki, M.S.; Angelidis, A.S. Isolation and molecular identification of yeasts in Greek kefir. Int. J. Dairy Tech. 2016, 70, 261–268.
- Simova, E.; Beshkova, D.; Angelov, A.; Hristozova, T.; Frengova, G.; Spasov, Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biotechnol. 2002, 28, 1–6.
- Kesmen, Z.; Kacmaz, N. Determination of Lactic Microflora of Kefir Grains and Kefir Beverage by Using Culture-Dependent and Culture-Independent Methods. J. Food Sci. 2011, 76, M276–M283.
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequencing-based analysis of the bacterial and fungal composition of kefir grains and milks from multiple sources. PLoS ONE 2013, 8, e69371.
- Dobson, A.; O’Sullivan, O.; Cotter, P.D.; Ross, P.; Hill, C. High-throughput sequence-based analysis of the bacterial composition of kefir and an associated kefir grain. FEMS Microbiol. Lett. 2011, 320, 56–62.
- Zamberi, N.R.; Mohamad, N.E.; Yeap, S.K.; Ky, H.; Beh, B.K.; Liew, W.C.; Tan, S.W.; Ho, W.Y.; Boo, S.Y.; Chua, Y.H.; et al. 16S Metagenomic Microbial Composition Analysis of Kefir Grain using MEGAN and BaseSpace. Food Biotechnol. 2016, 30, 219–230.
- Gao, W.; Zhang, L. Comparative analysis of the microbial community composition between Tibetan kefir grains and milks. Food Res. Int. 2019, 116, 137–144.
- Korsak, N.; Taminiau, B.; Leclercq, M.; Nezer, C.; Crevecoeur, S.; Ferauche, C.; Detry, E.; Delcenserie, V.; Daube, G. Short communication: Evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J. Dairy Sci. 2015, 98, 3684–3689.





