Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stephen H. Safe | -- | 1406 | 2022-12-01 23:40:56 | | | |
| 2 | Rita Xu | -4 word(s) | 1402 | 2022-12-02 03:57:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Safe, S.; Zhang, L. Aryl Hydrocarbon Receptor in Breast Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/37695 (accessed on 07 February 2026).
Safe S, Zhang L. Aryl Hydrocarbon Receptor in Breast Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/37695. Accessed February 07, 2026.
Safe, Stephen, Lei Zhang. "Aryl Hydrocarbon Receptor in Breast Cancer" Encyclopedia, https://encyclopedia.pub/entry/37695 (accessed February 07, 2026).
Safe, S., & Zhang, L. (2022, December 01). Aryl Hydrocarbon Receptor in Breast Cancer. In Encyclopedia. https://encyclopedia.pub/entry/37695
Safe, Stephen and Lei Zhang. "Aryl Hydrocarbon Receptor in Breast Cancer." Encyclopedia. Web. 01 December, 2022.
Copy Citation
The aryl hydrocarbon receptor (AhR) is expressed in breast cancer cells and tumors and in some studies, the AhR is a negative prognostic factor for patient survival. Structurally diverse AhR ligands have been extensively investigated as anticancer agents in breast cancer cells and tumors and show efficacy in both estrogen receptor (ER)-positive and ER -negative breast cancer cells. Moreover, synthetic AhR ligands are being developed and have been in clinical trials for treating breast cancer.
AhR
breast cancer
agonist
ligand
1. Introduction
The aryl hydrocarbon receptor (AhR) is a basic helix-loop-helix protein that binds the environmental toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with high affinity and mediates the toxic and biologic effects induced by this compound and structurally-related halogenated aromatics [1][2][3][4][5]. The classical mechanism of AhR-mediated gene expression and functions involves the ligand-dependent formation of the AhR and the AhR nuclear translocator (ARNT) protein as a heterodimer, which in turn binds cognate cis elements in target gene promoters [6]. The cis elements or xenobiotic response elements (XREs) contain a core GCGTG pentanucleotide sequence and variable flanking nucleotides [6][7][8]. This classical mechanism of action of the AHR:ARNT complex which targets sequence-specific cis-elements is similar to that described for many members of the nuclear receptor (NR) superfamily of intracellular receptors such as estrogen receptors (ERs, ESR1) [9][10][11].
Among all intracellular receptors, the AhR is the only receptor identified in molecular toxicology studies focused on determining the mechanism of action of TCDD and structurally related compounds [1][2]. The discovery of the AhR as a “toxicant” receptor has subsequently been a significant hindrance in development and clinical applications of AhR ligands for the treatment of multiple diseases. Overcoming the concerns regarding the potential toxicity of AhR ligands was due to several factors, including the development of AhR knockout (AhRKO) mouse models [12][13][14] and discoveries showing that many AhR ligands are “health promoting” compounds [15][16][17][18][19]. Differences in AhR ligand persistence may be related to their dioxin-like toxicities. The role of the AhR and its ligands as inhibitors of breast cancer in cellular and in vivo models will be investigated. Several studies support a role for AhR ligands as inhibitors of breast cancer and this includes some studies in this laboratory on TCDD and related compounds as antiestrogens associated with ligand-dependent inhibitory AhR-ERα crosstalk [20][21][22]. There is extensive support from cell culture and in vivo studies indicating that the AhR is a target for breast cancer therapy [23] and human clinical trials using the AhR ligand “aminoflavone” have been carried out for treating breast and other cancers [24][25][26][27]. Moreover, studies from several laboratories show that many structural classes of AhR ligands also inhibit some aspects of mammary carcinogenesis [23]. In contrast, there are also reports showing that AhR ligands enhance breast cancer growth and development [28][29][30][31] and there are other examples of AhR/AhR ligands exhibiting both tumor suppressive and tumor promoter-like activities for specific cancers [32][33][34][35][36].
2. Selective AhR Modulators (SAhRMs)
Initial studies on the AhR and the steroid hormone NRs identified exogenous (e.g.,: TCDD) or endogenous (e.g., steroid hormones) ligands that act primarily as receptor agonists. However, for the AhR and other nuclear receptors, it was soon recognized that many different structural classes of ligands also bound the receptor and could act as tissue/cell-and even gene specific agonists, antagonists or partial agonists/antagonists. For example, the AhR binds structurally diverse industrial and synthetic compounds, PAHs, pharmaceuticals, mycotoxins, multiple classes of health promoting phytochemicals including flavonoids, polyphenolics, heteroaromatics such as indole-3-carbinol (I3C), microbial metabolites, and 1,4-dihyroxy-2-naphthoic acid (DHNA) [15][16][17][18][19] (Figure 1 and Figure 2). In addition, some endogenous compounds, including 6-formyl (3,2-b) carbazole (FICZ), 2-(1′-H-indole-3-carbonyl) thiazole-4-carboxylic acid methyl ester (ITE), tryptophan metabolites such as kynurenine and other gut microbial products, and leukotrienes may play a role as endogenous ligands for the AhR [37][38][39][40][41][42].
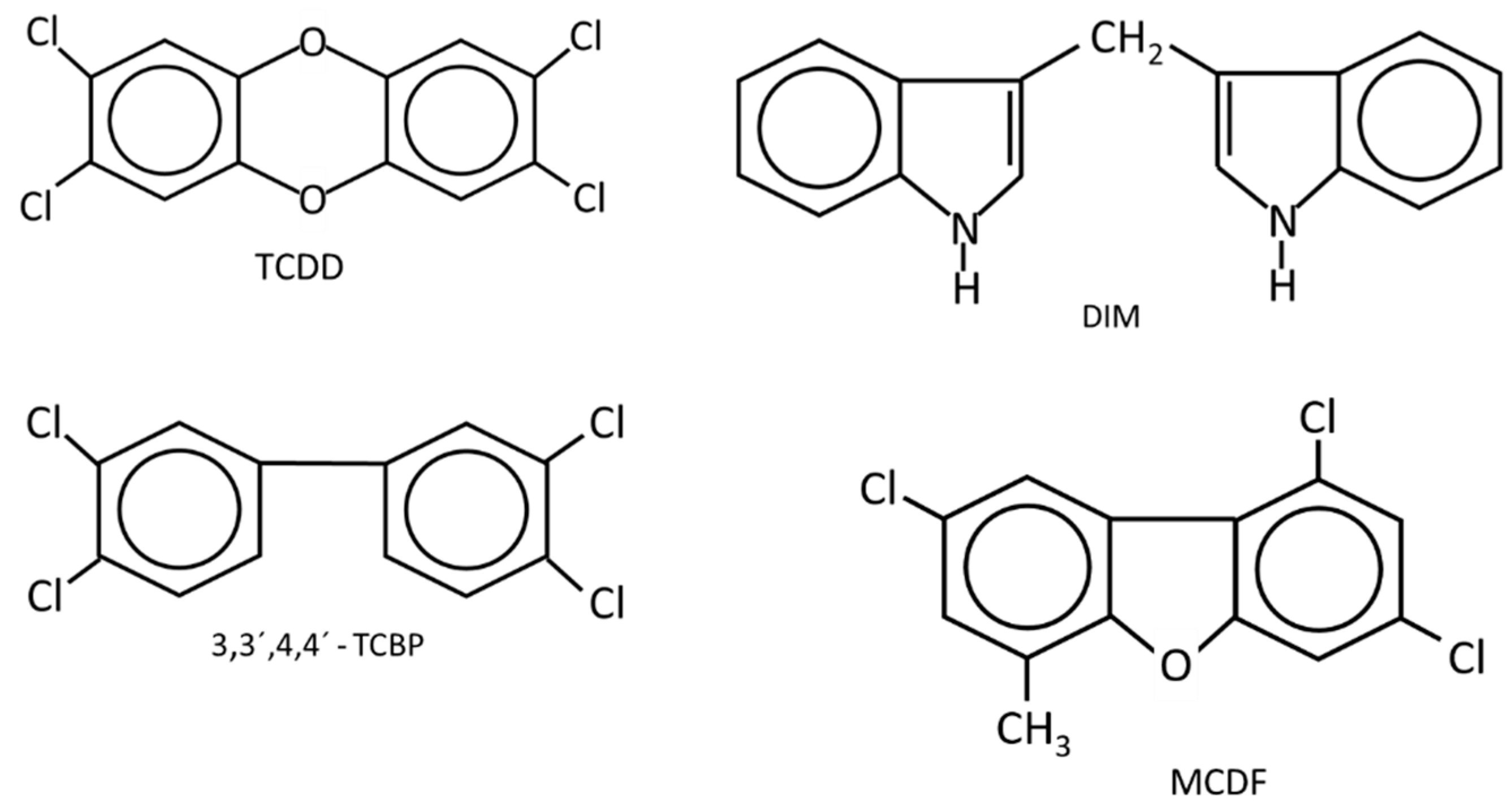
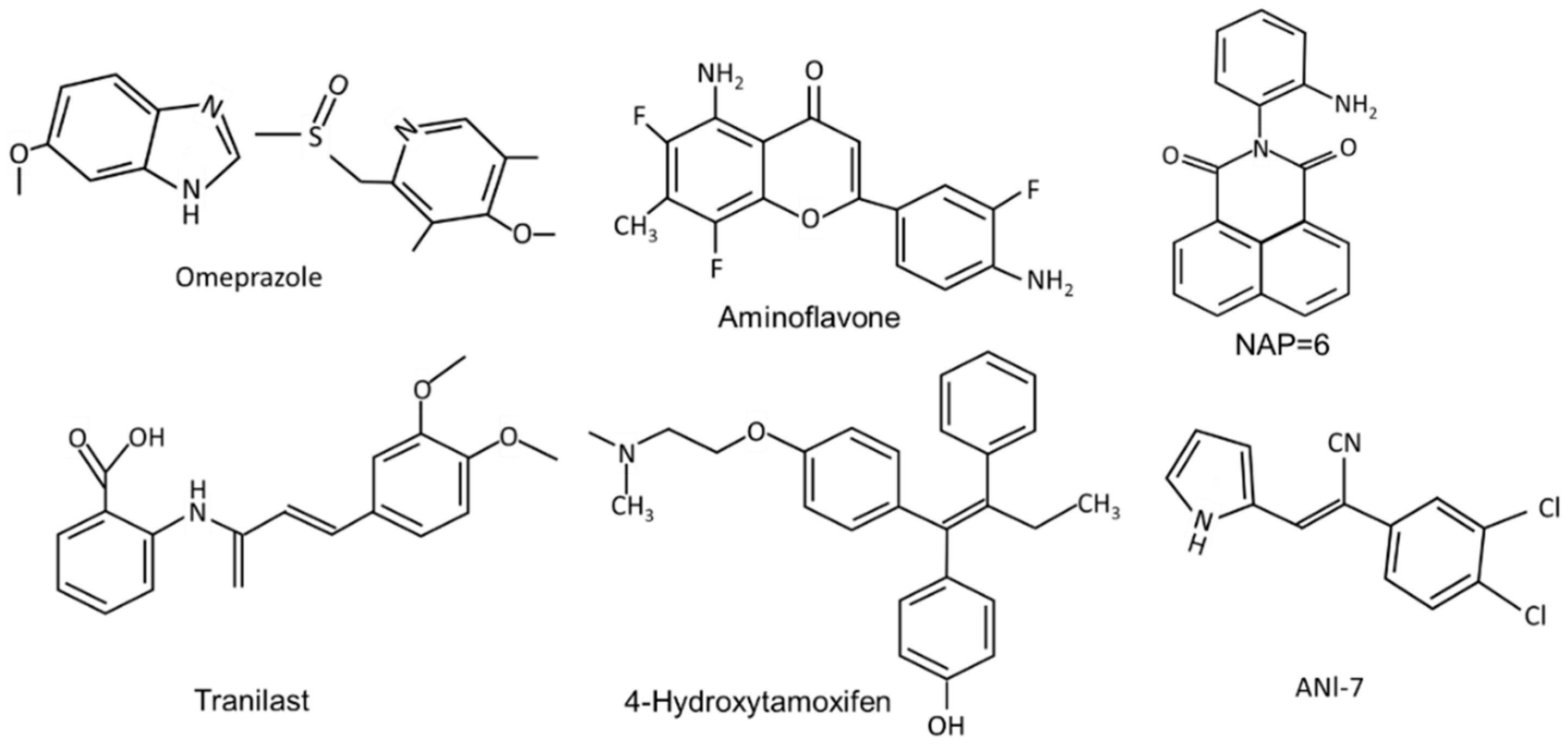
Figure 2. The structures of synthetic compounds, including pharmaceuticals that are AhR ligands.
The selectivity of AhR ligands in terms of their tissue-specific agonist or antagonist activity has been reported in breast and other cancer cell lines and also has been recently reviewed [15]. It is striking that the RNAseq analysis of TCDD and related toxicants are highly variable with respect to their differentially expressed genes. A landmark study of 596 drug-related compounds identified a sub-set of 147 compounds that were evaluated in several Ah-responsive assays, including receptor binding, reporter gene activation and CYP1A1 gene expression [50]. They observed multiple differences among these pharmaceutical compounds to activate putative Ah-responsive endpoints. For example, 59% (81/137) of the compounds induced hepatic CYP1A1 mRNA in mice but did not bind or activate the AhR in vitro. Only nine of these compounds exhibited both in vitro and in vivo activity as AhR agonists in the complete panel of assays which included cytochrome P450 induction in mouse cancer cell lines and liver (in vivo). The selectivity of these AhR-active pharmaceuticals has been further investigated in the laboratory in breast and pancreatic cancer cells [51][52][53].
The pharmaceutical-derived AhR agonists identified in the screening study [51], including flutamide, leflunomide, mexiletine hydrochloride, nimodipine, omeprazole, sulindac and tranilast were investigated in breast cancer cells. Their activity as inducers of CYP1A1 and CYP1B1 in MDA-MB-468 and BT474 breast cancer cells was structure, response and cell type-specific. Compared to TCDD, induction of CYP1A1 was more robust in BT474 than MDA-MB-468 cells, whereas for most of these compounds the reverse was true for the induction of CYP1B1. 4-Hydroxytamoxifen induced minimal (<25% of maximal induction observed for 10 nM TCDD) CYP1A1 and CYP1B1 expression in both cell lines and with the exception of induction of CYP1B1 (50% of maximal induction observed for 10 nM TCDD), tranilast was also a weak inducer of CYP1A1 and CYP1B1 [51]. The pattern of CYP1A1/CYP1B1 induction in MDA-MB-231 cells also differed from that observed in MDA-MB-468 and BT474 cells. The selectivity was also observed for the effects of these pharmaceuticals on the invasion (Boyden chamber) of MDA-MB-231 cells. TCDD (minimal), omeprazole and tranilast inhibited invasion at sub-toxic concentrations, whereas no effects were observed for 4-hydroxytamoxifen, flutamide, leflunomide, mexiletine, nimodipine, and sulindac [52]. Using omeprazole as a model, it was also shown that inhibition of MDA-MB-231 cell invasion by omeprazole was reversed by AhR antagonists and AhR knockdown (siAhR) and inhibition of invasion was primarily due to AhR-dependent downregulation of CXCR4, which was observed both in vitro and in vivo [52]. These highly variable ligand-dependent results in breast cancer cells were observed for pharmaceuticals that were all AhR-active in liver and liver cancer cells, demonstrating that these compounds are SAhRMs. Moreover, there is evidence for SAhRM-like activity for many other structural classes of AhR ligands [15][16].
3. AhR in Breast Cancer: Prognostic Significance
There are multiple genes/gene products expressed in breast cancer and other tumors that can predict overall survival or recurrence of disease and they also may be useful for selecting appropriate treatment regimens [54]. These markers, which include receptors, may or may not be indicative of their functional activity or predict effects of therapeutic regimens. There were some differences in the nuclear and extranuclear distribution of AhR protein in non-pathological breast ductal epithelial cells and invasive ductal carcinoma and AhR overexpression was associated with better prognosis of ER-negative and ER-positive invasive ductal carcinoma patients [55]. In another study on 436 breast cancer cases, it was concluded “that AhR expression is not a prognostic factor in breast cancer” [56]. There were correlations between AhR, and levels of several genes associated with inflammation and high levels of AhR repressor (AhRR) mRNA which predicted enhanced patient survival. This might suggest that since AhRR inhibits AhR function due to competition for ARNT, then high AhR levels would be negative prognostic factors; however, this was not observed in a prospective study of 1116 patients where correlations between multiple prognostic factors and their combination with AhR expression were evaluated for their prognostic value. Low cytosolic AhR levels and positive aromatase were associated with more aggressive ER negative (ER-) tumors; however, AhR tumor genotypes did not correlate with AhR protein levels [57]. Another report indicated that the predictive value of the AhR was dependent on the lymph node status of the patient and concluded “that AhR is a marker of poor prognosis for patients with LN-negative luminal-like BCs” [58]. The results suggest that the prognostic value of AhR levels (mRNA and protein), intracellular location and AhR polymorphisms with respect to patient survival/disease recurrence is complex and dependent on many other factors, including prior patient treatment protocols [55][56][57][58].
References
- Poland, A.; Glover, E.; Kende, A.S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol: Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 1976, 251, 4936–4946.
- Poland, A.; Knutson, J.C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: Examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 1982, 22, 517–554.
- Avilla, M.N.; Malecki, K.M.C.; Hahn, M.E.; Wilson, R.H.; Bradfield, C.A. The Ah Receptor: Adaptive Metabolism, Ligand Diversity, and the Xenokine Model. Chem. Res. Toxicol. 2020, 33, 860–879.
- Schmidt, J.V.; Bradfield, C.A. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 1996, 12, 55–89.
- Beischlag, T.V.; Luis Morales, J.; Hollingshead, B.D.; Perdew, G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 207–250.
- Whitlock, J.P., Jr.; Okino, S.T.; Dong, L.; Ko, H.P.; Clarke-Katzenberg, R.; Ma, Q.; Li, H. Cytochromes P450 5: Induction of cytochrome P4501A1: A model for analyzing mammalian gene transcription. FASEB J. 1996, 10, 809–818.
- Whitlock Jr, J.P. Genetic and molecular aspects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin action. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 251–277.
- Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 307–340.
- Evans, R.M. The nuclear receptor superfamily: A rosetta stone for physiology. Mol. Endocrinol. 2005, 19, 1429–1438.
- Chambon, P. The nuclear receptor superfamily: A personal retrospect on the first two decades. Mol. Endocrinol. 2005, 19, 1418–1428.
- Simpson, E.; Santen, R.J. Celebrating 75 years of oestradiol. J. Mol. Endocrinol. 2015, 55, T1–T20.
- Schmidt, J.V.; Su, G.H.; Reddy, J.K.; Simon, M.C.; Bradfield, C.A. Characterization of a murine AhR null allele: Involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. USA 1996, 93, 6731–6736.
- Mimura, J.; Yamashita, K.; Nakamura, K.; Morita, M.; Takagi, T.N.; Nakao, K.; Ema, M.; Sogawa, K.; Yasuda, M.; Katsuki, M.; et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 1997, 2, 645–654.
- Gonzalez, F.J.; Fernandez-Salguero, P. The aryl hydrocarbon receptor: Studies using the AHR-null mice. Drug Metab Dispos. 1998, 26, 1194–1198.
- Safe, S.; Jin, U.H.; Park, H.; Chapkin, R.S.; Jayaraman, A. Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs). Int. J. Mol. Sci. 2020, 21, 6654.
- Safe, S.; Han, H.; Goldsby, J.; Mohankumar, K.; Chapkin, R.S. Aryl Hydrocarbon Receptor (AhR) Ligands as Selective AhR Modulators: Genomic Studies. Curr. Opin. Toxicol. 2018, 11–12, 10–20.
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334.
- Denison, M.S.; Seidel, S.D.; Rogers, W.J.; Ziccardi, M.H.; Winter, G.M.; Heath-Pagliuso, S. Natural and synthetic ligands for the Ah receptor. In Molecular Bioloy Approaches to Toxicology; Puga, A., Kendall, K.B., Eds.; Taylor and Francis: London, UK, 1998; pp. 3–33.
- Safe, S. Selective Ah receptor modulators (SAhRMs): Progress towards development of a new class of inhibitors of breast cancer growth. J. Women’s Cancer 2001, 3, 37–45.
- Wormke, M.; Stoner, M.; Saville, B.; Walker, K.; Abdelrahim, M.; Burghardt, R.; Safe, S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol. Cell Biol. 2003, 23, 1843–1855.
- Krishnan, V.; Porter, W.; Santostefano, M.; Wang, X.; Safe, S. Molecular mechanism of inhibition of estrogen-induced cathepsin D gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in MCF-7 cells. Mol. Cell Biol. 1995, 15, 6710–6719.
- Safe, S.; Wormke, M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem. Res. Toxicol. 2003, 16, 807–816.
- Baker, J.R.; Sakoff, J.A.; McCluskey, A. The aryl hydrocarbon receptor (AhR) as a breast cancer drug target. Med. Res. Rev. 2020, 40, 972–1001.
- Meng, L.-h.; Kohlhagen, G.; Liao, Z.-y.; Antony, S.; Sausville, E.; Pommier, Y. DNA-protein cross-links and replication-dependent histone H2AX phosphorylation induced by aminoflavone (NSC 686288), a novel anticancer agent active against human breast cancer cells. Cancer Res. 2005, 65, 5337–5343.
- Terzuoli, E.; Puppo, M.; Rapisarda, A.; Uranchimeg, B.; Cao, L.; Burger, A.M.; Ziche, M.; Melillo, G. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1α expression in an AhR-independent fashion. Cancer Res. 2010, 70, 6837–6848.
- Callero, M.A.; Suarez, G.V.; Luzzani, G.; Itkin, B.; Nguyen, B.; Loaiza-Perez, A.I. Aryl hydrocarbon receptor activation by aminoflavone: New molecular target for renal cancer treatment. Int. J. Oncol. 2012, 41, 125–134.
- Kuffel, M.J.; Schroeder, J.C.; Pobst, L.J.; Naylor, S.; Reid, J.M.; Kaufmann, S.H.; Ames, M.M. Activation of the antitumor agent aminoflavone (NSC 686288) is mediated by induction of tumor cell cytochrome P450 1A1/1A2. Mol. Pharmacol. 2002, 62, 143–153.
- Guarnieri, T. Aryl Hydrocarbon Receptor Connects Inflammation to Breast Cancer. Int. J. Mol. Sci. 2020, 21, 5264.
- Schlezinger, J.J.; Liu, D.; Farago, M.; Seldin, D.C.; Belguise, K.; Sonenshein, G.E.; Sherr, D.H. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol. Chem. 2006, 387, 1175–1187.
- Yang, X.; Liu, D.; Murray, T.J.; Mitchell, G.C.; Hesterman, E.V.; Karchner, S.I.; Merson, R.R.; Hahn, M.E.; Sherr, D.H. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene 2005, 24, 7869–7881.
- Narasimhan, S.; Stanford Zulick, E.; Novikov, O.; Parks, A.J.; Schlezinger, J.J.; Wang, Z.; Laroche, F.; Feng, H.; Mulas, F.; Monti, S.; et al. Towards Resolving the Pro- and Anti-Tumor Effects of the Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2018, 19, 1388.
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814.
- Kolluri, S.K.; Jin, U.H.; Safe, S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch. Toxicol. 2017, 91, 2497–2513.
- Jin, U.-H.; Karki, K.; Cheng, Y.; Michelhaugh, S.K.; Mittal, S.; Safe, S. The aryl hydrocarbon receptor is a tumor suppressor–like gene in glioblastoma. J. Biol. Chem. 2019, 294, 11342–11353.
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203.
- Abdelrahim, M.; Ariazi, E.; Kim, K.; Khan, S.; Barhoumi, R.; Burghardt, R.; Liu, S.; Hill, D.; Finnell, R.; Wlodarczyk, B. 3-Methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor α. Cancer Res. 2006, 66, 2459–2467.
- Rannug, A.; Rannug, U.; Rosenkranz, H.S.; Winqvist, L.; Westerholm, R.; Agurell, E.; Grafstrom, A.K. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J. Biol. Chem. 1987, 262, 15422–15427.
- Song, J.; Clagett-Dame, M.; Peterson, R.E.; Hahn, M.E.; Westler, W.M.; Sicinski, R.R.; DeLuca, H.F. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc. Natl. Acad. Sci. USA 2002, 99, 14694–14699.
- Chiaro, C.R.; Morales, J.L.; Prabhu, K.S.; Perdew, G.H. Leukotriene A4 metabolites are endogenous ligands for the Ah receptor. Biochemistry 2008, 47, 8445–8455.
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385.
- Fukumoto, S.; Toshimitsu, T.; Matsuoka, S.; Maruyama, A.; Oh-Oka, K.; Takamura, T.; Nakamura, Y.; Ishimaru, K.; Fujii-Kuriyama, Y.; Ikegami, S.; et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol. Cell Biol. 2014, 92, 460–465.
- Mexia, N.; Gaitanis, G.; Velegraki, A.; Soshilov, A.; Denison, M.S.; Magiatis, P. Pityriazepin and other potent AhR ligands isolated from Malassezia furfur yeast. Arch. Biochem. Biophys. 2015, 571, 16–20.
- Holcomb, M.; Safe, S. Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Lett. 1994, 82, 43–47.
- Ramamoorthy, K.; Gupta, M.S.; Sun, G.; McDougal, A.; Safe, S. 3,3’,4,4’-Tetrachlorobiphenyl exhibits antiestrogenic and antitumorigenic activity in the rodent uterus and mammary and in human breast cancer cells. Carcinogenesis 1999, 20, 115–123.
- Chen, I.; McDougal, A.; Wang, F.; Safe, S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis 1998, 19, 1631–1639.
- McDougal, A.; Wilson, C.; Safe, S. Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by aryl hydrocarbon receptor agonists. Cancer Lett. 1997, 120, 53–63.
- McDougal, A.; Sethi Gupta, M.; Ramamoorthy, K.; Sun, G.; Safe, S.H. Inhibition of carcinogen-induced rat mammary tumor growth and other estrogen-dependent responses by symmetrical dihalo-substituted analogs of diindolylmethane. Cancer Lett. 2000, 151, 169–179.
- McDougal, A.; Wormke, M.; Calvin, J.; Safe, S. Tamoxifen-induced antitumorigenic/antiestrogenic action synergized by a selective aryl hydrocarbon receptor modulator. Cancer Res. 2001, 61, 3902–3907.
- Huggins, C.B. Selective induction of hormone-dependent mammary adenocarcinoma in the rat. J. Lab. Clin. Med. 1987, 109, 262–266.
- Hu, W.; Sorrentino, C.; Denison, M.S.; Kolaja, K.; Fielden, M.R. Induction of cyp1a1 is a nonspecific biomarker of aryl hydrocarbon receptor activation: Results of large scale screening of pharmaceuticals and toxicants in vivo and in vitro. Mol. Pharmacol. 2007, 71, 1475–1486.
- Jin, U.H.; Lee, S.O.; Safe, S. Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. J. Pharm. Exp. 2012, 343, 333–341.
- Jin, U.H.; Lee, S.O.; Pfent, C.; Safe, S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer 2014, 14, 498.
- Jin, U.H.; Kim, S.B.; Safe, S. Omeprazole Inhibits Pancreatic Cancer Cell Invasion through a Nongenomic Aryl Hydrocarbon Receptor Pathway. Chem. Res. Toxicol. 2015, 28, 907–918.
- Saito, R.; Miki, Y.; Hata, S.; Takagi, K.; Iida, S.; Oba, Y.; Ono, K.; Ishida, T.; Suzuki, T.; Ohuchi, N. Aryl hydrocarbon receptor in breast cancer—A newly defined prognostic marker. Horm. Cancer 2014, 5, 11–21.
- Vacher, S.; Castagnet, P.; Chemlali, W.; Lallemand, F.; Meseure, D.; Pocard, M.; Bieche, I.; Perrot-Applanat, M. High AHR expression in breast tumors correlates with expression of genes from several signaling pathways namely inflammation and endogenous tryptophan metabolism. PLoS ONE 2018, 13, e0190619.
- Tryggvadottir, H.; Sandén, E.; Björner, S.; Bressan, A.; Ygland Rödström, M.; Khazaei, S.; Edwards, D.P.; Nodin, B.; Jirström, K.; Isaksson, K. The Prognostic Impact of Intratumoral Aryl Hydrocarbon Receptor in Primary Breast Cancer Depends on the Type of Endocrine Therapy: A Population-Based Cohort Study. Front. Oncol. 2021, 11, 1671.
- Jeschke, U.; Zhang, X.; Kuhn, C.; Jalaguier, S.; Colinge, J.; Pfender, K.; Mayr, D.; Ditsch, N.; Harbeck, N.; Mahner, S. The prognostic impact of the aryl hydrocarbon receptor (AhR) in primary breast cancer depends on the lymph node status. Int. J. Mol. Sci. 2019, 20, 1016.
- Li, Y.; Qin, H.Z.; Song, Q.; Wu, X.D.; Zhu, J.H. Lack of association between the aryl hydrocarbon receptor rs2066853 polymorphism and breast cancer: A meta-analysis on Ahr polymorphism and breast cancer. Genet. Mol. Res. 2015, 14, 16162–16168.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
851
Revisions:
2 times
(View History)
Update Date:
02 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




