Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mozaffar Abdollahifar | -- | 1196 | 2022-12-01 22:48:29 | | | |
| 2 | Vivi Li | Meta information modification | 1196 | 2022-12-02 05:13:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Abdollahifar, M.; Molaiyan, P.; Perovic, M.; Kwade, A. Artificial Solid Electrolyte Interface in Anode Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/37694 (accessed on 07 February 2026).
Abdollahifar M, Molaiyan P, Perovic M, Kwade A. Artificial Solid Electrolyte Interface in Anode Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/37694. Accessed February 07, 2026.
Abdollahifar, Mozaffar, Palanivel Molaiyan, Milena Perovic, Arno Kwade. "Artificial Solid Electrolyte Interface in Anode Materials" Encyclopedia, https://encyclopedia.pub/entry/37694 (accessed February 07, 2026).
Abdollahifar, M., Molaiyan, P., Perovic, M., & Kwade, A. (2022, December 01). Artificial Solid Electrolyte Interface in Anode Materials. In Encyclopedia. https://encyclopedia.pub/entry/37694
Abdollahifar, Mozaffar, et al. "Artificial Solid Electrolyte Interface in Anode Materials." Encyclopedia. Web. 01 December, 2022.
Copy Citation
Due to the ever-growing importance of rechargeable lithium-ion batteries, the development of electrode materials and their processing techniques remains a hot topic in academia and industry. Even the well-developed and widely utilized active materials present issues, such as surface reactivity, irreversible capacity in the first cycle, and ageing. Thus, there have been many efforts to modify and coat the surface of active materials to enhance the electrochemical performance of the resulting electrodes and cells. This type of coating stands out because of the possibility of acting as an artificial solid electrolyte interphase (A-SEI), serving as an anode protective layer.
polymer coating
anode materials
lithium-ion batteries
artificial solid electrolyte interphase
1. Introduction
The development of rechargeable lithium-ion batteries (LIBs) began in the early 1990s and received remarkable attention, as they are employed in many modern portable electronic devices, as well as hybrid electric vehicles (HEVs) and electric vehicles (EVs) because of their high energy densities [1]. The progress is very fast regarding the development of new active anode and cathode materials, electrolyte formulation, electrode composition, and cell design with consideration of safety and costs [2][3]. The interest in developing active materials for LIBs has been considerably increasing to enhance specific capacity and enable higher rate capability and long cycle life stability—the critical parameters for practical applications. Various material modification methods have been introduced in academia and industry to improve the general properties of active materials without changing the elemental or crystal structure, and thereby, the intrinsic properties. One of the well-known methods is surface architecture, which represents surface coating and etching, which protects the active material particles from direct contact with the electrolyte. In LIBs containing liquid (or polymer) electrolytes, the electrode surface is covered by a passivating layer called the solid electrolyte interface (SEI) on the anode materials and the cathode electrolyte interface (CEI) [4] on the cathode particles. The SEI, in particular, has been regarded as a crucial interface in the battery related to the capacity fade, cycle life, and other key performance parameters. The SEI has a protective role, blocking the electrons that could further reduce the electrolyte, but it also consumes the valuable electrolyte and Li-ions, leading to irreversible capacity loss [5][6]. During the first few cycles, the SEI film forms from electrolyte decomposition and reduction reactions with the lithium salts on the anode particle surface [7]. However, the SEI film is usually not stable, particularly for anode materials with massive volume expansion during the lithiation step. Therefore, the electrolyte ions are consumed over the cycles, resulting in capacity degradation [8].
Coating the thin layer of “protective” material onto the active material particle can increase its micro-structure stability, resulting in improved electrochemical properties. Many modifications to the electrode [9][10][11][12][13] and active material particles have been investigated, such as surface modification by carbon, metal oxides, and polymers [14][15][16][17][18][19][20][21][22][23][24]. Coatings consisting of inorganic materials, such as Al2O3, MgO, ZrO2, SiO2, TiO2, and others, commonly provide a Li+-conducting protective layer on the active material, which reduces the chemical reaction between the active material and the electrolyte. However, their deposition usually requires a high-temperature treatment, and the formation of uniformly distributed coating is very challenging.
The SEI should allow the rapid Li-ion transfer between the electrolyte and the electrode without blocking the electron pathway on the electrode current collector interface. Ideally, this layer should self-heal when the changes in the electrode surface occur due to volume expansion. A thin polymer layer on active anode materials can theoretically fulfill these criteria and could act as an artificial SEI. The properties of this layer, such as its thickness, ion-diffusion capability, chemical composition, and mechanical properties, are vital for having a stable electrode.
2. Polymer Coating on Anode Materials
In the following sections, researchers discuss the effect of polymer coating on the electrochemical performance and morphology of different anode materials with mainly three different electrochemical (de)lithiation mechanisms; the advantages and drawbacks for these anode materials are summarized in Table 1:
Table 1. An overview of the (dis)advantages of different types of anodes.
| Types of Anodes | Benefits | Drawbacks |
|---|---|---|
| Intercalation-type anodes | Low-cost materials, high electronic conductivity, good safety for Ti-based anodes, long cycle life, high power capability | Low specific capacity, safety issue for Gr, low energy density |
| Alloy-type anodes | High specific capacity, good safety, low-cost materials and abundance for Si, high energy density | Large volume changes, low electronic conductivity, poor CE, poor cycling |
| Conversion-type anodes | High specific capacity, low-cost materials, low operation potential | Poor CE, unstable SEI, poor cycling |
- −
-
Intercalation anodes, such as Graphite (Gr) and Titanium oxides.
- −
-
Alloy anodes, for instance, Silicon (Si), Germanium (Ge), and Tin (Sn).
- −
-
Conversion anodes, for example, transition metal oxides (Fe3O4, Co3O4, CuO, etc.).
Figure 1 illustrates the advantages and drawbacks of these three types of materials and the connection between working potentials and the specific capacity of the anode materials [25]. Generally, depending on the anode active material, polymer coating on the particles could help resolve some critical challenges, such as poor cycle life and C-rate capability, low Coulombic efficiency (CE), unstable SEI, and high irreversible capacity, which will be addressed here in detail for the specific polymers and anode materials.
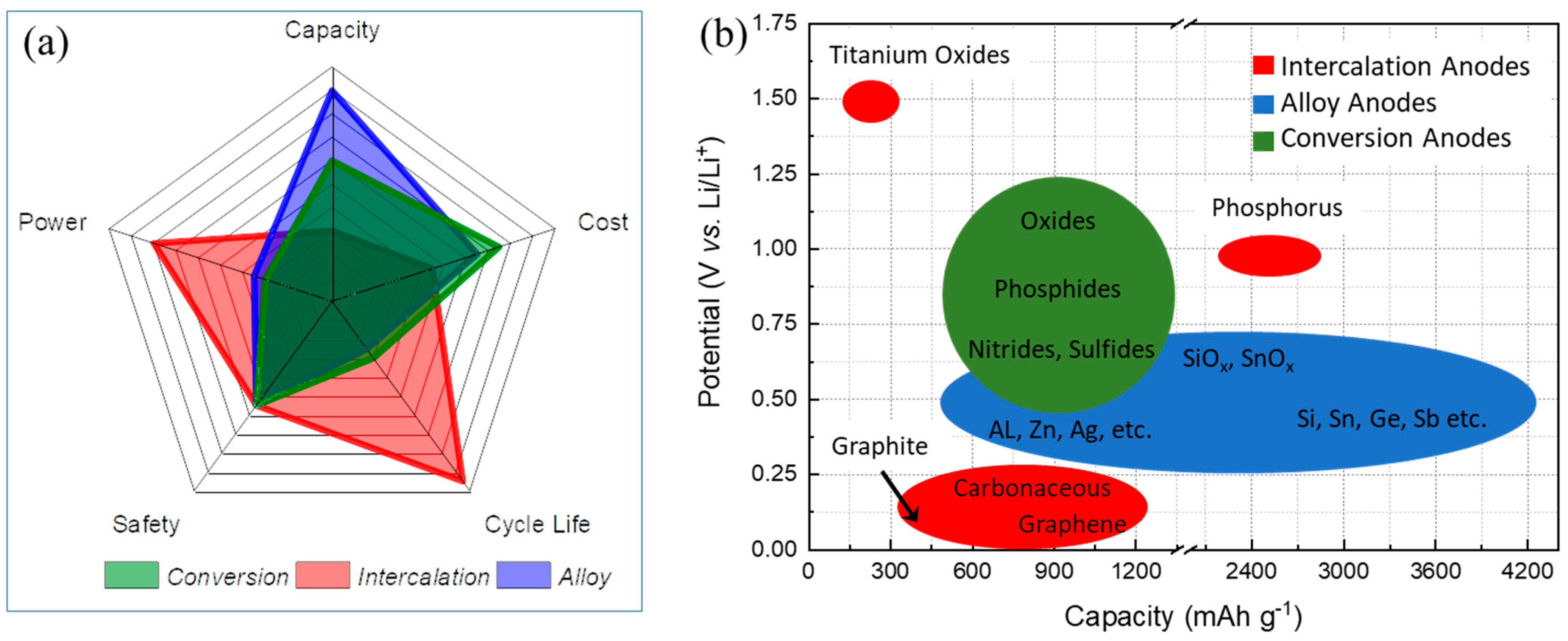
Figure 1. Anode active materials with three different primary electrochemical (de)lithiation mechanisms: (a) Radar plot comparing five critical categories of capacity, cost, cycle life, safety, and power. (b) Schematic illustration comparing potential vs. capacity of certain anode materials.
A thin polymer layer on active anode materials could act as an artificial SEI. This layer can be fabricated for mechanical flexibility to maintain the passivation of active anode materials. The polymer film could be synthesized via different techniques [26][27][28][29][30][31][32][33][34][35][36][37][38][39]. Generally, thin polymer films could accommodate volume expansion (unlike glass or ceramic layers) while simultaneously demonstrating good chemical and structural stabilities during (de)lithiation processes. The polymer film thickness is typically about 2–25 nm [10][40][41][42][43][44][45][46][47][48][49][50][51][52][53].
Conductive polymers are attractive additive materials for LIBs because of their outstanding electrochemical properties: enhancing the electronic conductivity, inhibiting the phase transition, increasing structural stability, decreasing active material dissolution, leading to a remarkable improvement in reversible capacity, rate capability, and cycle stability. Conductive polymers, such as polypyrrole (PPy) [44], polyaniline (PANi) [54], poly(3,4-ethylenedioxythiophene) (PEDOT) [55], PEDOT:poly(styrenesulfonate) (PEDOT:PSS) [31], and polythiophene (PT) [33], but also other polymers, such as polyvinylidene fluoride (PVDF) [12][32] and Polydopamine (PDA) [56], have been used as attractive coating agents for active anode materials to improve the mechanical flexibility and the electrochemical performance of LIBs. Figure 2 demonstrates the contribution of polymers reported in the literature chosen here for coating active anode materials. The category “others” comprises the literature using polyvinylpyrrolidone and polyacrylonitrile [49], poly(1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane) [51], poly(vinyl alcohol) [52][57], polyether, polyethylene glycol tert -octylphenylether and polymer polyallyl amine [17], poly(diallyl dimethylammonium chloride) and poly(sodium 4-styrenesulfonate) [58], polyacrylic acid and polymethacrylic acid [9], PVDF [12][32], 1,3,5-trivinyl-1,3,5-trimethylcyclotrisiloxane [13], PEDOT:PSS [31][59], PDA [56], poly(dimethyldiallylammonium chloride) and poly(methyl methacralate) (PMMA), poly(sodium-p-styrenesulfonate) [60], 1,3,5-trimethylcyclotrisiloxane (V3D3) [13] and poly(ethylene oxide) [61]. These polymers may serve as a host for Li-ion (de)intercalation and enhance the electron transfer in the electrode, particularly with the electrodeposition method. In this entry, researchers will discuss the effects of polymer coating on the electrochemical performance of anode materials.

Figure 2. The percentage of various polymers (PPy, PEDOT, PT, PANi, and others) that have been used to coat anode materials so far reported in the literature.
References
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300.
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264.
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278.
- Cavers, H.; Molaiyan, P.; Abdollahifar, M.; Lassi, U.; Kwade, A. Perspectives on Improving the Safety and Sustainability of High Voltage Lithium-Ion Batteries Through the Electrolyte and Separator Region. Adv. Energy Mater. 2022, 12, 2200147.
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 1997, 144, L208.
- Aurbach, D.; Markovsky, B.; Levi, M.D.; Levi, E.; Schechter, A.; Moshkovich, M.; Cohen, Y. New insights into the interactions between electrode materials and electrolyte solutions for advanced nonaqueous batteries. J. Power Sources 1999, 81, 95–111.
- Meda, U.S.; Lal, L.; Sushantha, M.; Garg, P. Solid Electrolyte Interphase (SEI), a boon or a bane for lithium batteries: A review on the recent advances. J. Energy Storage 2021, 47, 103564.
- Kim, J.; Chae, O.B.; Lucht, B.L. Perspective—structure and stability of the solid electrolyte interphase on silicon anodes of lithium-ion batteries. J. Electrochem. Soc. 2021, 168, 30521.
- Komaba, S.; Ozeki, T.; Okushi, K. Functional interface of polymer modified graphite anode. J. Power Sources 2009, 189, 197–203.
- Wang, C.; Wu, H.; Chen, Z.; McDowell, M.T.; Cui, Y.; Bao, Z. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 2013, 5, 1042.
- Kim, J.-M.; Park, H.-S.; Park, J.-H.; Kim, T.-H.; Song, H.-K.; Lee, S.-Y. Conducting polymer-skinned electroactive materials of lithium-ion batteries: Ready for monocomponent electrodes without additional binders and conductive agents. ACS Appl. Mater. Interfaces 2014, 6, 12789–12797.
- Luo, J.; Wu, C.-E.; Su, L.-Y.; Huang, S.-S.; Fang, C.-C.; Wu, Y.-S.; Chou, J.; Wu, N.-L. A proof-of-concept graphite anode with a lithium dendrite suppressing polymer coating. J. Power Sources 2018, 406, 63–69.
- Carter, R.; Parker, J.F.; Sassin, M.B.; Klein, E.J.; Wolak, M.A.; Love, C.T.; Long, J.W. Initiated Chemical Vapor Deposition of Ultrathin Polymer Coatings at Graphite Electrodes for Enhanced Performance in Li-Ion Batteries. J. Electrochem. Soc. 2020, 167, 60510.
- Li, C.; Zhang, H.P.; Fu, L.J.; Liu, H.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Cathode materials modified by surface coating for lithium ion batteries. Electrochim. Acta 2006, 51, 3872–3883.
- Mauger, A.; Julien, C. Surface modifications of electrode materials for lithium-ion batteries: Status and trends. Ionics 2014, 20, 751–787.
- Oriňáková, R.; Fedorková, A.; Oriňák, A. Effect of PPy/PEG conducting polymer film on electrochemical performance of LiFePO4 cathode material for Li-ion batteries. Chem. Pap. 2013, 67, 860–875.
- Li, F.-S.; Wu, Y.-S.; Chou, J.; Winter, M.; Wu, N.-L. A Mechanically Robust and Highly Ion-Conductive Polymer-Blend Coating for High-Power and Long-Life Lithium-Ion Battery Anodes. Adv. Mater. 2015, 27, 130–137.
- Cao, Z.; Xu, P.; Zhai, H.; Du, S.; Mandal, J.; Dontigny, M.; Zaghib, K.; Yang, Y. Ambient-Air Stable Lithiated Anode for Rechargeable Li-Ion Batteries with High Energy Density. Nano Lett. 2016, 16, 7235–7240.
- Liu, Y.; Zhou, G.; Liu, K.; Cui, Y. Design of Complex Nanomaterials for Energy Storage: Past Success and Future Opportunity. Acc. Chem. Res. 2017, 50, 2895–2905.
- Wang, F.; Chen, G.; Zhang, N.; Liu, X.; Ma, R. Engineering of carbon and other protective coating layers for stabilizing silicon anode materials. Carbon Energy 2019, 1, 219–245.
- Zhou, H.; Yu, S.; Liu, H.; Liu, P. Protective coatings for lithium metal anodes: Recent progress and future perspectives. J. Power Sources 2020, 450, 227632.
- Fedorov, R.G.; Maletti, S.; Heubner, C.; Michaelis, A.; Ein-Eli, Y. Molecular Engineering Approaches to Fabricate Artificial Solid-Electrolyte Interphases on Anodes for Li-Ion Batteries: A Critical Review. Adv. Energy Mater. 2021, 11, 2101173.
- Pham, Q.-T.; Chern, C.-S. Applications of polymers in lithium-ion batteries with enhanced safety and cycle life. J. Polym. Res. 2022, 29, 124.
- Chen, Z.; Soltani, A.; Chen, Y.; Zhang, Q.; Davoodi, A.; Hosseinpour, S.; Peukert, W.; Liu, W. Emerging Organic Surface Chemistry for Si Anodes in Lithium-Ion Batteries: Advances, Prospects, and Beyond. Adv. Energy Mater. 2022, 12, 2200924.
- Lu, J.; Chen, Z.; Pan, F.; Cui, Y.; Amine, K. High-performance anode materials for rechargeable lithium-ion batteries. Electrochem. Energy Rev. 2018, 1, 35–53.
- Yuan, L.; Wang, J.; Chew, S.Y.; Chen, J.; Guo, Z.P.; Zhao, L.; Konstantinov, K.; Liu, H.-K. Synthesis and characterization of SnO2–polypyrrole composite for lithium-ion battery. J. Power Sources 2007, 174, 1183–1187.
- Shao, Q.-G.; Chen, W.-M.; Wang, Z.-H.; Qie, L.; Yuan, L.-X.; Zhang, W.-X.; Hu, X.-L.; Huang, Y.-H. SnO2-based composite coaxial nanocables with multi-walled carbon nanotube and polypyrrole as anode materials for lithium-ion batteries. Electrochem. Commun. 2011, 13, 1431–1434.
- Liu, R.; Li, D.; Wang, C.; Li, N.; Li, Q.; Lü, X.; Spendelow, J.S.; Wu, G. Core–shell structured hollow SnO2–polypyrrole nanocomposite anodes with enhanced cyclic performance for lithium-ion batteries. Nano Energy 2014, 6, 73–81.
- Cao, Z.; Yang, H.; Dou, P.; Wang, C.; Zheng, J.; Xu, X. Synthesis of three-dimensional hollow SnO2@PPy nanotube arrays via template-assisted method and chemical vapor-phase polymerization as high performance anodes for lithium-ion batteries. Electrochim. Acta 2016, 209, 700–708.
- Guan, B.Y.; Yu, L.; Li, J.; Lou, X.W. A universal cooperative assembly-directed method for coating of mesoporous TiO2 nanoshells with enhanced lithium storage properties. Sci. Adv. 2016, 2, e1501554.
- Liu, Y.; Tang, D.; Zhong, H.; Zhang, Q.; Yang, J.; Zhang, L. Facile synthesis of nanostructured Li4Ti5O12/PEDOT:PSS composite as anode material for lithium-ion batteries. RSC Adv. 2016, 6, 95512–95517.
- Sun, X.; Lu, X.; Huang, S.; Xi, L.; Liu, L.; Liu, B.; Weng, Q.; Zhang, L.; Schmidt, O.G. Reinforcing Germanium Electrode with Polymer Matrix Decoration for Long Cycle Life Rechargeable Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 38556–38566.
- Xu, D.; Wang, P.; Yang, R. Conducting polythiophene-wrapped Li4Ti5O12 spinel anode material for ultralong cycle-life Li-ion batteries. Ceram. Int. 2017, 43, 4712–4715.
- Zhou, Y.; Jin, X.; Ni, J.; Zhang, S.; Yang, J.; Liu, P.; Wang, Z.; Lei, J. Evaporation induced uniform polypyrrole coating on CuO arrays for free-standing high lithium storage anode. J. Solid State Electrochem. 2019, 23, 1829–1836.
- Ding, Z.; Zhang, Q.; Chen, Y.; Liu, G.; Xin, X.; He, H.; Cai, B.; Wu, J.; Yao, X. PEDOT-PSS coated VS2 nanosheet anodes for high rate and ultrastable lithium-ion batteries. New J. Chem. 2019, 43, 1681–1687.
- Tolganbek, N.; Mentbayeva, A.; Serik, N.; Batyrgali, N.; Naizakarayev, M.; Kanamura, K.; Bakenov, Z. Design and preparation of thin film gel polymer electrolyte for 3D Li-ion battery. J. Power Sources 2021, 493, 229686.
- Zhang, Z.; Wu, D.; Jiang, L.; Liang, F.; Rui, Y.; Tang, B. One-step synthesis based on non-aqueous sol-gel conductive polymer-coated SnO2 nanoparticles as advanced anode materials for lithium-ion batteries. J. Alloys Compd. 2022, 899, 163274.
- Abdollahifar, M.; Vinograd, A.; Lu, C.-Y.; Chang, S.-J.; Müller, J.; Frankenstein, L.; Placke, T.; Kwade, A.; Winter, M.; Chao, C.-Y. Enabling Long-Cycling Life of Si-on-Graphite Composite Anodes via Fabrication of a Multifunctional Polymeric Artificial Solid–Electrolyte Interphase Protective Layer. ACS Appl. Mater. Interfaces 2022, 34, 38824–38834.
- Seo, J.; Hyun, S.; Moon, J.; Lee, J.Y.; Kim, C. High Performance of a Polydopamine-Coated Graphite Anode with a Stable SEI Layer. ACS Appl. Energy Mater. 2022, 5, 5610–5616.
- Liu, Y.; Matsumura, T.; Imanishi, N.; Hirano, A.; Ichikawa, T.; Takeda, Y. Preparation and Characterization of Si/C Composite Coated with Polyaniline as Novel Anodes for Li-Ion Batteries. Electrochem. Solid State Lett. 2005, 8, A599.
- Xiao, W.; Chen, J.S.; Lu, Q.; Lou, X.W. Porous spheres assembled from polythiophene (PTh)-coated ultrathin MnO2 nanosheets with enhanced lithium storage capabilities. J. Phys. Chem. C 2010, 114, 12048–12051.
- Jeong, J.-M.; Choi, B.G.; Lee, S.C.; Lee, K.G.; Chang, S.-J.; Han, Y.-K.; Lee, Y.B.; Lee, H.U.; Kwon, S.; Lee, G. Hierarchical hollow spheres of Fe2O3@ polyaniline for lithium ion battery anodes. Adv. Mater. 2013, 25, 6250–6255.
- Han, F.; Li, D.; Li, W.-C.; Lei, C.; Sun, Q.; Lu, A.-H. Nanoengineered Polypyrrole-Coated Fe2O3@ C Multifunctional Composites with an Improved Cycle Stability as Lithium-Ion Anodes. Adv. Funct. Mater. 2013, 23, 1692–1700.
- Du, F.-H.; Li, B.; Fu, W.; Xiong, Y.-J.; Wang, K.-X.; Chen, J.-S. Surface binding of polypyrrole on porous Silicon hollow nanospheres for Li-ion battery anodes with high structure Stability. Adv. Mater. 2014, 26, 6145–6150.
- Luo, L.; Zhao, P.; Yang, H.; Liu, B.; Zhang, J.-G.; Cui, Y.; Yu, G.; Zhang, S.; Wang, C.-M. Surface Coating Constraint Induced Self-Discharging of Silicon Nanoparticles as Anodes for Lithium Ion Batteries. Nano Lett. 2015, 15, 7016–7022.
- Yin, Z.; Fan, W.; Ding, Y.; Li, J.; Guan, L.; Zheng, Q. Shell Structure Control of PPy-Modified CuO Composite Nanoleaves for Lithium Batteries with Improved Cyclic Performance. ACS Sustain. Chem. Eng. 2015, 3, 507–517.
- Ma, H.; Liu, X.; Zhang, D.; Xiang, J. Synthesis of polyaniline shell on nickel oxide nanoflake arrays for enhanced lithium ion storage. Mater. Res. Bull. 2017, 96, 301–305.
- Gu, H.; Chen, F.; Liu, C.; Qian, J.; Ni, M.; Liu, T. Scalable fabrication of core-shell structured Li4Ti5O12/PPy particles embedded in N-doped graphene networks as advanced anode for lithium-ion batteries. J. Power Sources 2017, 369, 42–49.
- Attia, E.N.; Hassan, F.M.; Li, M.; Batmaz, R.; Elkamel, A.; Chen, Z. Tailoring the chemistry of blend copolymers boosting the electrochemical performance of Si-based anodes for lithium ion batteries. J. Mater. Chem. A 2017, 5, 24159–24167.
- Fu, R.; Nie, P.; Shi, M.; Wang, J.; Jiang, J.; Zhang, Y.; Wu, Y.; Fang, S.; Dou, H.; Zhang, X. Rigid Polyimide Buffering Layer Enabling Silicon Nanoparticles Prolonged Cycling Life for Lithium Storage. ACS Appl. Energy Mater. 2018, 1, 948–955.
- Shen, B.H.; Wang, S.; Tenhaeff, W.E. Ultrathin conformal polycyclosiloxane films to improve silicon cycling stability. Sci. Adv. 2019, 5, eaaw4856.
- Shi, W.; Wu, H.B.; Baucom, J.; Li, X.; Ma, S.; Chen, G.; Lu, Y. Covalently Bonded Si–Polymer Nanocomposites Enabled by Mechanochemical Synthesis as Durable Anode Materials. ACS Appl. Mater. Interfaces 2020, 12, 39127–39134.
- Hou, L.; Bao, R.; kionga Denis, D.; Sun, X.; Zhang, J.; uz Zaman, F.; Yuan, C. Synthesis of ultralong ZnFe2O4@ polypyrrole nanowires with enhanced electrochemical Li-storage behaviors for lithium-ion batteries. Electrochim. Acta 2019, 306, 198–208.
- Li, Z.-F.; Zhang, H.; Liu, Q.; Liu, Y.; Stanciu, L.; Xie, J. Novel pyrolyzed polyaniline-grafted silicon nanoparticles encapsulated in graphene sheets as Li-ion battery anodes. ACS Appl. Mater. Interfaces 2014, 6, 5996–6002.
- Chen, X.; Cao, Z.; Xing, L.; Liao, Y.; Qiu, Y.; Li, W. Improved Li-storage performance with PEDOT-decorated MnO2 nanoboxes. Nanoscale 2017, 9, 18467–18473.
- Yue, H.; Du, T.; Wang, Q.; Shi, Z.; Dong, H.; Cao, Z.; Qiao, Y.; Yin, Y.; Xing, R.; Yang, S. Biomimetic Synthesis of Polydopamine Coated ZnFe2O4 Composites as Anode Materials for Lithium-Ion Batteries. ACS Omega 2018, 3, 2699–2705.
- Kim, Y.S.; Kim, S.H.; Kim, G.; Heo, S.; Mun, J.; Han, S.; Jung, H.; Kyoung, Y.K.; Yun, D.J.; Baek, W.J.; et al. Protective Oxide Coating for Ionic Conductive Solid Electrolyte Interphase. ACS Appl. Mater. Interfaces 2016, 8, 30980–30984.
- Li, F.-S.; Wu, Y.-S.; Chou, J.; Wu, N.-L. A dimensionally stable and fast-discharging graphite–silicon composite Li-ion battery anode enabled by electrostatically self-assembled multifunctional polymer-blend coating. Chem. Commun. 2015, 51, 8429–8431.
- Liu, J.; Xu, J.; Chen, Y.; Sun, W.; Zhou, X.; Ke, J. Synthesis and electrochemical performance of a PEDOT: Ge composite as the anode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2019, 14, 359–370.
- Wu, Y.S.; Lyu, P.R. Double-Coated Hard Carbon as an Anode Material for High C-Rate Lithium Ion Batteries. Mater. Sci. Forum 2018, 921, 105–110.
- Nieradko, M.; Eskandarian, L.; Semenikhin, O.A. Aluminum anodes coated with polymer electrolyte show improved reversibility and cycling ability in Li-Ion batteries. Electrochim. Acta 2019, 327, 135023.
More
Information
Subjects:
Electrochemistry; Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
02 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




