Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mozaffar Abdollahifar and Version 2 by Vivi Li.
Due to the ever-growing importance of rechargeable lithium-ion batteries, the development of electrode materials and their processing techniques remains a hot topic in academia and industry. Even the well-developed and widely utilized active materials present issues, such as surface reactivity, irreversible capacity in the first cycle, and ageing. Thus, there have been many efforts to modify and coat the surface of active materials to enhance the electrochemical performance of the resulting electrodes and cells. This type of coating stands out because of the possibility of acting as an artificial solid electrolyte interphase (A-SEI), serving as an anode protective layer.
- polymer coating
- anode materials
- lithium-ion batteries
- artificial solid electrolyte interphase
1. Introduction
The development of rechargeable lithium-ion batteries (LIBs) began in the early 1990s and received remarkable attention, as they are employed in many modern portable electronic devices, as well as hybrid electric vehicles (HEVs) and electric vehicles (EVs) because of their high energy densities [1]. The progress is very fast regarding the development of new active anode and cathode materials, electrolyte formulation, electrode composition, and cell design with consideration of safety and costs [2][3][2,3]. The interest in developing active materials for LIBs has been considerably increasing to enhance specific capacity and enable higher rate capability and long cycle life stability—the critical parameters for practical applications. Various material modification methods have been introduced in academia and industry to improve the general properties of active materials without changing the elemental or crystal structure, and thereby, the intrinsic properties. One of the well-known methods is surface architecture, which represents surface coating and etching, which protects the active material particles from direct contact with the electrolyte. In LIBs containing liquid (or polymer) electrolytes, the electrode surface is covered by a passivating layer called the solid electrolyte interface (SEI) on the anode materials and the cathode electrolyte interface (CEI) [4] on the cathode particles. The SEI, in particular, has been regarded as a crucial interface in the battery related to the capacity fade, cycle life, and other key performance parameters. The SEI has a protective role, blocking the electrons that could further reduce the electrolyte, but it also consumes the valuable electrolyte and Li-ions, leading to irreversible capacity loss [5][6][5,6]. During the first few cycles, the SEI film forms from electrolyte decomposition and reduction reactions with the lithium salts on the anode particle surface [7]. However, the SEI film is usually not stable, particularly for anode materials with massive volume expansion during the lithiation step. Therefore, the electrolyte ions are consumed over the cycles, resulting in capacity degradation [8].
Coating the thin layer of “protective” material onto the active material particle can increase its micro-structure stability, resulting in improved electrochemical properties. Many modifications to the electrode [9][10][11][12][13][9,10,11,12,13] and active material particles have been investigated, such as surface modification by carbon, metal oxides, and polymers [14][15][16][17][18][19][20][21][22][23][24][14,15,16,17,18,19,20,21,22,23,24]. Coatings consisting of inorganic materials, such as Al2O3, MgO, ZrO2, SiO2, TiO2, and others, commonly provide a Li+-conducting protective layer on the active material, which reduces the chemical reaction between the active material and the electrolyte. However, their deposition usually requires a high-temperature treatment, and the formation of uniformly distributed coating is very challenging.
The SEI should allow the rapid Li-ion transfer between the electrolyte and the electrode without blocking the electron pathway on the electrode current collector interface. Ideally, this layer should self-heal when the changes in the electrode surface occur due to volume expansion. A thin polymer layer on active anode materials can theoretically fulfill these criteria and could act as an artificial SEI. The properties of this layer, such as its thickness, ion-diffusion capability, chemical composition, and mechanical properties, are vital for having a stable electrode.
2. Polymer Coating on Anode Materials
In the following sections, rwesearchers discuss the effect of polymer coating on the electrochemical performance and morphology of different anode materials with mainly three different electrochemical (de)lithiation mechanisms; the advantages and drawbacks for these anode materials are summarized in Table 1:Table 1.
An overview of the (dis)advantages of different types of anodes.
| Types of Anodes | Benefits | Drawbacks |
|---|---|---|
| Intercalation-type anodes | Low-cost materials, high electronic conductivity, good safety for Ti-based anodes, long cycle life, high power capability | Low specific capacity, safety issue for Gr, low energy density |
| Alloy-type anodes | High specific capacity, good safety, low-cost materials and abundance for Si, high energy density | Large volume changes, low electronic conductivity, poor CE, poor cycling |
| Conversion-type anodes | High specific capacity, low-cost materials, low operation potential | Poor CE, unstable SEI, poor cycling |
- −
-
Intercalation anodes, such as Graphite (Gr) and Titanium oxides.
- −
-
Alloy anodes, for instance, Silicon (Si), Germanium (Ge), and Tin (Sn).
- −
-
Conversion anodes, for example, transition metal oxides (Fe3O4, Co3O4, CuO, etc.).A thin polymer layer on active anode materials could act as an artificial SEI. This layer can be fabricated for mechanical flexibility to maintain the passivation of active anode materials. The polymer film could be synthesized via different techniques [26][27][28][29][30][31][32][33][34][35][36][37][38][39][26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Generally, thin polymer films could accommodate volume expansion (unlike glass or ceramic layers) while simultaneously demonstrating good chemical and structural stabilities during (de)lithiation processes. The polymer film thickness is typically about 2–25 nm [10][40][41][42][43][44][45][46][47][48][49][50][51][52][53][10,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Conductive polymers are attractive additive materials for LIBs because of their outstanding electrochemical properties: enhancing the electronic conductivity, inhibiting the phase transition, increasing structural stability, decreasing active material dissolution, leading to a remarkable improvement in reversible capacity, rate capability, and cycle stability. Conductive polymers, such as polypyrrole (PPy) [44], polyaniline (PANi) [54], poly(3,4-ethylenedioxythiophene) (PEDOT) [55], PEDOT:poly(styrenesulfonate) (PEDOT:PSS) [31], and polythiophene (PT) [33], but also other polymers, such as polyvinylidene fluoride (PVDF) [12][32][12,32] and Polydopamine (PDA) [56], have been used as attractive coating agents for active anode materials to improve the mechanical flexibility and the electrochemical performance of LIBs. Figure 2 demonstrates the contribution of polymers reported in the literature chosen here for coating active anode materials. The category “others” comprises the literature using polyvinylpyrrolidone and polyacrylonitrile [49], poly(1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane) [51], poly(vinyl alcohol) [52][57][52,57], polyether, polyethylene glycol tert -octylphenylether and polymer polyallyl amine [17], poly(diallyl dimethylammonium chloride) and poly(sodium 4-styrenesulfonate) [58], polyacrylic acid and polymethacrylic acid [9], PVDF [12][32][12,32], 1,3,5-trivinyl-1,3,5-trimethylcyclotrisiloxane [13], PEDOT:PSS [31][59][31,59], PDA [56], poly(dimethyldiallylammonium chloride) and poly(methyl methacralate) (PMMA), poly(sodium-p-styrenesulfonate) [60], 1,3,5-trimethylcyclotrisiloxane (V3D3) [13] and poly(ethylene oxide) [61]. These polymers may serve as a host for Li-ion (de)intercalation and enhance the electron transfer in the electrode, particularly with the electrodeposition method. In this papentry, researchersr, we will discuss the effects of polymer coating on the electrochemical performance of anode materials.
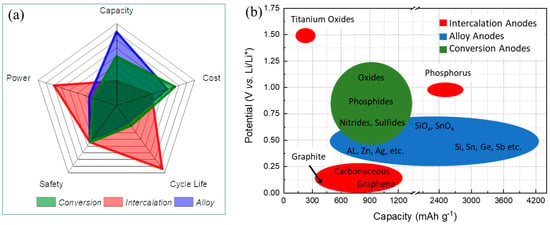 Figure 1. Anode active materials with three different primary electrochemical (de)lithiation mechanisms: (a) Radar plot comparing five critical categories of capacity, cost, cycle life, safety, and power. (b) Schematic illustration comparing potential vs. capacity of certain anode materials.
Figure 1. Anode active materials with three different primary electrochemical (de)lithiation mechanisms: (a) Radar plot comparing five critical categories of capacity, cost, cycle life, safety, and power. (b) Schematic illustration comparing potential vs. capacity of certain anode materials. Figure 2.The percentage of various polymers (PPy, PEDOT, PT, PANi, and others) that have been used to coat anode materials so far reported in the literature.
Figure 2.The percentage of various polymers (PPy, PEDOT, PT, PANi, and others) that have been used to coat anode materials so far reported in the literature.
