Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuanyuan Ma | -- | 3743 | 2022-12-01 15:09:49 | | | |
| 2 | Rita Xu | -19 word(s) | 3724 | 2022-12-02 03:06:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Duan, Y.; Liang, Y.; Yang, F.; Ma, Y. Neural Regulations in Tooth Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/37675 (accessed on 07 February 2026).
Duan Y, Liang Y, Yang F, Ma Y. Neural Regulations in Tooth Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/37675. Accessed February 07, 2026.
Duan, Yihong, Yongfeng Liang, Fangyi Yang, Yuanyuan Ma. "Neural Regulations in Tooth Development" Encyclopedia, https://encyclopedia.pub/entry/37675 (accessed February 07, 2026).
Duan, Y., Liang, Y., Yang, F., & Ma, Y. (2022, December 01). Neural Regulations in Tooth Development. In Encyclopedia. https://encyclopedia.pub/entry/37675
Duan, Yihong, et al. "Neural Regulations in Tooth Development." Encyclopedia. Web. 01 December, 2022.
Copy Citation
The tooth–periodontium complex and its nerves have active reciprocal regulation during development and homeostasis. These effects are predominantly mediated by a range of molecules secreted from either the nervous system or the tooth–periodontium complex. Different strategies mimicking tooth development or physiological reparation have been applied to tooth regeneration studies, where the application of these nerve- or tooth-derived molecules has been proven effective.
tooth
neural regulation
development
homeostasis
1. Introduction
Innervation regulates craniofacial development and homeostasis while being reciprocally affected by target tissues. For example, the absence of innervation causes target organ deformation, as in individuals with Moebius syndrome, a disease caused by the absence or underdevelopment of VI and VII cranial nerves, a series of craniofacial deformations is present [1]. Rabbit submandibular glands undergo atrophy upon denervation, which can be reversed by redistribution of autonomic nerves [2].
The tooth–periodontium complex, including teeth, gingiva, periodontal ligament (PDL), and alveolar bone, is one of the densely innervated cranial structures containing trigeminal sensory nerves and autonomic nerves. An array of evidence has demonstrated the mutual regulation of tooth and dental nerves in development [3]. The mutual regulation also exists in tooth and periodontal homeostasis. For instance, innervation orchestrates inflammation [4], promotes periodontal healing [5], and regulates the physiological reparation and regeneration of dental tissue [6]. These interactions in development and diseases are mediated by a variety of nerve- or tooth-derived molecules and will probably inspire advances in tooth regeneration.
Currently, researchers have employed various strategies for tooth regeneration using fetal tissue [7][8] and tooth germ cells [9] or using adult mesenchymal stem cells (MSCs) [10]. They imitate tooth development and physiological reparation, respectively. However, the innervation of bio-engineered teeth does not fully replicate the highly asymmetric and organized pattern of the natural tooth–periodontium complex [7]. Better restoring tooth innervation requires deeper understanding of the mutual regulation between tooth and dental nerves in development and homeostasis. Understanding this will also benefit dental tissue engineering, as some neurogenic molecules are effective in promoting dental tissue formation [11].
2. Anatomical and Embryological Basis
A tooth can be divided into two parts: the crown visible in the mouth after eruption and the root anchored in alveolar bone by the periodontal ligament. The dental pulp, within the tooth center, is extensively filled with nerves composed of sensory and sympathetic fibers, which arise from the trigeminal ganglion (TG) and the superior cervical ganglion (SCG) respectively (Table 1). Whether parasympathetic nerves innervate the dental pulp is still a subject of debate, but parasympathetic neuropeptides are observed in the dental pulp [4]. The axons mainly accumulate in the coronal pulp to form the subodontoblastic Raschkow plexus, implicating possible crosstalk between odontoblasts and pulpal nerves [4]. The periodontal ligament is densely innervated by mainly sensory fibers and a few autonomic fibers (Table 2). The sensory endings include various proprioceptors, such as Ruffini ending, differing from the sensory innervation in the pulp.
Table 1. Nerves in the pulp.
| Nerve Fibers | Regional Distribution | Function |
|---|---|---|
| sensory | Raschkow plexus Throughout the pulp In the dentinal tubule |
Highly specialized nociceptors |
| sympathetic | SCG→TG→sensory fibers→pulp SCG→inferior/superior alveolar artery→pulp Around pulp arterioles Raschkow plexus |
Regulate pulp blood flow |
Table 2. Nerves in the periodontal ligament.
| Nerve Fibers | Regional Distribution | Function |
|---|---|---|
| sensory | Throughout the PDL As bundles around blood vessels near the alveolar bone As free endings near the cementum |
Nociceptors and mechanoreceptors Regulate blood flow |
| autonomic | Few | Regulate blood flow |
In recent years, extensive studies have suggested the significance of local sensory and sympathetic nerves in maintaining dental and periodontal health and promoting the recovery of local diseases. This not only is because of their anatomical localization but also is demonstrated through inferior alveolar nerve resection (IANr), which not only reduces incisor dentin/enamel formation and injury repair, but also suppresses alveolar bone remodeling and periodontal defect repair [12][13][14]. In addition, sympathetic innervation also appears to take a regulatory role in osteoclast infiltration in rat periapical lesions [15], and alveolar bone remodeling in oral challenge with P. gingivalis [16]. Reversely, carotid sinus nerve stimulation plays a protective role in rat periodontitis, attenuating alveolar bone loss and inflammation [17]. Altogether, these studies agree that normal sensory and sympathetic innervation are critical for tooth–periodontium complex.
The tooth is also capable of influencing nerves, which will be discussed in detail in the following section. The foundation of this mutual regulation between nerves and the tooth–periodontium complex probably lies in their embryological origin. The dental mesenchyme origins from the cranial neural crest (CNC) cells and is developmentally associated with the neural system. One study has revealed that the mesenchymal stem cells of dental pulp (DPSCs) can still be differentiated into functional dopaminergic neurons by midbrain cues in vitro [18]. This remaining responsiveness indicates dental mesenchymal cells have preserved some hereditary similarity with neural cells. More convincingly, RNA-seq analysis has revealed neural/glial cells have a closer origin relation with skeletal cells than pigment cells on the genetic level [19]. This developmental homology suggests there may still be reciprocal regulation between the tooth and the nervous system.
3. Tooth Influences Neurophysiology during Development Process
3.1. Tooth Innervation Is Spatiotemporally Regulated
The tooth sequentially develops as the result of epithelial–mesenchymal interactions. During the embryonic (E) stage for crown formation, the tooth experiences bud, cap, and bell stages, followed by root formation including crown–root transition, root elongation, eruption, and full development after birth (Figure 1). Tooth innervation happens concomitantly with tooth formation, and appears to strongly correlate with tooth developmental stage.
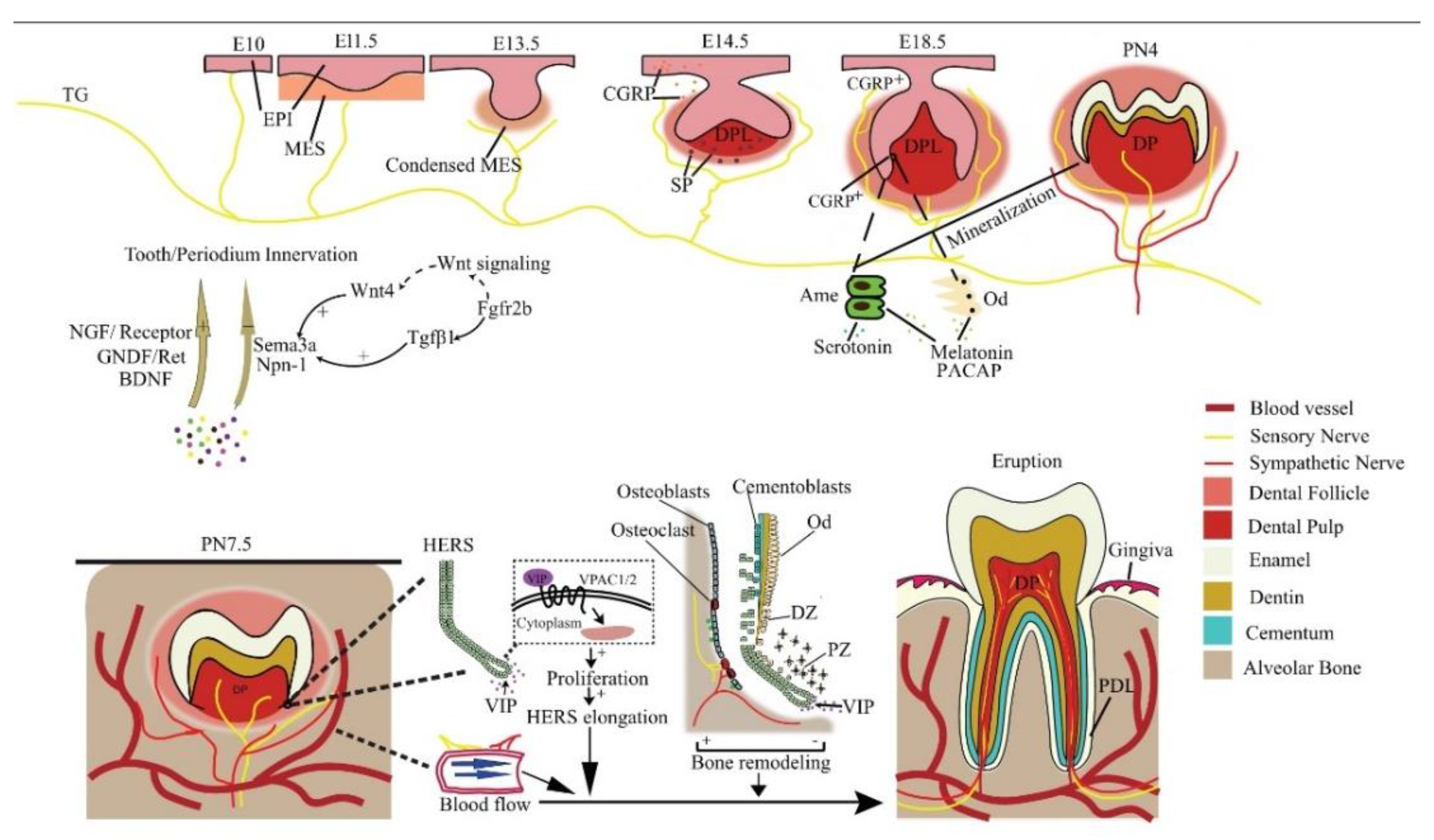
Figure 1. Reciprocal regulation between developing tooth and its innervation. NGF, GNDF, and BDNF promote nerve growth, while Sema3a and Npn-1 suppress it and ensure normal patterning. In a mouse embryo, the pioneering trigeminal ganglion (TG) fibers first arrive at the maxillary oral epithelium on E10. On E14.5, SP is detected and is essential for tooth development to continue. The sensory fibers grow into the dental follicle on E18.5. CGRP, PACAP, melatonin, and serotonin regulate tooth mineralization. Sensory fibers enter the dental pulp on PN4, while the sympathetic fibers enter it on PN 9, after root formation has begun. Neural VIP promotes the elongation of HERS to stimulate the differentiation of DF cells, resulting in root growth. Periodontal nerves regulate bone remodeling as well as the local blood flow in alveolar sockets to affect tooth eruption. EPI = epithelium; MES = mesenchyme; Ame = ameloblast; Od = odontoblast; NGF = nerve growth factor; DPL = dental papilla; DP = dental pulp; PDL = periodontal ligament; CGRP+ = CGRP positive cells; DZ = differentiation zone; PZ = proliferation zone.
At E11.5, mouse molar tooth formation is initiated as a local thickening of dental epithelium, while the pioneering trigeminal fibers first arrive at the maxillary oral epithelium at E10 [20]. However, the trigeminal fibers do not infringe on the presumptive dental mesenchyme or developing tooth germ but only navigate their periphery from initiation to the cap stage and grow into the dental follicle (DF) at the bell stage [20]. At the cap stage, the sensory fibers form a plexus under the dental papilla, which later gives rise to the fine DF branches, but will not enter the pulp until a thin layer of enamel has been deposited at postnatal (PN) day 4. Intriguingly, the trigeminal fibers enter the pulp through presumptive root apices in a multi-rooted tooth, in the absence of any physical barrier such as the pulp floor [20], implicating a predetermined mesenchymal route for nerve ingrowth. The sympathetic fibers, which previously only exist around blood vessels outside the dental pulp, enter the dental pulp following sensory fibers on PN9, after root formation has begun [21]. This indicates that the ingrowth of dental nerves follows developmentally regulated timing, order, and routes.
Evidence shows that the developing dental tissues can regulate their own innervation by either attracting or repelling neurites (Figure 1). For example, semaphorin 3a (Sema3a) is a nerve repellent expressed in tooth germs. Its distribution in tissue shifts as tooth development proceeds, thus controlling the timing and route of tooth innervation [20]. On the other hand, auto-transplanted tooth germs can induce their own innervation [22], and adult denervated teeth can be automatically reinnervated [23][24]. In line with these findings, an in vitro co-culture study reveals tooth germ mesenchyme differentially attracts or repels neurites from TG explants depending on the developmental stage [25], meaning both the attracting and repelling are developmentally regulated.
3.2. The Molecular Guidance Cues for Tooth–Periodontium Innervation
Many molecules, mainly neurotrophins and semaphorins, expressed in and around the developing tooth germ modulate tooth innervation and other aspects of tooth development, such as cytodifferentiation. Many of them are also implied in periodontal innervation. Studies of tooth regeneration do not monitor the expression of these molecules [7][9][10], but their proper spatiotemporal expression is vital for the normal patterning of tooth nerves. Proper application of these molecules may benefit tooth nerve regeneration.
3.2.1. Neurotrophins
Neurotrophins are identified as a family of growth factors important for neuronal survival, development, and function, as well as for the immune and reproductive systems. NGF as a prototypic neurotrophin is a significant target-derived promotive factor for peripheral sensory and sympathetic innervation. NGF immunoreactivity is mainly located in the dental mesenchyme in the bud stage, while in the cap and bell stages, it is mainly expressed in the dental epithelium and adjacent odontoblast layer. Its gene expression pattern correlates with pulpal neurite growth in postnatal mouse molars, suggesting that NGF is involved in the local sprouting, guidance, and arrangement of trigeminal axons in developing teeth [26]. This can explain why neonatal exposure to anti-NGF reduces the number of trigeminal neurons projecting to rat molar dental pulp [27].
NGF receptors TrkA and p75NTR (Figure 1) are concentrated in nerve fibers approaching the tooth germ and mediate innervation throughout the whole development process [28]. It is reported that TrkA knockout mice lack both sympathetic and sensory pupal nerves [29] and the deletion of p75NTR in mice causes weaker osteogenic ability [30]. Furthermore, the p75NTR gene positively correlates with mineralization-related genes in mouse ectomesenchymal stem cells [31], possibly involved in the melanoma-associated antigen-D1 (Mage-D1) and NF-κB pathway [32][33]. Therefore, the dental NGF receptors are important not only for innervation but also for tooth morphogenesis and hard tissue formation.
Other neurotrophic factors, including brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and glial-derived neurotrophic factor (GDNF) are also implicated in the innervating of dental nerves (Figure 1). Ablation of GDNF/Ret signaling before pulpal innervation significantly reduces pulpal neurites in Ret knockout mice, as well in mice treated with Ret inhibitor [34]. Interestingly, in an adult tooth, Ret+ fibers only make up a portion of pulpal afferents and are predominantly expressed in a mutually exclusive manner with TrkA in trigeminal sensory neurons, although all the small, medium, or large diameter trigeminal sensory neurons may contain them both concomitantly [34]. Even so, GDNF and NGF work in synergy to promote the innervation of all types of neurons classified by size. However, their individual target neurons have little overlapping, meaning they cannot be regarded as functionally equivalent agents. A similar phenomenon might also exist with other neurotrophins mentioned above. Recently, increasing studies are pointing to the morphological and even functional heterogeneity among dental pulp afferents [35][36][37][38]. Therefore, neurotrophins may have a predilection for specific subpopulations of pulp afferents with a mutually exclusive expression.
Neurotrophins are also involved in periodontal innervation. Periodontal mechanoreceptive Ruffini ending shows immunoreactivity for TrkB, an affinity receptor for BDNF and neurotrophin-4/5. BDNF knockdown reduces periodontal nerve density and causes Ruffini ending deformation in mice [39] more severely than neurotrophin-4/5 depletion does at the early stage [40]. The cellular distribution and expression patterns of GDNF and its receptors in the periodontium coincide with the development of Ruffini endings. Ablation of GDNF/Ret signaling also results in abnormal periodontal fibers [34]. This indicates that various neurotrophins regulate periodontal innervation.
3.2.2. Semaphorins
The semaphorin family is a class of secreted, membrane-bound proteins that regulate many developmental processes [41]. Most semaphorins are chemorepellent against neurites, but some may promote axonal growth, such as semaphorin7A, which is expressed in odontoblasts and promotes dentin–pulp complex terminal innervation.
Sema3a is the most studied in dental development studies. Sema3a expression is absent from the distribution domains of dental nerves in embryonic mouse tooth germ, while the expression of its receptor, neuropilin-1 (Npn1), is restricted to dental axons. In Sema3a−/− and Npn1−/− mice embryos, nerve fibers appear prematurely in the presumptive dental mesenchyme and later ectopically in the dental papilla mesenchyme [42][43]. In postnatal mouse molar, Sema3a is observed in the future pulp floor area, but absent from the future apical foramen, which illustrates its control of normal pulpal nerve pathways through the apical foramen [20]. Interestingly, the expression pattern of multiple neurotrophins is not altered in Sema3a−/− mice, indicating the attracting and repelling regulations in tooth innervation are exclusive but coordinated [20][42][43].
Sema3a also impacts periodontal nerve distribution and structure. In Sema3a−/− mouse incisors, the number of nerves and arborizations abnormally increases in the developing dental follicle target field and periodontium, and there are abundant nerve fibers in the labial periodontium [42]. Unlike in the periodontium, whether Sema3a affects pulpal nerve structure is contingent. In Sema3a null mice, pulpal nerve thickness and fasciculation are notably reduced in molars but have no apparent changes in incisors [42][43]. This is probably due to the subtle differences between incisor and molar innervation, which is also the explanation for why Sema3a-knockout-induced premature innervation happens slightly later in incisors than in molars [42].
Notably, Sema3a modulation is discrepant between teeth and other hard tissue. Its knockout does not trigger dental hard tissue defects but causes skeletal deformation and osteopenia [44]. Speculatively, this is probably because other semaphorins that possess similar functions, mainly class 3, 4, 5, and 6 molecules [25], also potentially regulate tooth development and may compensate for the lost non-neuronal function of Sema3a in developing teeth.
The upstream signaling of Sema3a is seldom studied. It appears that Wnt4 and Tgf-β1 in the dental epithelium induce early Sema3a expression in the dental mesenchyme on E10-E11 via epithelial–mesenchymal interactions (Figure 1). In addition, Fgfr2b−/− molar germ is devoid of Tgf-β1, which accounts for the abnormal Sema3a expression from the E13.5 late bud stage onwards [45]. The canonical Wnt signaling pathway is also altered in Fgfr2b−/− teeth, but not via Wnt4. Evidence shows that Wnt4 stimulates jaw mesenchymal Msx1 expression, which is indispensable for tooth morphogenesis beyond the bud stage, whereas Tgf-β1 promotes dental mesenchymal cell proliferation. Thereby Wnt, Tgf-β, and Fgf signaling conjugate tooth innervation with tooth formation [20].
Conclusively, Sema3a is a potent repellent for pulpal and periodontal innervation. Local injection of a Sema3a inhibitor alone is sufficient to promote innervation of bio-engineered teeth [46], revealing its potency as a regulatory target for bio-engineered teeth.
3.3. Related Application in Tooth Regeneration
The molecules controlling tooth innervation may be applied for achieving better regeneration of the tooth neural network. In a study of preserving immature teeth with non-vital pulp, the application of amelogenin after root canal treatment successfully induces pulp regeneration with a dense neural network [47]. Amelogenin is a vital component of the enamel matrix. This confirms molecules other than neurotrophins and semaphorins may also regulate tooth innervation and again demonstrates that a developing tooth controls its own innervation.
Similarly, neurotrophins and semaphorins may also be applied in tooth and periodontium regeneration. However, like many other regeneration studies, did not compare the distribution, pattern, and function of the regenerated pulp neural network with those of a natural one, meaning the regeneration may not be fully successful. Therefore, if neurotrophins and semaphorins are really to be applied, their physiological expression pattern during tooth development should be carefully scrutinized to instruct proper application so that the tooth neural network can best mimic a natural one.
4. The Nervous System Regulates Tooth Development
4.1. Neural Regulations in Tooth Development at Pre-Eruptive Stage
Controversy exists with regard to the participation of nerves in tooth initiation. In polyphyodont fish, denervation completely abolished tooth germ formation. However, studies on mice suggest the opposite. Mouse diastema is the edentulous region between incisors and molars which contains three unerupted tooth primordia that develop until the bud stage. It is devoid of peripheral nerves, indicating innervation is not required for mouse tooth initiation [48]. Consistently, mouse embryonic mandible explants with or without TG attached produce similar tooth formation incidence [49]. However, these conclusions are flawed in that they cannot factor out the pioneer trigeminal fibers that arrive near the oral epithelium prior to dental placode formation [50]. These pioneer fibers might prime the oral epithelium to form a dental placode (Figure 1). In fact, a recent study detected calcitonin gene-related peptide (CGRP), a common sensory nerve marker, from E14.5 to E17.5 with epithelium predilection in mice [51]. In addition, substance P (SP), a well-known member of the tachykinin family released by sensory neurons, is transiently expressed in developing mouse tooth germ. Its receptor antagonist blocks the development of ex vivo mouse tooth germs at E14, which is reversed by exogenous SP [52]. These pieces of evidence endorse the speculation that some early-arriving sensory fibers interact with the oral epithelium to initiate tooth development. Above all, controversy persists on the role of innervation in tooth initiation and early development before mineralization. In view of the pioneering trigeminal fibers to the oral epithelium, it would be desirable to use animal models whose dental innervation is completely ablated with genetic tools to obtain more compelling evidence.
In the mineralizing phase of tooth development, innervation clearly regulates the normal structure and morphology of the tooth. In cap and bell stage mouse molars, CGRP is mainly detected around the dental epithelium–mesenchyme interface, and its expression suddenly increases during E17.5 to E18.5, when odontoblasts and ameloblasts differentiate from the dental papilla and inner enamel epithelium, respectively [51]. This coincidence indicates a role of sensory fibers in tooth development, but how the nerves elicit normal dentinogenesis and amelogenesis remains to be explored.
Some studies have highlighted specific neurogenic factors in the regulation of tooth development. Nerve fibers containing PACAP, a pleiotropic neuropeptide, have been observed in the odontoblastic and subodontoblastic layers of the dental pulp [53] (Figure 1). PACAP-deficient mice exhibit aberrant enamel or dentine structure, accompanied by smaller incisors and thinner molar dentin [54]. This is most likely due to the regulation of PACAP on Sonic hedgehog (Shh) and Notch signaling which are critical in tooth morphogenesis [55][56].
In addition, neurohormones released in the central nervous system (CNS) also regulate tooth development. Melatonin can enhance odontogenic differentiation of dental papilla cells in the late bell stage [57] by upregulating RORα and altering mitochondrial function and biogenesis in these cells [58][59]. Melatonin is also reported to promote the normal survival and mineralization of ameloblasts in postnatal mouse molar germs [60] by activating the MAPK signaling via repressing β-arrestin [61], and the Wnt pathway may also be involved [62]. Moreover, the precursor of melatonin, serotonin, stimulates the development of tooth germs in an embryonic mouse mandibular explant culture beyond the bud stage, meaning it may promote the differentiation of tooth germ cells [63]. Further analysis reveals this may be achieved by direct activation of serotonin receptors [64]. In addition, serotonin 2B receptor knockdown leads to abnormal enamel volume and structure in mouse molars [65]. Serotonin regulates periodontal development too. The intake of a serotonin re-uptake inhibitor, fluoxetine, decreases periodontium-forming cells [66].
Together, these studies suggest the importance of innervation and neuron-derived factors in regulating normal tooth morphogenesis. These factors may prove helpful in regenerating teeth with tooth germ cells or fetal tissue with respect to their developmental roles.
4.2. Neural Regulations in Tooth Eruption
Many hypotheses have emerged trying to elucidate the underlying mechanism for tooth eruption, but none have gained entire recognition. However, it is widely accepted tooth eruption depends on the development and remodeling of the surrounding jaw bone and the extent of tooth root development [67]. Therefore, neuroregulation probably regulates eruption by targeting these physiological processes (Figure 1).
Alveolar bone remodeling: Both sensory and autonomic nerves can actively regulate bone remodeling. During experimental tooth movement, nerve bundles are found to co-localize with osteoclasts and Howship’s resorption lacunae [68], providing ponderable evidence of neural participation in alveolar bone resorption. In light of this, alveolar nerves might also participate in tooth eruption. Although the researchers did not further specify the biomolecular event, intrabone nerves regulate bone remodeling mainly through secreting various molecules targeting bone cells [69]. For instance, the sympathetic neurotransmitter norepinephrine overall suppresses bone formation and promotes bone resorption [69].
Dental follicle (DF): The DF plays a critical role in tooth eruption by regulating jaw bone remodeling and tooth root growth. Eruption totally fails after surgically removing DF, while the removal of the developing tooth crown or root did not disrupt normal eruption [70]. However, a direct investigation into the neural regulation of DF function is still absent to date. Nevertheless, it seems dental innervation can regulate DF function indirectly via Hertwig’s epithelial root sheath (HERS), an important guide for root growth. Growing HERS expresses a receptor for vasoactive intestinal peptide (VIP), which enhances HERS elongation in ex vivo tooth germs by promoting cell proliferation [71]. In turn, HERS cells can stimulate the differentiation of cementoblasts or osteoblasts and the mineralization of DF cells via the Wnt signaling pathway [72].
Local blood flow: The vasculature within the alveolar socket is also thought to affect tooth eruption. The local injection of vasoactive drugs alters socket blood flow as well as tooth eruption rate in rats dose-dependently [73]. Systemic volume expansion by injection of Ringer’s solution also increases socket blood flow and tooth eruption rate by decreasing systemic arterial pressure, not by local nutrition [74]. Both sensory and autonomic innervations in the periodontium regulate local blood flow, thereby regulating tooth eruption.
Overall, although neural participation in tooth eruption is abundantly implicated, a concrete mechanism is still waiting to be unveiled, and further research in this direction will help decipher the mystery of the general tooth eruption mechanism as well. For rodent incisors, tooth eruption is continuous throughout the whole life. Researchers will discuss stem cells and neural regulation in incisor homeostasis and injury repair in detail in Section 7.
4.3. Related Application in Tooth Regeneration
Some of the neurogenic molecules implicated in tooth development are applicable to tooth regeneration, imitating their developmental roles. For example, serotonin is used to treat murine oral keratinocytes (MOKs), which also arise from embryonic mouse oral epithelium as the enamel organ does. MOKs growing in the presence of serotonin after reprogramming with Tgf-β1 successfully produced amelogenin, and in a 3D culture, they formed enamel-producing organoids. After densification with pressure and vacuum, the enamel formed by MOKs possesses a similar structure and mechanical properties to natural enamel, suggesting the potential application of serotonin in enamel regeneration via tissue engineering methods [11]. Other molecules discussed here may also help regenerate dental tissues, but their efficacy and application methods need to be explored.
References
- Ruge-Peña, N.O.; Valencia, C.; Cabrera, D.; Aguirre, D.C.; Lopera, F. Moebius syndrome: Craniofacial clinical manifestations and their association with prenatal exposure to misoprostol. Laryngoscope Investig. Otolaryngol. 2020, 5, 727–733.
- Zhang, S.-E.; Su, Y.-X.; Zheng, G.-S.; Liang, Y.-J.; Liao, G.-Q. Reinnervated nerves contribute to the secretion function and regeneration of denervated submandibular glands in rabbits. Eur. J. Oral Sci. 2014, 122, 372–381.
- Luukko, K.; Kettunen, P. Coordination of tooth morphogenesis and neuronal development through tissue interactions: Lessons from mouse models. Exp. Cell Res. 2014, 325, 72–77.
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. Dental nerves: A neglected mediator of pulpitis. Int. Endod. J. 2021, 54, 85–99.
- Nonaka, S.; Kitaura, H.; Kimura, K.; Ishida, M.; Takano-Yamamoto, T. Expression of pituitary adenylate cyclase-activating peptide (PACAP) and PAC1 in the periodontal ligament after tooth luxation. Cell. Mol. Neurobiol. 2013, 33, 885–892.
- Diogenes, A. Trigeminal Sensory Neurons and Pulp Regeneration. J. Endod. 2020, 46, S71–S80.
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230.
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480.
- Duailibi, M.T.; Duailibi, S.E.; Young, C.S.; Bartlett, J.D.; Vacanti, J.P.; Yelick, P.C. Bioengineered teeth from cultured rat tooth bud cells. J. Dent. Res. 2004, 83, 523–528.
- Hung, C.-N.; Mar, K.; Chang, H.-C.; Chiang, Y.-L.; Hu, H.-Y.; Lai, C.-C.; Chu, R.-M.; Ma, C.M. A comparison between adipose tissue and dental pulp as sources of MSCs for tooth regeneration. Biomaterials 2011, 32, 6995–7005.
- Bazina, F.; Brouxhon, S.M.; Graham, U.M.; Kyrkanides, S. Serotonin contributes to the in vitro production of a biomimetic enamel-like material from reprogrammed oral epithelial keratinocytes. Orthod. Craniofac. Res. 2021, 24, 494–501.
- Liu, A.Q.; Zhang, L.S.; Fei, D.D.; Guo, H.; Wu, M.L.; Liu, J.; He, X.N.; Zhang, Y.J.; Xuan, K.; Li, B. Sensory nerve-deficient microenvironment impairs tooth homeostasis by inducing apoptosis of dental pulp stem cells. Cell Prolif. 2020, 53, e12803.
- Hayano, S.; Fukui, Y.; Kawanabe, N.; Kono, K.; Nakamura, M.; Ishihara, Y.; Kamioka, H. Role of the Inferior Alveolar Nerve in Rodent Lower Incisor Stem Cells. J. Dent. Res. 2018, 97, 954–961.
- Xu, Y.; Xia, M.; Chen, T.; Yang, Y.; Fu, G.; Ji, P.; Wu, Q. Inferior alveolar nerve transection disturbs innate immune responses and bone healing after tooth extraction. Ann. N. Y. Acad. Sci. 2019, 1448, 52–64.
- Haug, S.R.; Heyeraas, K.J. Effects of sympathectomy on experimentally induced pulpal inflammation and periapical lesions in rats. Neuroscience 2003, 120, 827–836.
- Kim, Y.; Hamada, N.; Takahashi, Y.; Sasaguri, K.; Tsukinoki, K.; Onozuka, M.; Sato, S. Cervical sympathectomy causes alveolar bone loss in an experimental rat model. J. Periodontal Res. 2009, 44, 695–703.
- Ribeiro, A.B.; Brognara, F.; da Silva, J.F.; Castania, J.A.; Fernandes, P.G.; Tostes, R.C.; Salgado, H.C. Carotid sinus nerve stimulation attenuates alveolar bone loss and inflammation in experimental periodontitis. Sci. Rep. 2020, 10, 19258.
- Kanafi, M.; Majumdar, D.; Bhonde, R.; Gupta, P.; Datta, I. Midbrain cues dictate differentiation of human dental pulp stem cells towards functional dopaminergic neurons. J. Cell Physiol. 2014, 229, 1369–1377.
- Tatarakis, D.; Cang, Z.; Wu, X.; Sharma, P.P.; Karikomi, M.; MacLean, A.L.; Nie, Q.; Schilling, T.F. Single-cell transcriptomic analysis of zebrafish cranial neural crest reveals spatiotemporal regulation of lineage decisions during development. Cell Rep. 2021, 37, 110140.
- Kettunen, P.; Løes, S.; Furmanek, T.; Fjeld, K.; Kvinnsland, I.H.; Behar, O.; Yagi, T.; Fujisawa, H.; Vainio, S.; Taniguchi, M.; et al. Coordination of trigeminal axon navigation and patterning with tooth organ formation: Epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development 2005, 132, 323–334.
- Moe, K.; Kettunen, P.; Kvinnsland, I.H.; Luukko, K. Development of the pioneer sympathetic innervation into the dental pulp of the mouse mandibular first molar. Arch. Oral Biol. 2008, 53, 865–873.
- Erdélyi, G.; Fried, K.; Hildebrand, C. Nerve growth to tooth buds after homotopic or heterotopic autotransplantation. Brain Res. 1987, 430, 39–47.
- Fried, K.; Erdélyi, G. Inferior alveolar nerve regeneration and incisor pulpal reinnervation following intramandibular neurotomy in the cat. Brain Res. 1982, 244, 259–268.
- Holland, G.R.; Robinson, P.P. Reinnervation of the canine tooth pulp after section of the inferior alveolar nerve in the cat. Brain Res. 1985, 329, 300–303.
- Lillesaar, C.; Fried, K. Neurites from trigeminal ganglion explants grown in vitro are repelled or attracted by tooth-related tissues depending on developmental stage. Neuroscience 2004, 125, 149–161.
- Mahdee, A.; Eastham, J.; Whitworth, J.M.; Gillespie, J.I. Evidence for changing nerve growth factor signalling mechanisms during development, maturation and ageing in the rat molar pulp. Int. Endod. J. 2019, 52, 211–222.
- Qian, X.B.; Naftel, J.P. Effects of neonatal exposure to anti-nerve growth factor on the number and size distribution of trigeminal neurones projecting to the molar dental pulp in rats. Arch. Oral. Biol. 1996, 41, 359–367.
- Mitsiadis, T.A.; Pagella, P. Expression of Nerve Growth Factor (NGF), TrkA, and p75(NTR) in Developing Human Fetal Teeth. Front. Physiol. 2016, 7, 338.
- Matsuo, S.; Ichikawa, H.; Henderson, T.A.; Silos-Santiago, I.; Barbacid, M.; Arends, J.J.; Jacquin, M.F. trkA modulation of developing somatosensory neurons in oro-facial tissues: Tooth pulp fibers are absent in trkA knockout mice. Neuroscience 2001, 105, 747–760.
- Zhao, M.; Wang, Y.; Li, G.; Li, J.; Yang, K.; Liu, C.; Wen, X.; Song, J. The role and potential mechanism of p75NTR in mineralization via in vivo p75NTR knockout mice and in vitro ectomesenchymal stem cells. Cell Prolif. 2020, 53, e12758.
- Zhao, M.; Wen, X.; Li, G.; Ju, Y.; Wang, Y.; Zhou, Z.; Song, J. The spatiotemporal expression and mineralization regulation of p75 neurotrophin receptor in the early tooth development. Cell Prolif. 2019, 52, e12523.
- Yang, K.; Wang, Y.; Ju, Y.; Li, G.; Liu, C.; Liu, J.; Liu, Q.; Wen, X.; Liu, L.C. p75 neurotrophin receptor regulates differential mineralization of rat ectomesenchymal stem cells. Cell Prolif. 2017, 50, e12290.
- Shan, P.; Wang, X.; Zhang, Y.; Teng, Z.; Zhang, Y.; Jin, Q.; Liu, J.; Ma, J.; Nie, X. P75 neurotrophin receptor positively regulates the odontogenic/osteogenic differentiation of ectomesenchymal stem cells via nuclear factor kappa-B signaling pathway. Bioengineered 2022, 13, 11201–11213.
- Donnelly, C.R.; Shah, A.A.; Suh, E.B.; Pierchala, B.A. Ret Signaling Is Required for Tooth Pulp Innervation during Organogenesis. J. Dent. Res. 2019, 98, 705–712.
- Chung, M.K.; Jue, S.S.; Dong, X. Projection of non-peptidergic afferents to mouse tooth pulp. J. Dent. Res. 2012, 91, 777–782.
- Kim, H.Y.; Chung, G.; Jo, H.J.; Kim, Y.S.; Bae, Y.C.; Jung, S.J.; Kim, J.S.; Oh, S.B. Characterization of dental nociceptive neurons. J. Dent. Res. 2011, 90, 771–776.
- Vang, H.; Chung, G.; Kim, H.Y.; Park, S.-B.; Jung, S.J.; Kim, J.-S.; Oh, S.B. Neurochemical properties of dental primary afferent neurons. Exp. Neurobiol. 2012, 21, 68–74.
- Won, J.; Vang, H.; Lee, P.R.; Kim, Y.H.; Kim, H.W.; Kang, Y.; Oh, S.B. Piezo2 Expression in Mechanosensitive Dental Primary Afferent Neurons. J. Dent. Res. 2017, 96, 931–937.
- Hoshino, N.; Harada, F.; Alkhamrah, B.A.; Aita, M.; Kawano, Y.; Hanada, K.; Maeda, T. Involvement of brain-derived neurotrophic factor (BDNF) in the development of periodontal Ruffini endings. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 274, 807–816.
- Maruyama, Y.; Harada, F.; Jabbar, S.; Saito, I.; Aita, M.; Kawano, Y.; Suzuki, A.; Nozawa-Inoue, K.; Maeda, T. Neurotrophin-4/5-depletion induces a delay in maturation of the periodontal Ruffini endings in mice. Arch. Histol. Cytol. 2005, 68, 267–288.
- Jongbloets, B.C.; Pasterkamp, R.J. Semaphorin signalling during development. Development 2014, 141, 3292–3297.
- Shrestha, A.; Moe, K.; Luukko, K.; Taniguchi, M.; Kettunen, P. Sema3A chemorepellant regulates the timing and patterning of dental nerves during development of incisor tooth germ. Cell Tissue Res. 2014, 357, 15–29.
- Moe, K.; Sijaona, A.; Shrestha, A.; Kettunen, P.; Taniguchi, M.; Luukko, K. Semaphorin 3A controls timing and patterning of the dental pulp innervation. Differentiation 2012, 84, 371–379.
- Hayashi, M.; Nakashima, T.; Taniguchi, M.; Kodama, T.; Kumanogoh, A.; Takayanagi, H. Osteoprotection by semaphorin 3A. Nature 2012, 485, 69–74.
- Kettunen, P.; Spencer-Dene, B.; Furmanek, T.; Kvinnsland, I.H.; Dickson, C.; Thesleff, I.; Luukko, K. Fgfr2b mediated epithelial-mesenchymal interactions coordinate tooth morphogenesis and dental trigeminal axon patterning. Mech. Dev. 2007, 124, 868–883.
- Kuchler-Bopp, S.; Bagnard, D.; Van-Der-Heyden, M.; Idoux-Gillet, Y.; Strub, M.; Gegout, H.; Lesot, H.; Benkirane-Jessel, N.; Keller, L. Semaphorin 3A receptor inhibitor as a novel therapeutic to promote innervation of bioengineered teeth. J. Tissue Eng. Regen. Med. 2018, 12, e2151–e2161.
- Mounir, M.M.F.; Rashed, F.M.; Bukhary, S.M. Regeneration of Neural Networks in Immature Teeth with Non-Vital Pulp Following a Novel Regenerative Procedure. Int. J. Stem Cells 2019, 12, 410–418.
- Løes, S.; Kettunen, P.; Kvinnsland, H.; Luukko, K. Mouse rudimentary diastema tooth primordia are devoid of peripheral nerve fibers. Anat. Embryol. 2002, 205, 187–191.
- Lumsden, A.G.; Buchanan, J.A. An experimental study of timing and topography of early tooth development in the mouse embryo with an analysis of the role of innervation. Arch. Oral Biol. 1986, 31, 301–311.
- Stainier, D.Y.; Gilbert, W. Pioneer neurons in the mouse trigeminal sensory system. Proc. Natl. Acad. Sci. USA 1990, 87, 923–927.
- Maeda, Y.; Miwa, Y.; Sato, I. Distribution of the neuropeptide calcitonin gene-related peptide-α of tooth germ during formation of the mouse mandible. Ann. Anat. 2019, 221, 38–47.
- Weil, M.; Itin, A.; Keshet, E. A role for mesenchyme-derived tachykinins in tooth and mammary gland morphogenesis. Development 1995, 121, 2419–2428.
- Ichikawa, H.; Sugimoto, T. Pituitary adenylate cyclase-activating polypeptide-immunoreactive nerve fibers in rat and human tooth pulps. Brain Res. 2003, 980, 288–292.
- Sandor, B.; Fintor, K.; Reglodi, D.; Fulop, D.B.; Helyes, Z.; Szanto, I.; Nagy, P.; Hashimoto, H.; Tamas, A. Structural and Morphometric Comparison of Lower Incisors in PACAP-Deficient and Wild-Type Mice. J. Mol. Neurosci. 2016, 59, 300–308.
- Fulop, B.D.; Sandor, B.; Szentleleky, E.; Karanyicz, E.; Reglodi, D.; Gaszner, B.; Zakany, R.; Hashimoto, H.; Juhasz, T.; Tamas, A. Altered Notch Signaling in Developing Molar Teeth of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)-Deficient Mice. J. Mol. Neurosci. 2019, 68, 377–388.
- Cai, X.; Gong, P.; Huang, Y.; Lin, Y. Notch signalling pathway in tooth development and adult dental cells. Cell Prolif. 2011, 44, 495–507.
- Tachibana, R.; Tatehara, S.; Kumasaka, S.; Tokuyama, R.; Satomura, K. Effect of melatonin on human dental papilla cells. Int. J. Mol. Sci. 2014, 15, 17304–17317.
- Kang, J.; Chen, H.; Zhang, F.; Yan, T.; Fan, W.; Jiang, L.; He, H.; Huang, F. RORα Regulates Odontoblastic Differentiation and Mediates the Pro-Odontogenic Effect of Melatonin on Dental Papilla Cells. Molecules 2021, 26, 1098.
- Jiang, L.L.; Zhang, F.P.; He, Y.F.; Fan, W.G.; Zheng, M.M.; Kang, J.; Huang, F.; He, H.W. Melatonin regulates mitochondrial function and biogenesis during rat dental papilla cell differentiation. Eur. Rev. Med. Pharm. Sci. 2019, 23, 5967–5979.
- Tao, J.; Zhai, Y.; Park, H.; Han, J.; Dong, J.; Xie, M.; Gu, T.; Lewi, K.; Ji, F.; Jia, W. Circadian Rhythm Regulates Development of Enamel in Mouse Mandibular First Molar. PLoS ONE 2016, 11, e0159946.
- Ren, Q.; Pan, J.; Chen, Y.; Shen, Z.; Yang, Z.; Kwon, K.; Guo, Y.; Wang, Y.; Ji, F. Melatonin-Medicated Neural JNK3 Up-Regulation Promotes Ameloblastic Mineralization. Front. Cell Dev. Biol. 2021, 9, 749642.
- Pan, J.; Ren, Q.; Yang, Z.; Guo, Y.; Kwon, K.; Shen, C.; Wang, Y.; Ji, F. The effect of melatonin on the mouse ameloblast-lineage cell line ALCs. Sci. Rep. 2022, 12, 8225.
- Moiseiwitsch, J.R.; Lauder, J.M. Stimulation of murine tooth development in organotypic culture by the neurotransmitter serotonin. Arch. Oral Biol. 1996, 41, 161–165.
- Moiseiwitsch, J.R.; Raymond, J.R.; Tamir, H.; Lauder, J.M. Regulation by serotonin of tooth-germ morphogenesis and gene expression in mouse mandibular explant cultures. Arch. Oral Biol. 1998, 43, 789–800.
- Dimitrova-Nakov, S.; Baudry, A.; Harichane, Y.; Collet, C.; Marchadier, A.; Kellermann, O.; Goldberg, M. Deletion of serotonin 2B receptor provokes structural alterations of mouse dental tissues. Calcif. Tissue Int. 2014, 94, 293–300.
- Regueira, L.S.; de Marcelos, P.G.C.L.; Santiago-Jaegger, I.M.; Perez, D.E.d.C.; Evêncio, J.; Baratella-Evêncio, L. Fluoxetine effects on periodontogenesis: Histomorphometrical and immunohistochemical analyses in rats. J. Appl. Oral Sci. 2017, 25, 159–167.
- Kjær, I. Mechanism of human tooth eruption: Review article including a new theory for future studies on the eruption process. Scientifica 2014, 2014, 341905.
- Yang, L.; Kang, M.; He, R.; Meng, B.; Pal, A.; Chen, L.; Jheon, A.H.; Ho, S.P. Microanatomical changes and biomolecular expression at the PDL-entheses during experimental tooth movement. J. Periodontal Res. 2019, 54, 251–258.
- Wan, Q.-Q.; Qin, W.-P.; Ma, Y.-X.; Shen, M.-J.; Li, J.; Zhang, Z.-B.; Chen, J.-H.; Tay, F.R.; Niu, L.-N.; Jiao, K. Crosstalk between Bone and Nerves within Bone. Adv. Sci. 2021, 8, 2003390.
- Cahill, D.R.; Marks, S.C. Tooth eruption: Evidence for the central role of the dental follicle. J. Oral Pathol. 1980, 9, 189–200.
- Xu, J.; Kawashima, N.; Fujiwara, N.; Harada, H.; Ota, M.S.; Suda, H. Promotional effects of vasoactive intestinal peptide on the development of rodent Hertwig’s epithelial root sheath. Congenit. Anom. 2012, 52, 162–167.
- Yang, Y.; Ge, Y.; Chen, G.; Yan, Z.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; Tian, W. Hertwig’s epithelial root sheath cells regulate osteogenic differentiation of dental follicle cells through the Wnt pathway. Bone 2014, 63, 158–165.
- Shimada, A.; Komatsu, K.; Chiba, M. Effects of local injections of vasoactive drugs on eruption rate of incisor teeth in anaesthetized rats. Arch. Oral Biol. 2006, 51, 449–456.
- Shimada, A.; Komatsu, K.; Shibata, T.; Chiba, M. Effects of an intravenous infusion of Ringer’s solution on eruption rates of incisor teeth in anesthetized rats. Acta Odontol. Scand. 2006, 64, 16–20.
More
Information
Subjects:
Developmental Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
02 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




