2. Anatomical and Embryological Basis
A tooth can be divided into two parts: the crown visible in the mouth after eruption and the root anchored in alveolar bone by the periodontal ligament. The dental pulp, within the tooth center, is extensively filled with nerves composed of sensory and sympathetic fibers, which arise from the trigeminal ganglion (TG) and the superior cervical ganglion (SCG) respectively (
Table 1). Whether parasympathetic nerves innervate the dental pulp is still a subject of debate, but parasympathetic neuropeptides are observed in the dental pulp
[4]. The axons mainly accumulate in the coronal pulp to form the subodontoblastic Raschkow plexus, implicating possible crosstalk between odontoblasts and pulpal nerves
[4]. The periodontal ligament is densely innervated by mainly sensory fibers and a few autonomic fibers (
Table 2). The sensory endings include various proprioceptors, such as Ruffini ending, differing from the sensory innervation in the pulp.
A brief summary of pulpal and periodontal nerves is presented in the tables below.
Table 1. Nerves in the pulp.
Table 2. Nerves in the periodontal ligament.
In recent years, extensive studies have suggested the significance of local sensory and sympathetic nerves in maintaining dental and periodontal health and promoting the recovery of local diseases. This not only is because of their anatomical localization but also is demonstrated through inferior alveolar nerve resection (IANr), which not only reduces incisor dentin/enamel formation and injury repair, but also suppresses alveolar bone remodeling and periodontal defect repair
[12][13][14][12,13,14]. In addition, sympathetic innervation also appears to take a regulatory role in osteoclast infiltration in rat periapical lesions
[15], and alveolar bone remodeling in oral challenge with
P. gingivalis [16]. Reversely, carotid sinus nerve stimulation plays a protective role in rat periodontitis, attenuating alveolar bone loss and inflammation
[17]. Altogether, these studies agree that normal sensory and sympathetic innervation are critical for tooth–periodontium complex.
The tooth is also capable of influencing nerves, which will be discussed in detail in the following section. The foundation of this mutual regulation between nerves and the tooth–periodontium complex probably lies in their embryological origin. The dental mesenchyme origins from the cranial neural crest (CNC) cells and is developmentally associated with the neural system. One study has revealed that the mesenchymal stem cells of dental pulp (DPSCs) can still be differentiated into functional dopaminergic neurons by midbrain cues in vitro
[18]. This remaining responsiveness indicates dental mesenchymal cells have preserved some hereditary similarity with neural cells. More convincingly, RNA-seq analysis has revealed neural/glial cells have a closer origin relation with skeletal cells than pigment cells on the genetic level
[19]. This developmental homology suggests there may still be reciprocal regulation between the tooth and the nervous system.
3. Tooth Influences Neurophysiology during Development Process
3.1. Tooth Innervation Is Spatiotemporally Regulated
The tooth sequentially develops as the result of epithelial–mesenchymal interactions. During the embryonic (E) stage for crown formation, the tooth experiences bud, cap, and bell stages, followed by root formation including crown–root transition, root elongation, eruption, and full development after birth (
Figure 1). Tooth innervation happens concomitantly with tooth formation, and appears to strongly correlate with tooth developmental stage.
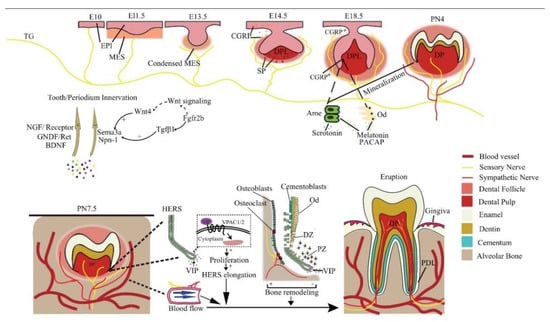
Figure 1. Reciprocal regulation between developing tooth and its innervation. NGF, GNDF, and BDNF promote nerve growth, while Sema3a and Npn-1 suppress it and ensure normal patterning. In a mouse embryo, the pioneering trigeminal ganglion (TG) fibers first arrive at the maxillary oral epithelium on E10. On E14.5, SP is detected and is essential for tooth development to continue. The sensory fibers grow into the dental follicle on E18.5. CGRP, PACAP, melatonin, and serotonin regulate tooth mineralization. Sensory fibers enter the dental pulp on PN4, while the sympathetic fibers enter it on PN 9, after root formation has begun. Neural VIP promotes the elongation of HERS to stimulate the differentiation of DF cells, resulting in root growth. Periodontal nerves regulate bone remodeling as well as the local blood flow in alveolar sockets to affect tooth eruption. EPI = epithelium; MES = mesenchyme; Ame = ameloblast; Od = odontoblast; NGF = nerve growth factor; DPL = dental papilla; DP = dental pulp; PDL = periodontal ligament; CGRP+ = CGRP positive cells; DZ = differentiation zone; PZ = proliferation zone.
At E11.5, mouse molar tooth formation is initiated as a local thickening of dental epithelium, while the pioneering trigeminal fibers first arrive at the maxillary oral epithelium at E10
[20]. However, the trigeminal fibers do not infringe on the presumptive dental mesenchyme or developing tooth germ but only navigate their periphery from initiation to the cap stage and grow into the dental follicle (DF) at the bell stage
[20]. At the cap stage, the sensory fibers form a plexus under the dental papilla, which later gives rise to the fine DF branches, but will not enter the pulp until a thin layer of enamel has been deposited at postnatal (PN) day 4. Intriguingly, the trigeminal fibers enter the pulp through presumptive root apices in a multi-rooted tooth, in the absence of any physical barrier such as the pulp floor
[20], implicating a predetermined mesenchymal route for nerve ingrowth. The sympathetic fibers, which previously only exist around blood vessels outside the dental pulp, enter the dental pulp following sensory fibers on PN9, after root formation has begun
[21]. This indicates that the ingrowth of dental nerves follows developmentally regulated timing, order, and routes.
Evidence shows that the developing dental tissues can regulate their own innervation by either attracting or repelling neurites (
Figure 1). For example, semaphorin 3a (Sema3a) is a nerve repellent expressed in tooth germs. Its distribution in tissue shifts as tooth development proceeds, thus controlling the timing and route of tooth innervation
[20]. On the other hand, auto-transplanted tooth germs can induce their own innervation
[22], and adult denervated teeth can be automatically reinnervated
[23][24][23,24]. In line with these findings, an in vitro co-culture study reveals tooth germ mesenchyme differentially attracts or repels neurites from TG explants depending on the developmental stage
[25], meaning both the attracting and repelling are developmentally regulated.
3.2. The Molecular Guidance Cues for Tooth–Periodontium Innervation
Many molecules, mainly neurotrophins and semaphorins, expressed in and around the developing tooth germ modulate tooth innervation and other aspects of tooth development, such as cytodifferentiation. Many of them are also implied in periodontal innervation. Studies of tooth regeneration do not monitor the expression of these molecules
[7][9][10][7,9,10], but their proper spatiotemporal expression is vital for the normal patterning of tooth nerves. Proper application of these molecules may benefit tooth nerve regeneration.
3.2.1. Neurotrophins
Neurotrophins are identified as a family of growth factors important for neuronal survival, development, and function, as well as for the immune and reproductive systems. NGF as a prototypic neurotrophin is a significant target-derived promotive factor for peripheral sensory and sympathetic innervation. NGF immunoreactivity is mainly located in the dental mesenchyme in the bud stage, while in the cap and bell stages, it is mainly expressed in the dental epithelium and adjacent odontoblast layer. Its gene expression pattern correlates with pulpal neurite growth in postnatal mouse molars, suggesting that NGF is involved in the local sprouting, guidance, and arrangement of trigeminal axons in developing teeth
[26]. This can explain why neonatal exposure to anti-NGF reduces the number of trigeminal neurons projecting to rat molar dental pulp
[27].
NGF receptors TrkA and p75NTR (
Figure 1) are concentrated in nerve fibers approaching the tooth germ and mediate innervation throughout the whole development process
[28]. It is reported that TrkA knockout mice lack both sympathetic and sensory pupal nerves
[29] and the deletion of p75NTR in mice causes weaker osteogenic ability
[30]. Furthermore, the p75NTR gene positively correlates with mineralization-related genes in mouse ectomesenchymal stem cells
[31], possibly involved in the melanoma-associated antigen-D1 (Mage-D1) and NF-κB pathway
[32][33][32,33]. Therefore, the dental NGF receptors are important not only for innervation but also for tooth morphogenesis and hard tissue formation.
Other neurotrophic factors, including brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and glial-derived neurotrophic factor (GDNF) are also implicated in the innervating of dental nerves (
Figure 1). Ablation of GDNF/Ret signaling before pulpal innervation significantly reduces pulpal neurites in Ret knockout mice, as well in mice treated with Ret inhibitor
[34]. Interestingly, in an adult tooth, Ret
+ fibers only make up a portion of pulpal afferents and are predominantly expressed in a mutually exclusive manner with TrkA in trigeminal sensory neurons, although all the small, medium, or large diameter trigeminal sensory neurons may contain them both concomitantly
[34]. Even so, GDNF and NGF work in synergy to promote the innervation of all types of neurons classified by size. However, their individual target neurons have little overlapping, meaning they cannot be regarded as functionally equivalent agents. A similar phenomenon might also exist with other neurotrophins mentioned above. Recently, increasing studies are pointing to the morphological and even functional heterogeneity among dental pulp afferents
[35][36][37][38][35,36,37,38]. Therefore, neurotrophins may have a predilection for specific subpopulations of pulp afferents with a mutually exclusive expression.
Neurotrophins are also involved in periodontal innervation. Periodontal mechanoreceptive Ruffini ending shows immunoreactivity for TrkB, an affinity receptor for BDNF and neurotrophin-4/5. BDNF knockdown reduces periodontal nerve density and causes Ruffini ending deformation in mice
[39] more severely than neurotrophin-4/5 depletion does at the early stage
[40]. The cellular distribution and expression patterns of GDNF and its receptors in the periodontium coincide with the development of Ruffini endings. Ablation of GDNF/Ret signaling also results in abnormal periodontal fibers
[34]. This indicates that various neurotrophins regulate periodontal innervation.
3.2.2. Semaphorins
The semaphorin family is a class of secreted, membrane-bound proteins that regulate many developmental processes
[41]. Most semaphorins are chemorepellent against neurites, but some may promote axonal growth, such as semaphorin7A, which is expressed in odontoblasts and promotes dentin–pulp complex terminal innervation.
Sema3a is the most studied in dental development studies. Sema3a expression is absent from the distribution domains of dental nerves in embryonic mouse tooth germ, while the expression of its receptor, neuropilin-1 (Npn1), is restricted to dental axons. In
Sema3a−/− and
Npn1−/− mice embryos, nerve fibers appear prematurely in the presumptive dental mesenchyme and later ectopically in the dental papilla mesenchyme
[42][43][42,43]. In postnatal mouse molar, Sema3a is observed in the future pulp floor area, but absent from the future apical foramen, which illustrates its control of normal pulpal nerve pathways through the apical foramen
[20]. Interestingly, the expression pattern of multiple neurotrophins is not altered in
Sema3a−/− mice, indicating the attracting and repelling regulations in tooth innervation are exclusive but coordinated
[20][42][43][20,42,43].
Sema3a also impacts periodontal nerve distribution and structure. In
Sema3a−/− mouse incisors, the number of nerves and arborizations abnormally increases in the developing dental follicle target field and periodontium, and there are abundant nerve fibers in the labial periodontium
[42]. Unlike in the periodontium, whether Sema3a affects pulpal nerve structure is contingent. In Sema3a null mice, pulpal nerve thickness and fasciculation are notably reduced in molars but have no apparent changes in incisors
[42][43][42,43]. This is probably due to the subtle differences between incisor and molar innervation, which is also the explanation for why Sema3a-knockout-induced premature innervation happens slightly later in incisors than in molars
[42].
Notably, Sema3a modulation is discrepant between teeth and other hard tissue. Its knockout does not trigger dental hard tissue defects but causes skeletal deformation and osteopenia
[44]. Speculatively, this is probably because other semaphorins that possess similar functions, mainly class 3, 4, 5, and 6 molecules
[25], also potentially regulate tooth development and may compensate for the lost non-neuronal function of Sema3a in developing teeth.
The upstream signaling of Sema3a is seldom studied. It appears that Wnt4 and Tgf-β1 in the dental epithelium induce early Sema3a expression in the dental mesenchyme on E10-E11 via epithelial–mesenchymal interactions (
Figure 1). In addition,
Fgfr2b−/− molar germ is devoid of Tgf-β1, which accounts for the abnormal Sema3a expression from the E13.5 late bud stage onwards
[45]. The canonical Wnt signaling pathway is also altered in
Fgfr2b−/− teeth, but not via Wnt4. Evidence shows that Wnt4 stimulates jaw mesenchymal Msx1 expression, which is indispensable for tooth morphogenesis beyond the bud stage, whereas Tgf-β1 promotes dental mesenchymal cell proliferation. Thereby Wnt, Tgf-β, and Fgf signaling conjugate tooth innervation with tooth formation
[20].
Conclusively, Sema3a is a potent repellent for pulpal and periodontal innervation. Local injection of a Sema3a inhibitor alone is sufficient to promote innervation of bio-engineered teeth
[46], revealing its potency as a regulatory target for bio-engineered teeth.
3.3. Related Application in Tooth Regeneration
The molecules controlling tooth innervation may be applied for achieving better regeneration of the tooth neural network. In a study of preserving immature teeth with non-vital pulp, the application of amelogenin after root canal treatment successfully induces pulp regeneration with a dense neural network
[47]. Amelogenin is a vital component of the enamel matrix. This confirms molecules other than neurotrophins and semaphorins may also regulate tooth innervation and again demonstrates that a developing tooth controls its own innervation.
Similarly, neurotrophins and semaphorins may also be applied in tooth and periodontium regeneration. However,
this study, like many other regeneration studies, did not compare the distribution, pattern, and function of the regenerated pulp neural network with those of a natural one, meaning the regeneration may not be fully successful. Therefore, if neurotrophins and semaphorins are really to be applied, their physiological expression pattern during tooth development should be carefully scrutinized to instruct proper application so that the tooth neural network can best mimic a natural one.
4. The Nervous System Regulates Tooth Development
4.1. Neural Regulations in Tooth Development at Pre-Eruptive Stage
Controversy exists with regard to the participation of nerves in tooth initiation. In polyphyodont fish, denervation completely abolished tooth germ formation. However, studies on mice suggest the opposite. Mouse diastema is the edentulous region between incisors and molars which contains three unerupted tooth primordia that develop until the bud stage. It is devoid of peripheral nerves, indicating innervation is not required for mouse tooth initiation
[48]. Consistently, mouse embryonic mandible explants with or without TG attached produce similar tooth formation incidence
[49]. However, these conclusions are flawed in that they cannot factor out the pioneer trigeminal fibers that arrive near the oral epithelium prior to dental placode formation
[50]. These pioneer fibers might prime the oral epithelium to form a dental placode (
Figure 1). In fact, a recent study detected calcitonin gene-related peptide (CGRP), a common sensory nerve marker, from E14.5 to E17.5 with epithelium predilection in mice
[51]. In addition, substance P (SP), a well-known member of the tachykinin family released by sensory neurons, is transiently expressed in developing mouse tooth germ. Its receptor antagonist blocks the development of ex vivo mouse tooth germs at E14, which is reversed by exogenous SP
[52]. These pieces of evidence endorse the speculation that some early-arriving sensory fibers interact with the oral epithelium to initiate tooth development. Above all, controversy persists on the role of innervation in tooth initiation and early development before mineralization. In view of the pioneering trigeminal fibers to the oral epithelium, it would be desirable to use animal models whose dental innervation is completely ablated with genetic tools to obtain more compelling evidence.
In the mineralizing phase of tooth development, innervation clearly regulates the normal structure and morphology of the tooth. In cap and bell stage mouse molars, CGRP is mainly detected around the dental epithelium–mesenchyme interface, and its expression suddenly increases during E17.5 to E18.5, when odontoblasts and ameloblasts differentiate from the dental papilla and inner enamel epithelium, respectively
[51]. This coincidence indicates a role of sensory fibers in tooth development, but how the nerves elicit normal dentinogenesis and amelogenesis remains to be explored.
Some studies have highlighted specific neurogenic factors in the regulation of tooth development. Nerve fibers containing PACAP, a pleiotropic neuropeptide, have been observed in the odontoblastic and subodontoblastic layers of the dental pulp
[53] (
Figure 1). PACAP-deficient mice exhibit aberrant enamel or dentine structure, accompanied by smaller incisors and thinner molar dentin
[54]. This is most likely due to the regulation of PACAP on Sonic hedgehog (Shh) and Notch signaling which are critical in tooth morphogenesis
[55][56][55,56].
In addition, neurohormones released in the central nervous system (CNS) also regulate tooth development. Melatonin can enhance odontogenic differentiation of dental papilla cells in the late bell stage
[57] by upregulating RORα and altering mitochondrial function and biogenesis in these cells
[58][59][58,59]. Melatonin is also reported to promote the normal survival and mineralization of ameloblasts in postnatal mouse molar germs
[60] by activating the MAPK signaling via repressing β-arrestin
[61], and the Wnt pathway may also be involved
[62]. Moreover, the precursor of melatonin, serotonin, stimulates the development of tooth germs in an embryonic mouse mandibular explant culture beyond the bud stage, meaning it may promote the differentiation of tooth germ cells
[63]. Further analysis reveals this may be achieved by direct activation of serotonin receptors
[64]. In addition, serotonin 2B receptor knockdown leads to abnormal enamel volume and structure in mouse molars
[65]. Serotonin regulates periodontal development too. The intake of a serotonin re-uptake inhibitor, fluoxetine, decreases periodontium-forming cells
[66].
Together, these studies suggest the importance of innervation and neuron-derived factors in regulating normal tooth morphogenesis. These factors may prove helpful in regenerating teeth with tooth germ cells or fetal tissue with respect to their developmental roles.
4.2. Neural Regulations in Tooth Eruption
Many hypotheses have emerged trying to elucidate the underlying mechanism for tooth eruption, but none have gained entire recognition. However, it is widely accepted tooth eruption depends on the development and remodeling of the surrounding jaw bone and the extent of tooth root development
[67]. Therefore, neuroregulation probably regulates eruption by targeting these physiological processes (
Figure 1).
Alveolar bone remodeling: Both sensory and autonomic nerves can actively regulate bone remodeling. During experimental tooth movement, nerve bundles are found to co-localize with osteoclasts and Howship’s resorption lacunae
[68], providing ponderable evidence of neural participation in alveolar bone resorption. In light of this, alveolar nerves might also participate in tooth eruption. Although the researchers did not further specify the biomolecular event
in this study, intrabone nerves regulate bone remodeling mainly through secreting various molecules targeting bone cells
[69]. For instance, the sympathetic neurotransmitter norepinephrine overall suppresses bone formation and promotes bone resorption
[69].
Dental follicle (DF): The DF plays a critical role in tooth eruption by regulating jaw bone remodeling and tooth root growth. Eruption totally fails after surgically removing DF, while the removal of the developing tooth crown or root did not disrupt normal eruption
[70]. However, a direct investigation into the neural regulation of DF function is still absent to date. Nevertheless, it seems dental innervation can regulate DF function indirectly via Hertwig’s epithelial root sheath (HERS), an important guide for root growth. Growing HERS expresses a receptor for vasoactive intestinal peptide (VIP), which enhances HERS elongation in ex vivo tooth germs by promoting cell proliferation
[71]. In turn, HERS cells can stimulate the differentiation of cementoblasts or osteoblasts and the mineralization of DF cells via the Wnt signaling pathway
[72].
Local blood flow: The vasculature within the alveolar socket is also thought to affect tooth eruption. The local injection of vasoactive drugs alters socket blood flow as well as tooth eruption rate in rats dose-dependently
[73]. Systemic volume expansion by injection of Ringer’s solution also increases socket blood flow and tooth eruption rate by decreasing systemic arterial pressure, not by local nutrition
[74]. Both sensory and autonomic innervations in the periodontium regulate local blood flow, thereby regulating tooth eruption.
Overall, although neural participation in tooth eruption is abundantly implicated, a concrete mechanism is still waiting to be unveiled, and further research in this direction will help decipher the mystery of the general tooth eruption mechanism as well. For rodent incisors, tooth eruption is continuous throughout the whole life.
RWe
searchers will discuss stem cells and neural regulation in incisor homeostasis and injury repair in detail in
Section 7.
4.3. Related Application in Tooth Regeneration
Some of the neurogenic molecules implicated in tooth development are applicable to tooth regeneration, imitating their developmental roles. For example, serotonin is used to treat murine oral keratinocytes (MOKs), which also arise from embryonic mouse oral epithelium as the enamel organ does. MOKs growing in the presence of serotonin after reprogramming with Tgf-β1 successfully produced amelogenin, and in a 3D culture, they formed enamel-producing organoids. After densification with pressure and vacuum, the enamel formed by MOKs possesses a similar structure and mechanical properties to natural enamel, suggesting the potential application of serotonin in enamel regeneration via tissue engineering methods
[11]. Other molecules discussed here may also help regenerate dental tissues, but their efficacy and application methods need to be explored.