Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Girolamo Casella | -- | 1428 | 2022-11-29 08:48:10 | | | |
| 2 | Conner Chen | Meta information modification | 1428 | 2022-12-06 06:41:11 | | | | |
| 3 | Conner Chen | Meta information modification | 1428 | 2022-12-09 05:43:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Casella, G.; Carlotto, S.; Lanero, F.; Mozzon, M.; Sgarbossa, P.; Bertani, R. Cyclo- and Polyphosphazenes for Biomedical Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/37639 (accessed on 04 March 2026).
Casella G, Carlotto S, Lanero F, Mozzon M, Sgarbossa P, Bertani R. Cyclo- and Polyphosphazenes for Biomedical Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/37639. Accessed March 04, 2026.
Casella, Girolamo, Silvia Carlotto, Francesco Lanero, Mirto Mozzon, Paolo Sgarbossa, Roberta Bertani. "Cyclo- and Polyphosphazenes for Biomedical Applications" Encyclopedia, https://encyclopedia.pub/entry/37639 (accessed March 04, 2026).
Casella, G., Carlotto, S., Lanero, F., Mozzon, M., Sgarbossa, P., & Bertani, R. (2022, December 01). Cyclo- and Polyphosphazenes for Biomedical Applications. In Encyclopedia. https://encyclopedia.pub/entry/37639
Casella, Girolamo, et al. "Cyclo- and Polyphosphazenes for Biomedical Applications." Encyclopedia. Web. 01 December, 2022.
Copy Citation
Cyclic and polyphosphazenes are extremely interesting and versatile substrates characterized by the presence of -P=N- repeating units. The chlorine atoms on the P atoms in the starting materials can be easily substituted with a variety of organic substituents, thus giving rise to a huge number of new materials for industrial applications. A wide variety of new phosphazene systems, either trimeric or polymeric, have been developed as biomaterials in view of different applications. The materials to apply in biomedical applications should be biocompatible.
cyclophosphazenes
polyphosphazenes
drug delivery
1. Introduction
Phosphazenes are interesting and versatile chemical substrates characterized by the presence of -P=N- repeating units giving rise to low-molecular-weight cyclic structures with three or four units up to polymers containing thousands of -P=N- moieties, where the P atom in the starting material (i.e., hexachlorocyclotriphosphazene, HCCP) bears two chlorine atoms [1].
The chemistry and the properties of phosphazenes, in view of industrial applications, have been reviewed in a series of books [2][3] and articles [4] stemming from papers published in 1964–1965, when Allcock and coworkers [5][6][7] first reported the synthesis of linear poly(organophosphazenes) (POPs) through the thermal-induced ring-opening of the HCCP and the subsequent substitution of the chlorine atoms with suitable organic groups to achieve a wide variety of new derivatives (Scheme 1 and Scheme 2).
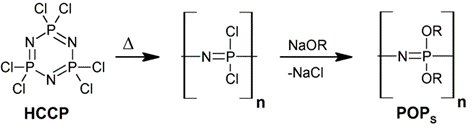
Scheme 1. From hexachlorocyclotriphosphazene (HCCP) to polyphosphazenes (POPs).
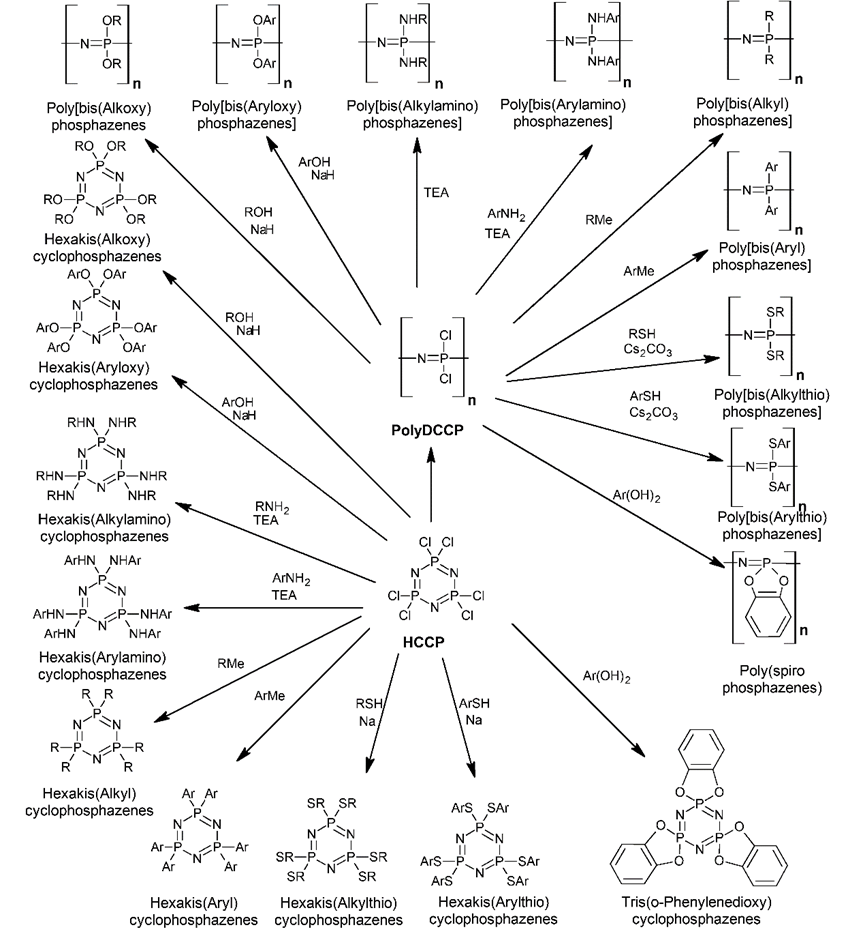
Scheme 2. From HCCP and polydichlorocyclophosphazene (PolyDCCP) to a wide variety of derivatives by nucleophilic substitution.
The scientific and applicative interest for phosphazenes arises from the relatively easy substitution of the chlorine atoms with an enormous variety of substituents, thus giving rise to an extremely wide number of new materials whose properties can be designed, in principle, based on the nature of the substituents in addition to the specific characteristics of the -P=N- backbone. Phosphorus is one of the most important elements preventing the combustion of organic materials, with a synergistic effect of nitrogen. Thus, not only is the -P=N- backbone nonflammable but it also quenches the combustion of other compounds in contact with it, likely due to both the interruption of the free radical processes and the formation of an intumescent barrier to the oxygen entrance [8][9]. Furthermore, the nature of the P-N bond guarantees an extremely low torsion barrier of the backbone, thus showing glass transition temperatures of some polyphosphazenes in the −100 °C region [10][11].
It is noteworthy that a lot of patents have been deposited over time based on phosphazenes, exhibiting specific properties of industrial interest. The most intriguing properties, which can be modulated on the bases of the molecular weight, structure, nature, and combination of substituents, range from the thermal resistance of the polymers, the tuneable low-glass-transition temperature, the hydrophilic/hydrophobic behavior and the water/solvent solubility to the compatibility with inorganic materials, owing to the possibility to introduce organosilicon moieties as substituents [11], and the formation of aerogels with various densities by the crosslinking of cyclotriphosphazenes and polysiloxanes [12].
Cyclophosphazenes have been proposed as hydraulic fluids, lubricant stabilizers and additives, in particular with trifluoromethylphenoxy substituents [3], as substrates for supramolecular assemblies [13][14][15], and as supports for metal catalysts, either through metal coordination by the nitrogen atoms of the backbone or through the presence of ligands as substituents [11][16][17].
Upon the polymerization and substitution of the reactive chlorine atoms, they give rise to a wide variety of new polyphosphazenes, containing from 100 to 15,000 or more repeating units (with molecular weights ranging from 2 to 10 × 106 Da) with an unusually broad range of useful properties. Polyphosphazenes with elastomeric [18][19], optical [20], proton-conducting [21], electrochemical [22], and fire-resistant [23] properties have been investigated and applied in the development of membranes [24], fuel cells [25], and hybrid materials [12]. New elastomeric inorganic silicon-based compounds, and specifically fire-resistant elastomers and plastics, have been studied for military purposes [26][27].
2. Biomedical Applications
During the last 20 years, a wide variety of new phosphazene systems, either trimeric or polymeric, have been developed as biomaterials in view of different applications. The materials to apply in biomedical applications should be biocompatible, in other words nontoxic themselves, as well as their degradation products. Moreover, both the materials and their degradation products also should not induce an inflammatory, carcinogenic, pyrogenic, or allergic response. The degradation products of many polyphosphazenes form a buffering system (ammonium phosphate) and maintain a neutral pH through the degradation [28][29]. The most important advantage of polyphosphazenes over other polymers is the possibility to introduce side groups with specific chemical–physical and biological behavior to design biomaterials for tailored applications. Fluorinated chains improve the hydrophobicity, giving rise to materials suitable for surface modifications, and have been approved as dental liner materials because of their antimicrobial properties and biological inertness [15][30]. The introduction of amino acid esters improves the degradation of the polymers: the backbone degradation gives rise to nontoxic products (phosphate and ammonia) and can be affected significantly by the presence of residual chlorine atoms and hydroxyl groups along the chain, together with the introduction of hydrolytically labile side groups: a number of amino acid esters have been introduced, also in combination, observing that their steric hindrance can modulate the hydrolysis rate. The materials can be used for drug delivery, tissue engineering, or shape–memory polymers for cardiovascular or bile duct stents, as examples, where the material can be either biostable or biodegradable into nontoxic end-products according to a modulable designed degradation rate (i.e., such as the rate of tissue growth or according to a desired therapeutic release rate) [31][32]. It has been observed that the presence of only small amino acids as substituents such as glycine and alanine induced a quicker degradation than phosphazenes bearing larger or phenoxy substituents. The modulation of the degradation rate could also be obtained with a combination of hydrophilic and hydrophobic side groups (i.e., carbohydrates or steroidal substituents) [33][34][35][36]. The degradation mechanism involves the attack of water molecules on organic side groups on the POPs, with the formation of P-OH units by the migration of protons from oxygen to nitrogen, thus sensitizing the polymer backbone to hydrolysis, yielding nontoxic degradation products which comprise mainly NH3, phosphate, and the corresponding side groups, as depicted in Scheme 3 [37][38].
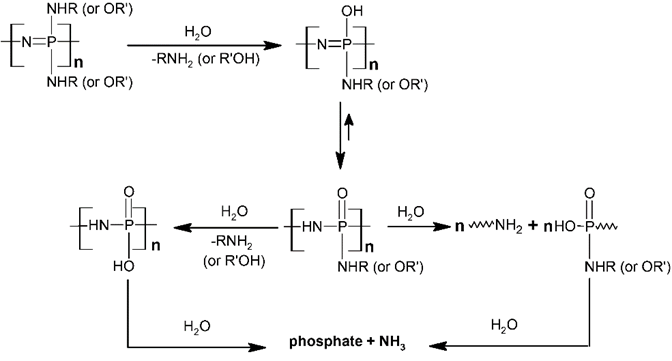
Scheme 3. Hydrolytic degradation mechanism of POPs.
It was observed that the degradation rates in polymers with side groups linked through the N-atom or the O-atom are different [39]. In the case of tyrosine, which can be attached to the polymer backbone either by amino or by phenolic moiety, only the polyphosphazene-bearing N-tyrosine side groups are biodegradable, while the phenolic group makes the polymers nondegradable but pH-sensitive (Scheme 4) [40][41][42][43]. The degradation rate of some water-soluble polyphosphazenes (bearing amino acid ester units, or pyrrolidinyl, or carboxylatophenoxy moieties) has been studied as a function of the pH, observing a considerably faster degradation at lower pH values. The hydrolytic stability can be tailored by the careful choice of the amino acid spacer and increased by the steric shielding of the polymeric backbone [44][45][46].
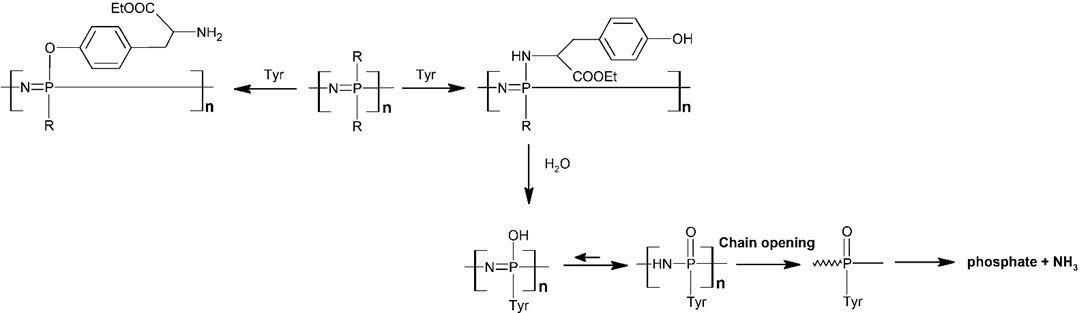
Scheme 4. Degradation process of the tyrosine substituted POP.
The introduction of functionalities into phosphazene pendant groups allows for the attachment of specific molecules into the system that can increase the affinity for the desired species. An example could be aminoethoxyethanol: the oxygen atom can be bonded to P and the amino unit can be used to bind, for instance, galactose or polyethylene glycol moieties, giving rise to materials able to interact with DNA [47][48].
As for tissue engineering applications, other properties must be explored:
- (i)
-
Glass transition temperature compared with the physiological temperature: as for bone tissue engineering, a glass transition temperature higher than the physiological one to maintain structural integrity in an in vivo environment is required [49];
- (ii)
-
Mechanical properties: substituents must be chosen in order to match the mechanical properties of the POPs (compressive and tensile strengths) and those of the native tissues;
- (iii)
-
Porosity and porous interconnectivity of biomaterials plays a key role either in drug delivery applications, due to their controlled degradability, or in tissue engineering, aging as materials scaffolds for cells proliferation;
- (iv)
-
Stimuli-responsive site behavior: temperature, ultrasound, light, pH, ionic strength, oxidative conditions, and enzyme presence are important stimuli for biomedical applications. Several stimuli-responsive materials have been prepared for tissue engineering and drug delivery due to the possibility of tuning the properties from combinations of different side groups [49][50][51][52]. The reaction of hexakis [4-(acrylamido)phenoxy]cyclotriohosphazene] with N-isopropylacrilamide and N-vinyl imidazole in the presence of ammoniumpersulfate gave crosslinked hydrogels which exhibited in vitro pH-responsive drug-release behavior [51].
In a quite recent review [53], the opportunity to combine the benefits of an inorganic backbone and a wide variety of organic (or organometallic) side groups in POPs have been considered for future bioapplications, such as the use of cyclomatrix polyphosphazenes to encapsulate particles suitable for imaging applications [54] or to apply POPs in a prototype of an artificial heart [55].
References
- Mark, J.E.; Allcock, H.R.; West, R. Inorganic Polymers, 2nd ed.; Oxford University Press: Oxford, UK, 2005; Volume 22, pp. 1–353.
- Gleria, M.; Jaeger, R. Phosphazenes: A Worldwide Insight; Science Publishing: Hauppage, NY, USA, 2004; p. 1047.
- Gleria, M.; de Jaeger, R. Applicative Aspects of Cyclophosphazenes; Science Publishing: Hauppage, NY, USA, 2004; p. 3.
- Gleria, M.; de Jaeger, R. Aspects of Phosphazene Research. J. Inorg. Organomet. Polym. 2001, 11, 1–45.
- Allcock, H.R.; Kugel, R.L. Synthesis of High Polymeric Alkoxy-and Aryloxyphosphonitriles. J. Am. Chem. Soc. 1965, 87, 4216–4217.
- Allcock, H.R.; Kugel, R.L.; Valan, K.J. Phosphonitrilic Compounds. VI. High Molecular Weight Poly(Alkoxy-and Aryloxyphosphazenes). Inorg. Chem. 1966, 5, 1709–1715.
- Allcock, H.R.; Kugel, R.L. Phosphonitrilic Compounds. VII. High Molecular Weight Poly(Diaminophosphazenes). Inorg. Chem. 1966, 5, 1716–1718.
- Sun, X.; Li, L.; Yang, Y.; Jia, C.; Zhang, X.; Wu, J.; Zhu, Z.; Wang, J.; Yang, J. Flame-Retardant Effect of Hyperbranched Phosphazene-Based Microspheres in Poly(L-Lactic Acid). J. Mater. Sci. 2022, 57, 1516–1535.
- Zarybnicka, L.; Machotova, J.; Kopecka, R.; Sevcik, R.; Hudakova, M.; Pokorny, J.; Sal, J. Effect of Cyclotriphosphazene-Based Curing Agents on the Flame Resistance of Epoxy Resins. Polymers 2020, 13, 8.
- Gleria, M.; de Jaeger, R. Polyphosphazenes: A Review. Top. Curr. Chem. 2005, 250, 165–251.
- Allcock, H.R. Chapter 7. Phosphazene High Polymers. In Phosphorus-Based Polymers: From Synthesis to Applications; RSC Polymer Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 125–150.
- Khanin, D.A.; Kononevich, Y.N.; Temnikov, M.N.; Morgalyuk, V.P.; Vasil’ev, V.G.; Popov, A.Y.; Brel, V.K.; Papkov, V.S.; Muzafarov, A.M. New Hybrid Materials Based on Cyclophosphazene and Polysiloxane Precursors: Synthesis and Properties. Polymer 2020, 186, 122011.
- Inoue, K.; Itaya, T. Synthesis and Functionality of Cyclophosphazene-Based Polymers. Bull. Chem. Soc. Jpn. 2001, 74, 1381–1395.
- Kato, F.; Chandra, A.; Tokita, M.; Asano, H.; Shimomoto, H.; Ihara, E.; Hayakawa, T. Self-Assembly of Hierarchical Structures Using Cyclotriphosphazene-Containing Poly(Substituted Methylene) Block Copolymers. ACS Macro. Lett. 2018, 7, 37–41.
- Andrianov, A.K. Polyphosphazenes for Biomedical Applications; John Wiley & Son: Hoboken, NJ, USA, 2009; p. 462.
- Belluco, U.; Bertani, R.; Michelin, R.A.; Mozzon, M.; Zingales, F.; Gleria, M. Organometallic Phosphazenes: Synthesis and Characterization of Pt(II) and Pt(0) Cinnammonitrile Cyclophosphazene Derivatives. Inorg. Chim. Acta 1995, 229, 13–15.
- Chistyakov, E.; Yudaev, P.; Nelyubina, Y. Crystallization of Nano-Sized Macromolecules by the Example of Hexakis-Cyclotriphosphazene. Nanomaterials 2022, 12, 2268.
- De Jaeger, R.; Gleria, M. Synthesis and Characterizations of Poly (Organophosphazenes); Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2004; p. 372.
- Allcock, H.R. A Perspective of Polyphosphazene Research. J. Inorg. Organomet. Polym. Mater. 2006, 16, 277–294.
- Zhang, L.; Shi, J.; Yang, Z.; Huang, M.; Chen, Z.; Gong, Q.; Cao, S. Photorefractive Properties of Polyphosphazenes Containing Carbazole-Based Multifunctional Chromophores. Polymer 2008, 49, 2107–2114.
- Burjanadze, M.; Paulsdorf, J.; Kaskhedikar, N.; Karatas, Y.; Wiemhöfer, H.D. Proton Conducting Membranes from Sulfonated Poly with an Interpenetrating Hydrophilic Network. Solid State Ion. 2006, 177, 2425–2430.
- Ali, Z.; Basharat, M.; Wu, Z. A Review on the Morphologically Controlled Synthesis of Polyphosphazenes for Electrochemical Applications. ChemElectroChem 2021, 8, 759–782.
- Gleria, M.; Bertani, R.; de Jaeger, R.; Lora, S. Fluorine Containing Phosphazene Polymers. J. Fluor. Chem. 2004, 125, 329–337.
- Allcock, H.R.; Phelps, M.V.B.; Barrett, E.W.; Pishko, M.V.; Koh, W.G. Ultraviolet Photolithographic Development of Polyphosphazene Hydrogel Microstructures for Potential Use in Microarray Biosensors. Chem. Mater. 2006, 18, 609–613.
- Wycisk, R.; Pintauro, P.N. Polyphosphazene Membranes for Fuel Cells. Adv. Polym. Sci. 2008, 216, 157–183.
- Amin, A.M.; Wang, L.; Wang, J.; Yu, H.; Huo, J.; Gao, J.; Xiao, A. Recent Research Progress in the Synthesis of Polyphosphazene and Their Applications. Des. Monomers Polym. 2009, 12, 357–375.
- Singler, R.E. Historical Overview of the Army Contributions to Phosphazene Chemistry. J. Inorg. Organomet. Polym. Mater. 2007, 16, 307–309.
- Marin, A.; Decollibus, D.P.; Andrianov, A.K. Protein Stabilization in Aqueous Solutions of Polyphosphazene Polyelectrolyte and Non-Ionic Surfactants. Biomacromolecules 2010, 11, 2268–2273.
- Decollibus, D.P.; Marin, A.; Andrianov, A.K. Effect of Environmental Factors on Hydrolytic Degradation of Water-Soluble Polyphosphazene Polyelectrolyte in Aqueous Solutions. Biomacromolecules 2010, 11, 2033–2038.
- Ogueri, K.S.; Ogueri, K.S.; Ude, C.C.; Allcock, H.R.; Laurencin, C.T. Biomedical Applications of Polyphosphazenes. Med. Devices Sens. 2020, 3, e10113.
- Khan, R.U.; Wang, L.; Yu, H.; Zain-ul-Abdin; Akram, M.; Wu, J.; Haroon, M.; Ullah, R.S.; Deng, Z.; Xia, X. Poly(Organo)Phosphazenes: Recent Progress in the Synthesis and Applications in Tissue Engineering and Drug Delivery. Russ. Chem. Rev. 2018, 87, 109–150.
- Singh, A.; Krogman, N.R.; Sethuraman, S.; Nair, L.S.; Sturgeon, J.L.; Brown, P.W.; Laurencin, C.T.; Allcock, H.R. Effect of Side Group Chemistry on the Properties of Biodegradable L-Alanine Cosubstituted Polyphosphazenes. Biomacromolecules 2006, 7, 914–918.
- Andrianov, A.K.; Marin, A. Degradation of Polyaminophosphazenes: Effects of Hydrolytic Environment and Polymer Processing. Biomacromolecules 2006, 7, 1581–1586.
- Lakshmi, S.; Katti, D.S.; Laurencin, C.T. Biodegradable Polyphosphazenes for Drug Delivery Applications. Adv. Drug Deliv. Rev. 2003, 55, 467–482.
- Baillargeon, A.L.; Penev, K.I.; Mequanint, K. One-Pot Substitution Approach for the Syntheses of Nonfunctional and Functional Poly Biomaterials. Macromol. Mater. Eng. 2017, 302, 1600318.
- Baillargeon, A.L.; Mequanint, K. Biodegradable Polyphosphazene Biomaterials for Tissue Engineering and Delivery of Therapeutics. Biomed. Res. Int. 2014, 2014.
- Teasdale, I.; Brüggemann, O. Polyphosphazenes: Multifunctional, Biodegradable Vehicles for Drug and Gene Delivery. Polymers 2013, 5, 161–187.
- James, R.; Deng, M.; Kumbar, S.G.; Laurencin, C.T. Polyphosphazenes. Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 193–206.
- Morozowich, N.L.; Mondschein, R.J.; Allcock, H.R. Comparison of the Synthesis and Bioerodible Properties of N-Linked Versus O-Linked Amino Acid Substituted Polyphosphazenes. J. Inorg. Organomet. Polym. Mater. 2014, 24, 164–172.
- Amin, A.M.; Shahid, S.A.; Li, W.; Haojie, Y.; Ali, Z.; Rehman, H.; Ghaffar, A.; Sarfraz, M.; Waqas, M. An Efficient Synthesis, Structural Characterization and Hydrolytic Degradation Studies of Poly as Potential Materials for Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1117–1121.
- Kumbar, S.G.; Bhattacharyya, S.; Nukavarapu, S.P.; Khan, Y.M.; Nair, L.S.; Laurencin, C.T. In Vitro and in Vivo Characterization of Biodegradable Poly(Organophosphazenes) for Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2006, 16, 365–385.
- Andrianov, A.K.; Marin, A.; Chen, J. Synthesis, Properties, and Biological Activity of Poly. Biomacromolecules 2006, 7, 394–399.
- Kumar, S.; Singh, R.K.; Prasad, D.N.; Bhardwaj, T.R. Synthesis and in Vitro Degradation Studies of Substituted Poly(Organophosphazenes) for Drug Delivery Applications. J. Drug Deliv. Sci. Technol. 2017, 38, 135–142.
- Andrianov, A.K. Water-Soluble Polyphosphazenes for Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2006, 16, 397–406.
- Andrianov, A.K.; Marin, A.; Peterson, P. Water-Soluble Biodegradable Polyphosphazenes Containing N-Ethylpyrrolidone Groups. Macromolecules 2005, 38, 7972–7976.
- Wilfert, S.; Iturmendi, A.; Schoefberger, W.; Kryeziu, K.; Heffeter, P.; Berger, W.; Brüggemann, O.; Teasdale, I. Water-Soluble, Biocompatible Polyphosphazenes with Controllable and PH-Promoted Degradation Behavior. J. Polym. Sci. A Polym. Chem. 2014, 52, 287–294.
- Heyde, M.; Claeyssens, M.; Schacht, E.H. Interaction between Proteins and Polyphosphazene Derivatives Having a Galactose Moiety. Biomacromolecules 2008, 9, 672–677.
- Stewart, F.F. Phosphazenes. Organophosphorus Chem. 2012, 41, 349–384.
- Nukavarapu, S.P.; Kumbar, S.G.; Brown, J.L.; Krogman, N.R.; Weikel, A.L.; Hindenlang, M.D.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene/Nano-Hydroxyapatite Composite Microsphere Scaffolds for Bone Tissue Engineering. Biomacromolecules 2008, 9, 1818–1825.
- Iturmendi, A.; Monkowius, U.; Teasdale, I. Oxidation Responsive Polymers with a Triggered Degradation via Arylboronate Self-Immolative Motifs on a Polyphosphazene Backbone. ACS Macro Lett. 2017, 6, 150–154.
- Ozay, H.; Sahin, O.; Koc, O.K.; Ozay, O. The Preparation and Applications of Novel Phosphazene Crosslinked Thermo and PH Responsive Hydrogels. J. Ind. Eng. Chem. 2016, 43, 28–35.
- Fu, J.; Liang, L.; Qiu, L.; Fu, J.; Liang, L.; Qiu, L. In Situ Generated Gold Nanoparticle Hybrid Polymersomes for Water-Soluble Chemotherapeutics: Inhibited Leakage and PH-Responsive Intracellular Release. Adv. Funct. Mater. 2017, 27, 1604981.
- Chen, F.; Teniola, O.R.; Ogueri, K.S.; Laurencin, C.T. Recent Trends in the Development of Polyphosphazenes for Bio-Applications. Regen. Eng. Transl. Med. 2022, 1, 1–22.
- Ding, G.; Wang, A.; Shi, X.; Li, J.; You, L.; Wang, S. Preparation of Multiple-Spectra Encoded Polyphosphazene Microspheres and Application for Antibody Detection. Polym. Bull. 2022, 79, 6409–6429.
- Wu, W.; Zhang, S.; Wu, Z.; Qin, S.; Li, F.; Song, T.; Cao, X.; Wang, Z.L.; Zhang, L. On the Understanding of Dielectric Elastomer and Its Application for All-Soft Artificial Heart. Sci. Bull. 2021, 66, 981–990.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
3 times
(View History)
Update Date:
09 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





