Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lina Kieush | -- | 7020 | 2022-12-01 08:11:01 | | | |
| 2 | Sirius Huang | -2 word(s) | 7018 | 2022-12-02 02:38:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kieush, L.; Rieger, J.; Schenk, J.; Brondi, C.; Rovelli, D.; Echterhof, T.; Cirilli, F.; Thaler, C.; Jaeger, N.; Snaet, D.; et al. Use of Secondary Carbon Bio-Carriers in Metallurgical Processes. Encyclopedia. Available online: https://encyclopedia.pub/entry/37588 (accessed on 07 February 2026).
Kieush L, Rieger J, Schenk J, Brondi C, Rovelli D, Echterhof T, et al. Use of Secondary Carbon Bio-Carriers in Metallurgical Processes. Encyclopedia. Available at: https://encyclopedia.pub/entry/37588. Accessed February 07, 2026.
Kieush, Lina, Johannes Rieger, Johannes Schenk, Carlo Brondi, Davide Rovelli, Thomas Echterhof, Filippo Cirilli, Christoph Thaler, Nils Jaeger, Delphine Snaet, et al. "Use of Secondary Carbon Bio-Carriers in Metallurgical Processes" Encyclopedia, https://encyclopedia.pub/entry/37588 (accessed February 07, 2026).
Kieush, L., Rieger, J., Schenk, J., Brondi, C., Rovelli, D., Echterhof, T., Cirilli, F., Thaler, C., Jaeger, N., Snaet, D., Peters, K., & Colla, V. (2022, December 01). Use of Secondary Carbon Bio-Carriers in Metallurgical Processes. In Encyclopedia. https://encyclopedia.pub/entry/37588
Kieush, Lina, et al. "Use of Secondary Carbon Bio-Carriers in Metallurgical Processes." Encyclopedia. Web. 01 December, 2022.
Copy Citation
The term ‘secondary carbon bio-carriers’ here refers to biomass, torrefied biomass, biochar, charcoal, or biocoke. The main focus is on torrefied biomass, which can act as a carbon source for partial or complete replacement of fossil fuel in various metallurgical processes. The material requirements for the use of secondary carbon bio-carriers in different metallurgical processes are systematized, and pathways for the use of secondary carbon bio-carriers in four main routes of steel production are described.
secondary carbon bio-carriers
biomass
torrefaction
biocoke
iron and steel industry
ferroalloys
1. Introduction
Iron and steelmaking can be principally conducted by four routes: BF/BOF, scrap/EAF, DRI/EAF, and SR/BOF [1][2]. The BF/BOF route includes cokemaking, iron ore sintering, iron ore pelletizing, BF-based ironmaking, casting, rolling, and power stations [3]. Current BFs operate with 70–80% sinter, 20–30% pellets, and 10–20% lump iron ore [4]. Figure 1 shows the four main iron and steelmaking routes using coal or coke that can be considered for the integrated use of secondary carbon bio-carriers.
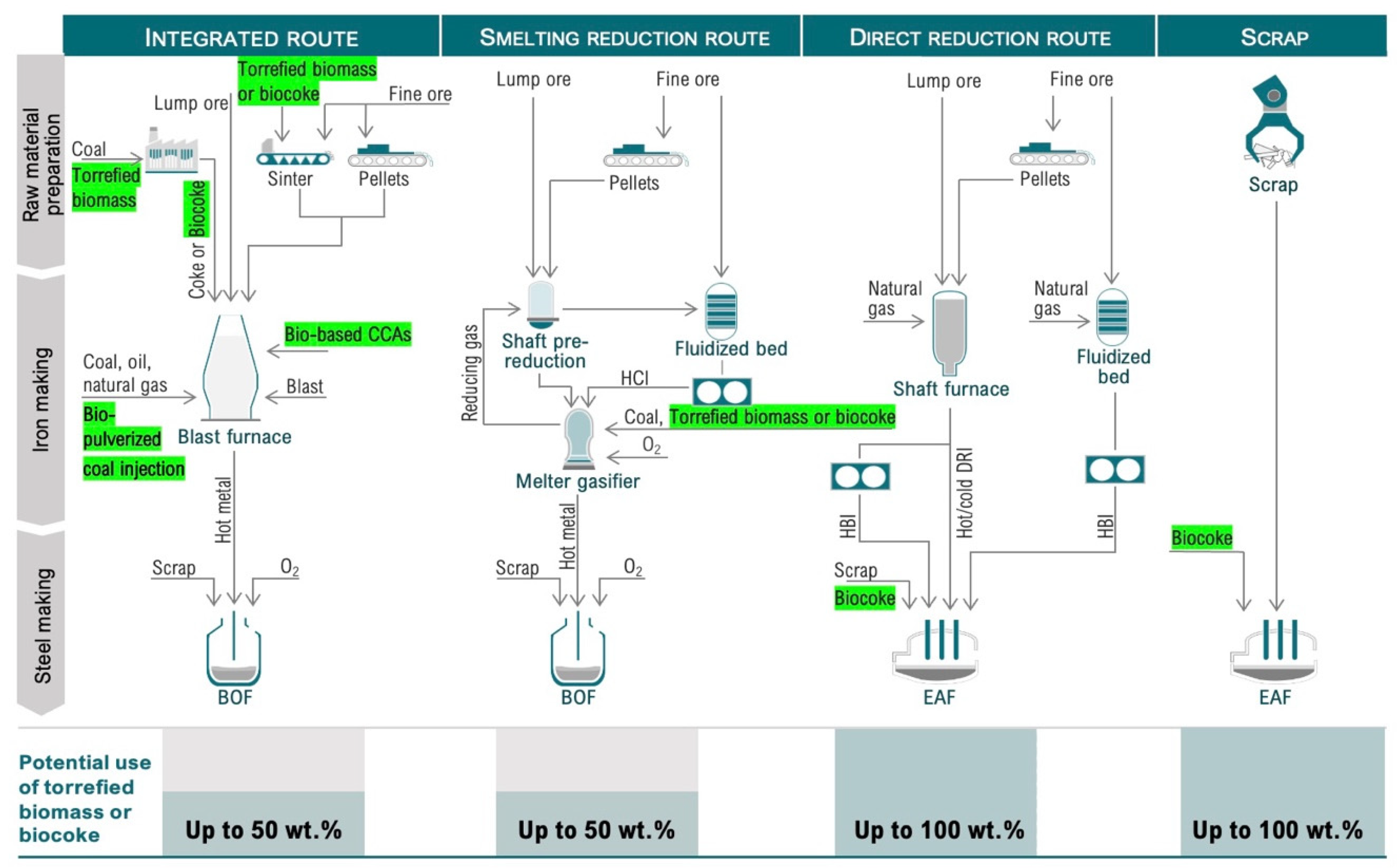
Figure 1. Main pathways for using secondary carbon bio-carriers in iron and steelmaking units.
As shown in Figure 1, torrefaction (light, medium, or severe, depending on the desired final properties of the solid product) can be used to improve the properties of the raw biomass. The torrefied biomass can either be directly used in cokemaking, sintering, and carbon composite agglomerates (CCAs) production, or it can be injected into the BF. It can be subjected to a further modification of properties through carbonization and compaction beneficiation by removing ash or adding minerals. Once the properties have been modified, secondary carbon bio-carriers can be directed to biocoke production. During cokemaking, the torrefied biomass can be used in a wide range from 3 to 50 wt.% of the feed mixture, depending on the requirements for the carbon-bearing material in a particular process. The biocoke obtained can be directed to:
- -
-
Sintering of iron ores to act as fuel;
- -
-
BF to carry out functions as a fuel and reducing agent (delivers chemical energy to melt the burden and contributes to the reduction of iron oxide to metallic iron), as a filter for entrained particles from the raceway, and provides the carbon for the carburization (saturate hot metal with carbon);
- -
-
EAF to enable carburizing and slag foaming;
- -
-
Melter gasifier to generate heat, to act as a reducing agent, to produce a reducing gas, to ensure the permeability of the burden, and to carburize the hot metal.
For each metallurgical route, the carbon-bearing material requirements and the possibility of using secondary carbon bio-carriers will be discussed in detail.
2. Cokemaking
2.1. Features of the Process and Requirements for the Carbon-Bearing Material
During the cokemaking process, coking coal undergoes several chemical and physical changes, including softening, swelling, shrinkage, and re-solidification, which are requirements for forming a strong coke structure. The coke quality should meet strict requirements for the application in the BF because it is the main consumer. Biocoke, in turn, should also meet these requirements, but they are more challenging to achieve, as part of the coking coal has been replaced by secondary carbon bio-carriers. Table 1 shows the main characteristics of conventional coke.
Table 1. Main properties required for conventional coke.
| Parameters | Conventional Coke | References |
|---|---|---|
| Ash, wt.% | <11 | [5] |
| Volatile matters, wt.% | <1.1 | [5][6] |
| Moisture by dry quenching, wt.% | <0.7 | [5] |
| Moisture by wet quenching, wt.% | <5 | [5] |
| C, wt.% | 96.5–97.5 | [5] |
| Fixed carbon, wt.% | 88.8 | [6] |
| H, wt.% | <0.8 | [5] |
| O, wt.% | <0.4 | [5] |
| N, wt.% | <1.3 | [5] |
| S, wt.% | 0.5–1.2 | [5] |
| Coke reactivity index, wt.% | <30 | [5] |
| Coke strength after reaction with CO2, wt.% | <65 | [5] |
| Structural strength, wt.% | <80 | [7] |
| Size distribution, mm | 40–80 | [5] |
| Bulk density, kg/m3 | 430–500 | [5] |
| Porosity, % | 45.0–55.0 | [5] |
| Electric resistivity, mΩ·m | 10–12 | [7] |
| Calorific value (MJ/kg) | app. 29.0 | [8] |
Table 2 compares the properties of torrefied biomass and biocoke. Biocoke is a coke in which part of the coal in the coal blend is replaced by biomass (biomass can be used in its original state or after heat treatment) and obtained at a temperature of 1100 °C like conventional coke. The values for biocoke (mentioned in Table 2) can be within a very wide range, as they depend on the type of biomass used, the amount of coal substituted, and the conditions under which the biocoke is produced. The use of torrefied biomass in cokemaking plants is limited because it adversely affects the coke quality. After all, biocoke increases the porosity, the coke reactivity index (CRI), and reduces strength after a reaction with CO2 (CSR). Increased CRI and porosity can be advantageous for some metallurgical processes, such as for injection in EAF. The low abrasion resistance and the chemical composition of the ash, which can accelerate its reactivity with CO2 in the BF, are limiting factors for the use of torrefied biomass [9].
| Parameters | Torrefied Biomass | Biocoke |
|---|---|---|
| Moisture, wt.% | 4.8 | 0.65 or 1.35 |
| Volatile matters, wt.% | 34–85 | 1.4–2.7 |
| Ash, wt.% | 0.4 | 5.8–10.8 |
| Fixed carbon, wt.% | 13–45 | 87.8–92.4 |
| C, wt.% | 45–68 | 86.38–91.65 |
| S, wt.% | traces | 0.22–0.23 |
| Coke reactivity index, wt.% | n/a | app. 25–50 |
| Strength after reaction with CO2, wt.% | n/a | app. 65–20 |
| Calorific value, (MJ/kg) | 16–29 | app. 18–32 |
n/a is not available.
Usually, biochar is characterized by a lower amount of ash and sulfur, which, compared to biocoke, has an advantage for use in metallurgical processes. However, biocoke has a sufficiently high FC and a low VM. It is worth noting that, compared to conventional coke, biocoke has a lower value of ash and sulfur due to the replacement of coal within the blend.
It should be noted that the pre-treatment of secondary carbon bio-carriers before adding them to the coal blend contributes to an increase in its amount. Thus, raw biomass can be added to the coal blend only in a small amount of up to 3 wt.%, which does not adversely affect the properties of the coke [15]. However, after preliminary heat treatment of the biomass, its use can be increased by up to 10% [16]. Further increase in torrefied biomass does not allow biocoke with the requirements necessary for use in a BF to be obtained. The torrefied biomass in the coal blend acts as an inert material, reducing the caking ability of the blend [17]. In addition, the size of the torrefied biomass is important for the process of coal caking. Thus, a smaller particle size reduces fluidity to a greater extent than larger particles [4]. Therefore, using the process of compaction of torrefied biomass can improve its properties and minimize the impact on the quality of the final product [7][12]. Comparing the results of various studies on CRI and CSR, it can be concluded that biocoke with the addition of charcoal is more reactive compared to biocoke from bio-briquettes. This is because, in the case of charcoal, there is a more active biomass structure within the coke.
Kudo et al. [18] carried out briquetting of solid biomass (bamboo, larch, and ground wood) at a temperature and high mechanical pressure of 130–200 °C and 114 MPa, respectively, with subsequent carbonization at 900 °C. As a result of the research, the authors obtained coke with a tensile strength (TS) of 5–19 MPa.
Castro-Díaz et al. [19] performed carbonization tests with hydrochars obtained after the hydrous pyrolysis at 350 °C for 6 h using pine kraft lignin, torrefied lignin, and a mixture of initial and torrefied lignins with a ratio of 50:50 wt.%/wt.%. The amount of ash was less than that of good coking coal. However, the reactivity of the obtained biocoke was high compared to coke from good coking coal, and the mechanical strength of biocoke was significantly lower than that of coke.
Kim et al. [2] studied the TS of cokes and their reactivity using ash-free coal (AFC) as a binder and added torrefied biomass. The TS of the coke containing the torrefied fuel slightly decreased. The reactivity of the coke containing AFC and torrefied fuel was higher than that of the coke containing only AFC.
Castro-Díaz et al. [9] found that blends containing 70 wt.% low-rank coal, 24 wt.% torrefied lignin (before or after demineralization), and 6 wt.% phenolic resin produced biocokes with a suitable mechanical strength. However, reactivity was higher compared to coke.
Table 3 provides a comparative analysis of the required amount of raw biomass and torrefied biomass to replace conventional coke in a blast furnace under the conditions of Lorraine, Saint-Gobain PAM plant [20]. It can be concluded that the torrefaction process allows for improving the properties of biomass and reducing its quantity to replace conventional coke.
Table 3. Amount of raw biomass and torrefied biomass required to substitute conventional coke in BF on the example of Lorraine, Saint-Gobain PAM plant [20].
| Source | Amount of Required Biomass (kt/Year) | |
|---|---|---|
| 20 wt.% | 50 wt.% | |
| Raw biomass | 192.5 | 481.3 |
| Torrefied biomass | 77.0 | 192.5 |
The use of torrefied biomass in the BF can be the most environmentally friendly option, with 14.7 % CO2-equivalent reduction, followed closely by pulverized biomass char injection and charcoal lumps loaded at the top of the furnace, with 14.5 and 14.4 % CO2-equivalent reduction, respectively [20].
2.2. Recommendations for the Use of Secondary Carbon Bio-Carriers
It can be concluded that, according to some research groups, secondary carbon bio-carriers can be considered an alternative to conventional metallurgical coke when finding the optimal conditions for obtaining biocoke. To mitigate the negative impact of torrefied biomass additives, the best way is to use biomass after torrefaction at the maximum possible temperature of 300 °C and in a compressed form, which will facilitate their local arrangement within the blend volume, and often not as uniform as when using the initial biomass. However, the use of compressed torrefied biomass in the coal blend may allow the use of a higher amount to replace coal without drastically degrading the properties of the biocoke. Compared to the existing conventional coke, the advantage of biocoke is reduced ash and sulfur. The amount of torrefied biomass may vary depending on the further purposes of using the biocoke and may reach 50 wt.%.
3. Iron Ore Sintering
3.1. Features of the Process and Requirements for the Carbon-Bearing Material
Sintering is the most economical and widely used process for preparing fine iron ore for use in a BF. Compared to pellets, sinter production is cheaper, and compared to lump ore, fluxed sinter is often more reducible with better softening characteristics [21]. At the same time, the sintering process accounts for about 10% of CO2 emissions from the entire metallurgical industry [22].
Sintering occurs at temperatures of 1200–1400 °C, during which a mixture of iron ore fines and other materials (e.g., sinter return fines, limestone) is used [23]. Coke breeze or coal with low volatiles are used as fuel for sintering in the amount of 3–5 wt.% [24]. Table 4 shows the main properties of coke breeze for iron ore sintering. For iron ore sintering, the fuel should have a low VM of <3 wt.%, a high density of >700 kg/m3, a small size of <0.3–3.0 mm, and an FC content of more than 76 wt.%.
Table 4. Main properties of coke breeze for iron ore sintering.
There are few studies in the literature [29][30][31] that have analyzed the substitution of coke breeze with 10–25% of raw biomass in the process of iron ore sintering. It has been found that adding biomass can lead to some adverse effects, such as a decrease in productivity, an increase in the total fuel consumption (coke breeze and biomass), which negatively affects the economics and environment, and a decrease in the strength of the sinter. The use of highly reactive charcoal can also increase sintering velocity.
When using renewable fuel in iron ore sintering, it is essential to find the optimal ratio [32] because substitution can deteriorate the strength of the iron ore sinter and reducibility index, and heat treatment of the biomass is also essential to improve its properties as a fuel. This allows fuel requirements for use in iron ore sintering to be met.
Many studies [33][34][35][36][37] have focused on biomass pyrolysis and charcoal after pyrolysis for iron ore sintering. Generally, all research results were based on using biochars after pyrolysis at 400–1000 °C. These studies found that, when using biochar with a relatively high FC content in the sintering process, a similar sinter yield and productivity to those obtained by using coke breeze can be achieved. Additionally, the application of biomass char in a sintering plant allows for a reduction of 6.7 % CO2-equivalent compared to the use of anthracite [20].
3.2. Recommendations for the Use of Secondary Carbon Bio-Carriers
Biomass, after severe torrefaction, does not gain the necessary properties to completely replace the coke breeze. Biomass torrefaction may allow partial replacement of coke breeze and/or anthracite at 20–25% [31][38] without degrading the properties of the iron ore sinter. Moreover, the use of torrefied biomass in a compressed form can be considered.
The use of biocoke produced using torrefied biomass up to 50 wt.% may allow the 50 wt.% replacement of conventional coke breeze. Generally, the advantage of using biocoke compared to coke breeze is that the ash content is much lower, which guarantees a smaller particulate matter content in the flue gas.
4. Bio-Based Carbon Composite Agglomerates (CCAs)
4.1. Features of the Process and Requirements for the Carbon-Bearing Material
CCAs are mainly used in the BF and the direct reduction process [39]. Conventional CCAs are produced as pellets by cold bonding with or without a binder or briquettes by hot or cold pressing. Special studies are needed on the production aspects of these pellets, especially since their use in a BF requires higher qualities in terms of strength. CCAs can be referred to as new raw materials for iron production when consisting of carbon-bearing biomaterial powder, iron ore powder, and a small amount of binder materials. Carbon-bearing biomaterials used in pellets can be used in the raw state or after heat treatment. However, there are several requirements for this type of CCA. For example, top-loaded CCAs should meet the minimum mechanical strength requirements for a BF. Otherwise, they can worsen the efficiency of the process [40]. In addition, carbon-bearing biomaterials directly affect the mechanisms of mass and heat transfer, temperature profile, and gas distribution inside the BF.
According to Ahmed et al. [41], the ash content should not increase when using new carbon-bearing components. In this regard, the use of torrefied biomass is a promising approach. Furthermore, agglomerates should have an FC content in a range to ensure the iron carburization process. The reactivity requirements for carbonaceous materials are not very stringent. Nevertheless, it is important that the carbon in the CCAs does not participate in any chemical reactions below the set temperature of the heat reserve zone, as this can reduce the efficiency of the process. One of the most important quality parameters is the strength of composite pellets. Chemical reactions lead to the formation of gasses within the pellet, increasing its porosity [41], and it has been reported by Mousa et al. [40] that the main disadvantage of CCAs is their low crushing strength.
According to Khanna et al. and Ueki et al. [27][42], up to 46% of biochar can be used in agglomerates in the direct reduction (DR) process. It has been pointed out that the reduction rate of iron oxide is higher in biochar-based CCAs due to its greater reactivity than in conventional coal or coke-based CCAs. For instance, Hu et al. [43] focused on using only biochar-iron ore composites. It was concluded that pellets with 60 wt.% iron ore content and a temperature higher than 800 °C promoted carbon conversion and iron ore reduction. Praes et al. [44] carried out tests on iron ore pellets firing, which consumed natural gas, coal (anthracite fines), and two different eucalyptus charcoal (partially replaced) with two diverse ranges of VM. The first charcoal had a VM range of 20.3–25.98%, and the second varied from 9.4% to 11.1%. It was concluded that replacing 7.5% anthracite fines with the two eucalyptus charcoal is possible; 10% anthracite fines replacement is possible with the charcoal with the lower VM.
In another research work [45], the effect of using palm kernel shells as a reducing agent was studied. Iron oxide in iron ore can be completely reduced to magnetite and partially reduced to wustite when up to 30 wt.% palm kernel shells are present in the blend. In addition, the degree of reduction increases with increasing temperature up to 900 °C, as evidenced by the mass loss of the composite pellet and the mass of oxygen removal.
The level of metallization of the reduced pellets is also an important factor. Srivastava et al. [46] studied the effect of 20 wt.% fine wood on the quality of the resulting pellets. Pellets were fired at different temperatures, and residence times were also studied. In most cases, the total iron loss in the slag from the original pellets was less than 1% by weight. Pellets that contained 97% Fe on average were obtained, and at the highest firing temperature, the pellets contained 98.10% Fe.
Han et al. [47] studied the application of bamboo char, charcoal, and straw fiber to produce DRI. The carbon content was 87.5%, 68.5%, and 20.89% for bamboo char, charcoal, and straw fiber, respectively. The research results showed that the metallization level increased with increasing temperature and, as a result, reached 91%. Furthermore, one of the interesting results was that, despite the low FC content in straw fiber, the rate of metallization of the pellets was higher than that of charcoal and bamboo charcoal pellets due to the increased amount of carbohydrates. Regarding the compressive strength of the pellets, it is possible to achieve production requirements, namely >1800 N, only when using a high firing temperature.
4.2. Recommendations for the Use of Secondary Carbon Bio-Carriers
Based on the above and the properties of torrefied biomass, torrefied biomass can be used after the maximum possible torrefaction temperature. This allows the maximum possible values of FC to be reached, which is important for ensuring the carburization process. As the VM of the fuel increases, the possibility of thermal decomposition becomes more likely. This means that secondary carbon bio-carriers with a relatively low VM are less likely to decompose at lower temperatures. It can be concluded that torrefied biomass has the potential to completely replace coal in the production of CCAs and to achieve the necessary production requirements, but finding the optimal technological parameters is also required.
5. Injection of Pulverized Secondary Carbon Bio-Carriers
In conventional pulverized coal injection (PCI) technology, non-coking or weakly coking coals are injected into the raceways of BFs to partially replace the coke [48]. This technology is the most efficient method of replacing non-renewable fuels in the BF with different secondary carbon carriers. For example, there is a practice of injecting about 20 kg/tHM of waste plastics at Voestalpine Stahl BF A [49].
Table 5 shows the selected properties of conventional pulverized coal.
The use of biomass in its raw state after torrefaction, as well as after pyrolysis, in PCI has been well studied [4][52][53][54][55][56][57], and the use of secondary carbon bio-carriers in this technology is proven. The papers mentioned above revealed the possibility of using 20–40% biomass injection, or even up to 100% replacement of injected coal. However, it should be noted that for the purposes of PCI, the secondary carbon bio-carriers should be evaluated through several properties, such as the fuel ratio, ignition temperature, and burnout [58].
Phanphanich et al. [59] reported that proximate and elemental compositions of torrefied biomass could be improved after torrefaction at temperatures ranging from 225 °C to 300 °C, and were comparable to coal.
Chen et al. [60] studied the effect of torrefaction on improving the physical and chemical properties of pulverized biomass for use in a BF. The authors concluded that the calorific value could be improved by subsequent compaction.
In another paper by Chen et al. [61], the torrefaction and burning characteristics of bamboo, oil palm, rice husk, bagasse, and Madagascar almonds were studied and compared to high-volatile bituminous coal. As a result, the authors emphasized that a torrefaction temperature of 300 °C is suitable for converting the initial biomass into biochar, which can subsequently be used for injection into a BF. These results are consistent with the results of studies by Du et al. [58], who reported that biomass torrefied at 300 °C or carbonized at temperatures below 500 °C could be applied with coals for PCI. However, torrefied biomass can only partially replace coal to keep a good burnout in raceways.
Recommendations for the Use of Secondary Carbon Bio-Carriers
The main limitation of the widespread use of torrefied biomass is its high yield of VM. Therefore, the partial replacement of coal from 20–50% with biomass after severe torrefaction can be considered. For a complete replacement of coal, it is possible to consider the option of using a blend of torrefied biomass at 300 °C and biochar obtained after carbonization of torrefied biomass at a ratio of 50:50.
6. Blast Furnace Process
6.1. Features of the Process and Requirements for the Carbon-Bearing Material
The BF is currently the most predominant technology to produce iron for steelmaking. The principle of the process is the conversion of iron oxides to iron using carbon-based reducing agents. The BF is a large countercurrent metallurgical shaft furnace in which iron oxides and coke fed into the furnace from above move downwards, and the reducing gasses move upwards [62]. The reducing conditions in the furnace are created by top-charged coke and tuyere-injected reducing agents; for instance, pulverized coal (PC). Using carbon-bearing materials is essential to operate a BF, and the requirements are strict [63].
Two of the most important parameters for using metallurgical coke in a BF are the CRI and CSR. According to Alvarez et al. [64], the industrial quality requirements for coke are a CRI under 30% and a CSR above 55%; according to Babich et al. [65], CRI and CSR requirements in European BFs are 23% and 65%, respectively. In addition to these two parameters, the coke should have good mechanical strength; M40 should be >88%, M25 should be >90%, and M10 should be <6% [25]. These indices represent the percentage of material grain sizes remaining >40 mm, >25 mm, and > 10 mm after mechanical treatment (100 revolutions in a drum) according to the Micum drum test [25]. All of this limits the use of secondary carbon bio-carriers for producing coke for BF purposes.
There is a current practice of using charcoal or a mixture of charcoal and PC in mini BFs with a production capacity of 40,000–350,000 t/year [66]. The advantages of the mini BF technology are low emissions, low sulfur content in the iron, and low slag volumes. The carbonization temperature for charcoal can range from 300–700 °C, depending on the quality requirements. For example, a relatively high VM can be useful in PCI technology.
Another study on the mini BF technology was carried out by de Castro et al. [67]. In this work, various scenarios for using charcoal and hydrogen-rich fuel gas were simulated. Some scenarios have shown that it is possible to increase productivity and, at the same time, reduce carbon consumption.
The use of charcoal has also demonstrated the potential to reduce CO2 emissions in steel production. However, according to Hanrot et al. [68], the successful use of charcoal can be implemented if local conditions and quality criteria permit, such as the availability of biomass cultivation and the production of charcoal in a sustainable manner.
6.2. Recommendations for the Use of Secondary Carbon Bio-Carriers
The main pathways for secondary carbon bio-carriers in the BF/BOF route are shown in Figure 2.
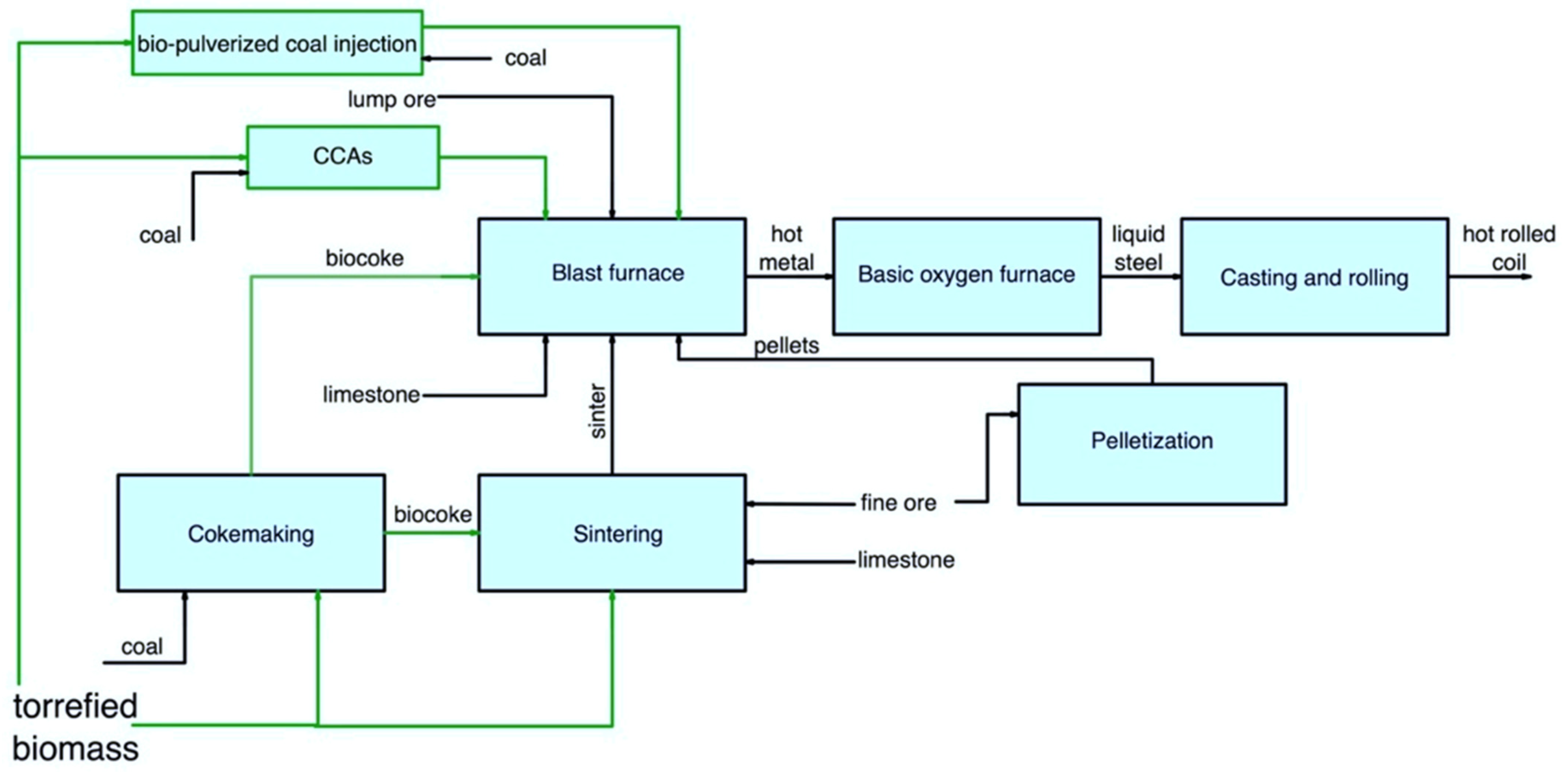
Figure 2. Main pathways for using secondary carbon bio-carriers in the BF/BOF route.
In a BF, the following ways of using secondary carbon bio-carriers can be considered:
- -
-
The use of biocoke produced with the addition of torrefied biomass, as discussed earlier in Cokemaking;
- -
-
The use of torrefied biomass or biocoke to produce sinter, which is afterwards used in the BF;
- -
-
The use of bio-pulverized coal injection technology with partial replacement of coal with up to 50% torrefied biomass or full replacement of coal with a mixture of torrefied biomass and torrefied biomass after carbonization;
- -
-
The use of torrefied biomass to produce CCAs, which are afterward used in the BF.
7. Electric Arc Furnace
7.1. Features of the Process and Requirements for the Carbon-Bearing Material
EAF-based steel production accounts for 28% of global output (~42% in the EU), according to the World Steel Association [69]. EAFs mainly use electricity with a small amount of carbon-bearing material [70]. Carbon-bearing material is added to the EAF route to perform the following functions: (a) charge carbon, with the main aim of adding chemical energy and creating a reducing atmosphere during smelting that minimizes the oxidation of alloys and metals; (b) injected carbon, also known as slag foaming carbon, where slag foaming technology in the EAF is used to increase energy efficiency and productivity, reduce operating costs, and improve the quality of steel produced [54]; and (c) afterward as a carburizer carbon in the ladle furnace for the carburizing process.
Norgate et al. [54] have shown that replacing a conventional carbon source from 50% to 100% is possible with charcoal after pyrolysis; also under life cycle assessment was charcoal production from Mallee eucalypt biomass. Yunos et al. [71] investigated the possibility of using biomass in the EAF; using palm shell char to partially replace coke in a laboratory-scale reactor at 1550 °C using the sessile-drop approach in an argon atmosphere. The test results showed an improved interaction with EAF slag compared to conventional coke.
In contrast, Huang et al. [72] concluded that the interaction between biochars and slag was weak compared to other carbon-bearing materials. The authors studied five carbon-bearing samples to replace conventional fuels: biochar obtained from wood biomass by slow pyrolysis at 900 °C, biochar obtained from wood biomass by fast pyrolysis at 400 °C, technical graphite, metallurgical coke, and semicoke obtained from waste tire pyrolysis at 700 °C.
Another study was carried out as part of the GreenEAF project (funded by the framework of the Research Fund for Coal and Steel, RFCS, 2009–2012, RFSR-CT-2009-00004). Bianco et al. [73] suggested a 1:1 substitution of coal (anthracite) and charcoal used in the EAF on an energy basis, assuming that charcoal is similar to or higher quality than coal and charcoal. The tests showed that charcoal could be used for both charge and slag foaming. Additionally, to achieve good foaming, the authors suggested several process approaches, such as improving the wettability of charcoal and slag, and the charcoal should be injected under the slag. Additionally, Fidalgo et al. [74] studied two biochars obtained from agricultural residues, grape seed and pumpkin seed chars, for EAF steelmaking. Hard coal and three types of anthracites were also used to compare the results. Biochars were obtained during the GreenEAF project for test runs. The temperatures used to produce biochar were 500 °C and 600 °C. A lower pyrolysis temperature of 500 °C was applied to obtain injected carbon with a higher VM to positively affect the foaming behavior. A pyrolysis temperature of 600 °C was applied to obtain charge carbon, for which it is more important to have a lower VM. The authors found that the biochar used in the project could replace coal with regard to reactivity. Furthermore, it was noted that the high VM of biochar is an adequate stimulation for slag foaming.
As a follow-up to the project mentioned above, Meier et al. [75] performed test runs with different carbon carriers in a dynamic process simulation model to investigate their application in an EAF within the framework of the GreenEAF2 project (RFCS, 2014–2017, RFSP-CT-2014-00003). Within this project, charcoal obtained from pyrolysis and torrefaction and virgin ligneous biomass (palm kernel shells) were used. The results using biomass showed a different behavior of the two materials studied; namely, anthracite and palm kernel shells. The mass fraction of waste gasses increased in the case of palm kernel shells, while melting the first portion of scrap because they have a higher VM content and are a more reactive material. It was concluded that it is possible to increase the use of post-combustion oxygen early in the process for palm kernel shells. It may subsequently lead to higher energy releases and increased melting rates.
Nwachukwu et al. [76] presented a model for the application of biofuels in steel production. According to the scheme, the amount of conventional fuel substitution ranged from 0% to 100%. However, it is worth noting that the model focused on using charcoal and bio-gas products.
Furthermore, Echterhof [77] presented a review of the utilization of alternative carbon sources by the EAF steel production route. The review showed that the use of alternative carbon sources in the EAF is required to produce fully environmentally friendly and carbon-neutral steel. Many research results have shown that it is fundamentally possible to use such substitutes.
7.2. Recommendations for the Use of Secondary Carbon Bio-Carriers
Figure 3 and Figure 4 show the pathways for secondary carbon bio-carriers in the EAF, where scrap is used as the main raw material, in the subsequent ladle furnace, and by DRI-EAF routes.
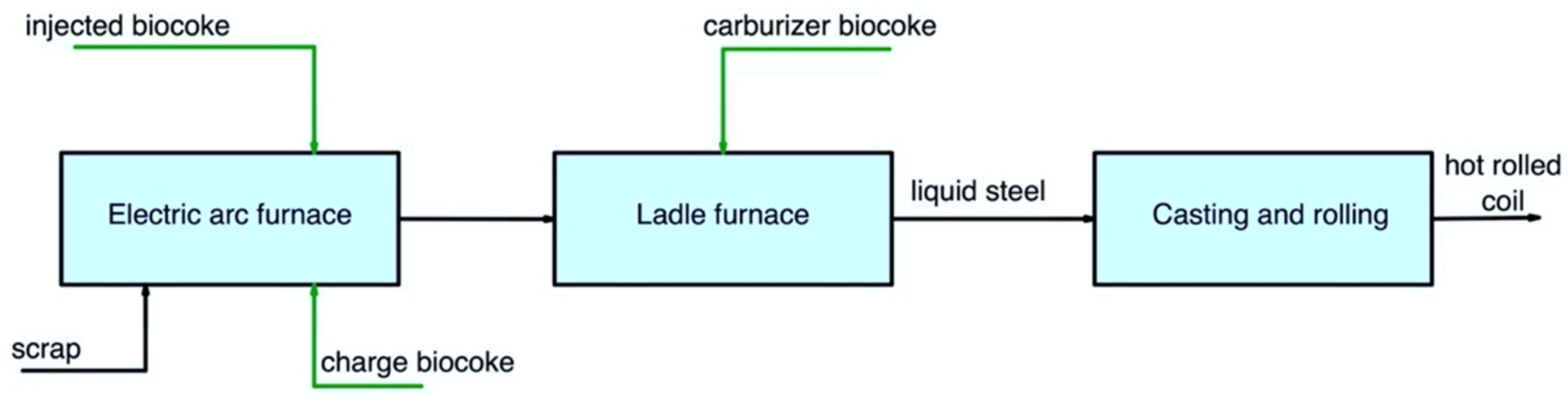
Figure 3. Main pathways for using secondary carbon bio-carriers in the EAF, where scrap is used as the main raw material.
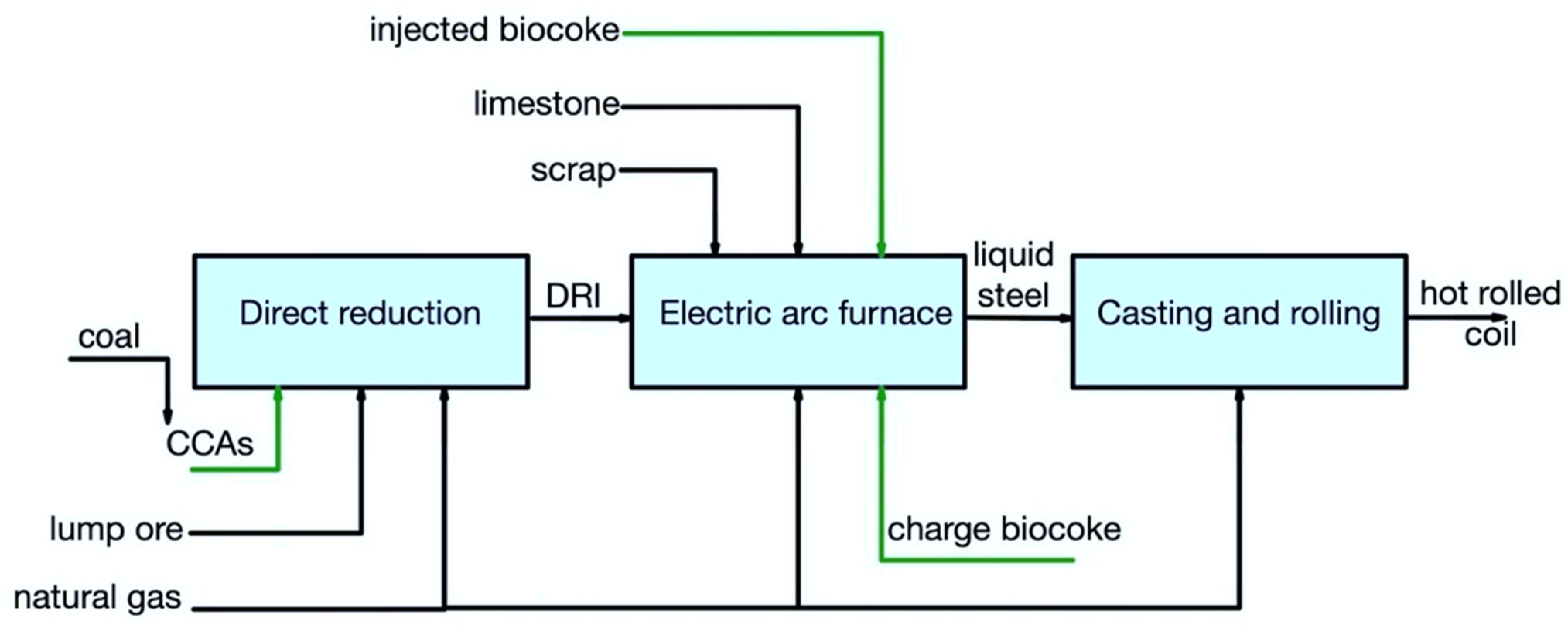
Figure 4. Main pathways for using secondary carbon bio-carriers in the DRI/EAF route.
There are several possible ways to apply secondary carbon bio-carriers (biocoke):
- -
-
a charge carbon in an EAF;
- -
-
an injected carbon in an EAF;
- -
-
a carburizer source in a ladle furnace.
It should be noted that, in the DRI/EAF route, it is also possible to consider using CCAs obtained using torrefied biomass.
Based on the above requirements, it can be concluded that the use of torrefied biomass is practically limited. However, the use of biocoke up to 100% as a carbon source is possible even with a high additive amount of torrefied biomass up to 50% within coal blend because it can meet the requirements for this process in terms of the VM and has a sufficient amount of FC > 85% for carburizing the steel or creating foaming slag to improve the energy efficiency of the melting process. Additionally, it is important to mention that there are no strict requirements for the strength of the carbon source for use in an EAF because of the features of the furnace [54]. Therefore, using biocoke with a high amount of torrefied biomass is a promising pathway for future research.
8. Smelting Reduction Processes
There are mainly two smelting reduction processes that are commercially proven, COREX and FINEX. Iron ores are heated and pre-reduced to DRI within these processes by the off-gas coming from the melter gasifier. The pre-reduction step could be implemented in a reduction shaft (COREX) or a fluidized bed reactor (FINEX). Pre-reduced iron ores are then melted in the melter gasifier. The melter gasifier uses oxygen and coal as a reducing agent. Subsequently, the hot metal is fed to the BOF for steelmaking [78][79][80]. These processes generally use non-coking coal as a fuel source, with the main requirements of FC > 40% and VM < 34%. Considering these requirements, secondary carbon bio-carriers can be used after torrefaction to the highest possible temperature, thus obtaining biochar with the highest possible values for FC and the lowest VM. Another option is to use biocoke, whereby it is possible to consider using biocoke obtained with a high amount of torrefied biomass of up to 50% in this route. An additional benefit of biocoke is that sulfur-containing compounds and ash formation are minimized.
8.1. COREX Process
COREX is a process that produces hot metal out of lumpy iron carriers (mainly pellets, but also lump ore and sinter). The main reducing agent is briquetted coal. The process mainly consists of two reactors; i.e., a reduction shaft and a melting gasifier [81]. For the COREX process, the most suitable conventional carbon source is non-coking coal with 55–70 wt.% FC content. There are also a number of requirements for the ash content and moisture content. The ash content should be lower than 12 wt.%, as a higher ash content increases the slag volume, resulting in high specific fuel consumption, poor drainage through the coal bed, and reduced productivity [82]. The moisture content should be as low as possible. The main requirements for the properties of the carbon-bearing material are given in Table 6.
Table 6. Main requirements for non-coking coal for COREX [82].
| Parameters | Values |
|---|---|
| Moisture, wt.% | <4 |
| Ash, wt.% | <12 |
| Volatile matters, wt.% | 25–27 |
| Sulfur, wt.% | <0.6 |
| Fixed carbon, wt.% | 55–70 |
| Calorific value, kJ kg−1 | >27,000 |
Although a significant amount of coal is used in the COREX process, 10–20% of metallurgical coke is required for heat generation, reducing gas production, and maintaining char bed permeability. The coke quality required for the COREX process is shown in Table 7.
| Parameters | Values |
|---|---|
| Coke reactivity index, wt.% | <35 |
| Coke strength after reaction, wt.% | >55 |
| Volatile matters, wt.% | app. 25 |
| Ash, wt.% | <15 |
| Sulfur, wt.% | <1 |
| Grain size, mm | 10–15 |
Several studies have investigated the use of torrefied biomass for the COREX ironmaking route. Adeleke et al. [84] investigated using coal briquettes and pre-treated biomass for use in COREX ironmaking processes. The initial biomass was ground to < 2 mm and subjected to a torrefaction process at a temperature of 260 °C and a residence time of 60 min. Carbon fines of 95 wt.%, torrefied biomass of 5 wt.%, and binder were homogeneously mixed, followed by the addition of water and proper mixing to activate the binder for agglomeration. The authors concluded that the coal fines-torrefied biomass briquettes satisfactorily met the required physical properties for the COREX ironmaking process.
Moreover, according to the scheme of mass, iron, and carbon balance for the Bio-COREX/BOF case by Yang et al. [85], the biochar substitution rate can be as high as 45 %LHV.
Figure 5 shows the main pathway for using secondary carbon bio-carriers in the COREX/BOF route. This layout can consider using the following:
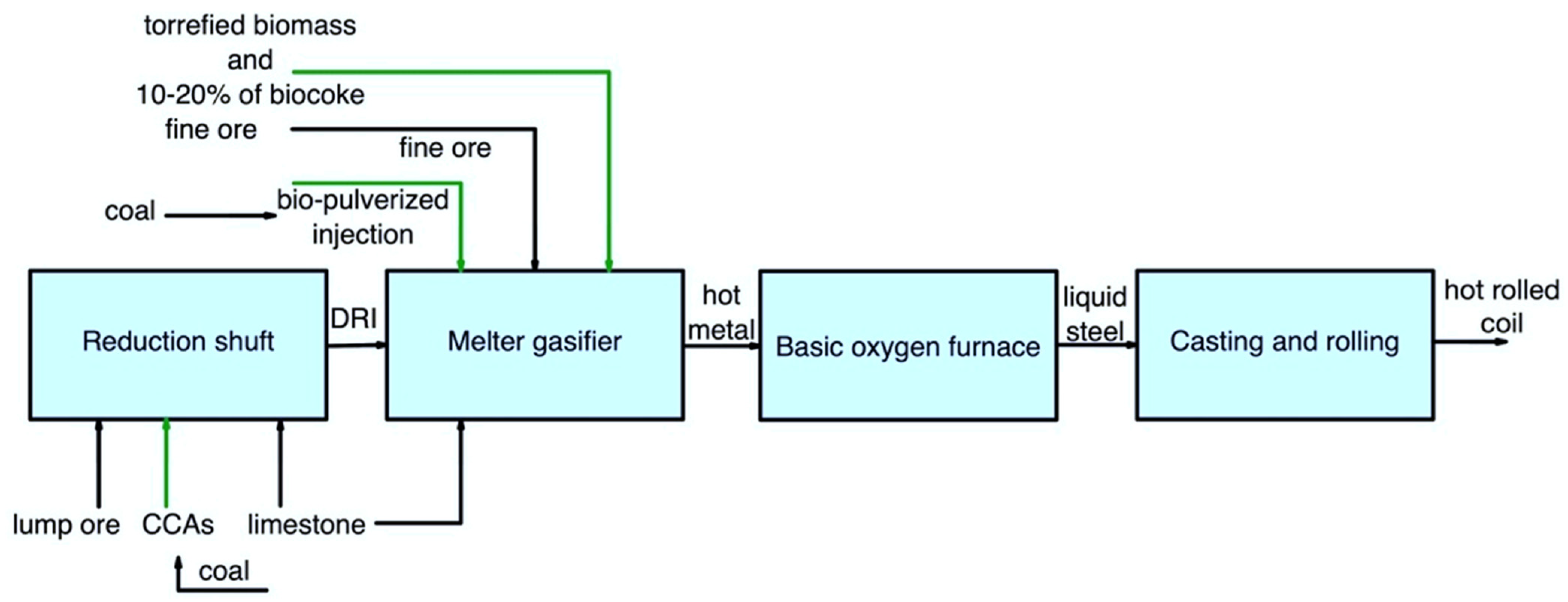
Figure 5. Main pathways for using secondary carbon bio-carriers in the COREX/BOF route.
- -
-
Biomass torrefied at the highest possible temperature can partially replace coal;
- -
-
Biocoke for complete replacement of the conventional coke. At the same time, it is possible to consider using biocoke with a high amount of torrefied biomass as a substitute for coal.
In addition, the use of bio-pulverized injection and CCAs should be considered possible options for this route.
8.2. FINEX Process
The FINEX smelting-reduction process is based on the direct use of non-coking coal and fine ore. The major difference between the COREX and FINEX processes is that the FINEX process can directly use sinter feed iron ore without an agglomeration [83]. The main FINEX process consists of a melter-gasifier and a series of fluidized bed reactors, forming a countercurrent system in which fine ore is reduced to DRI in three or four stages. The fine DRI is then compacted and loaded as hot compacted iron (HCI) into a gasifier melting unit. The HCl is then reduced and melted. The heat required for reduction and melting is provided by coal gasification. The reducing gas, also from coal gasification, passes through fluidized-bed reactors [83]. From the point of view of the fuel route, non-coking coals and coal briquettes are directly loaded into the melter-gasifier unit. The main fuel quality requirements for the FINEX process are shown in Table 8.
Table 8. Main requirements for fuel for FINEX.
| Fixed Carbon, wt.% |
Ash, wt.% |
Volatile Matters, wt.% | Sulfur, wt.% |
Reference |
|---|---|---|---|---|
| min. 55 | up to 25 | <35 | <1 | [86] |
Figure 6 shows the use of secondary carbon bio-carriers in the FINEX/BOF route.
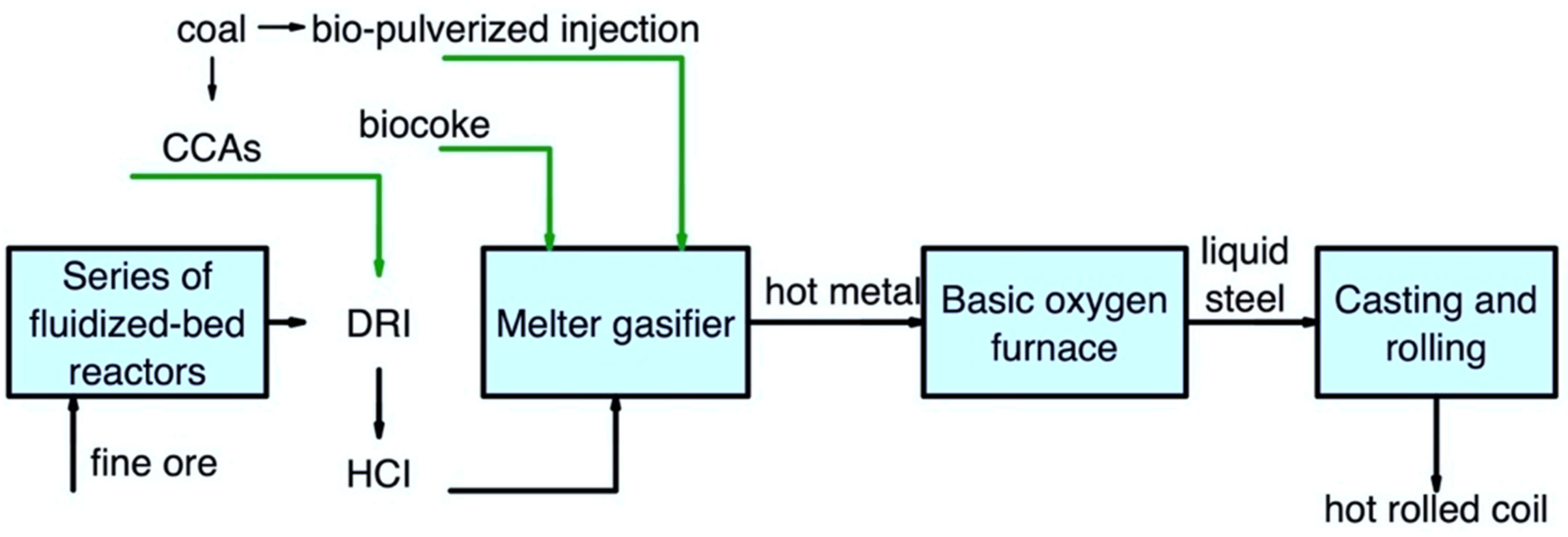
Figure 6. Main pathways for using secondary carbon bio-carriers in the FINEX/BOF route.
It is important to note that the use of secondary carbon bio-carriers in this scheme does not differ much from the COREX/BOF route. It is possible to partially replace coal briquettes using biomass torrefied at the highest possible temperature and subsequent compaction. The use of lumpy biocoke can be considered a complete replacement for coal. At the same time, it is also possible to consider the use of biocoke obtained with a high amount of torrefied biomass. Bio-pulverized injection and CCAs can be considered for using secondary carbon bio-carriers.
8.3. HIsarna and Hismelt Processes
HIsarna is a melt-in-bath technology that combines coal preheating and partial pyrolysis in a reactor [87]. This technology uses a melting vessel for the final reduction of ore and a melting cyclone for ore melting. Reduced CO2 emissions are enabled due to the absence of sintering and coking processes. The HIsarna technology reduces CO2 emissions by almost 70% by using biomass or natural gas instead of coal, flue gas treatment, CO2 storage, and thermal energy reuse [88]. Pulverized coal [89] and biomass [88] can be used as fuel in this process. Khasraw et al. [90] studied the properties of two samples of charcoal (birch wood and grass) compared to thermal coal. It was observed that chars showed a faster reaction compared to thermal coal. According to another paper by Khasraw et al. [87], torrefied grass contains a large amount of water and CO2 that is released at a very low temperature. Therefore, pre-treatment to approximately 400 °C is essential to obtain biochar with properties similar to the coal introduced into HIsarna.
Htet et al. [91] studied the properties of charcoal, thermal coal, and carbon black as reducing agents for the HIsarna process, and also studied their properties to understand how these materials can affect the process. In the case of using substitutes, the important parameters are the alkalinity index, VM content, and its amount, as well as the amount of ash.
Figure 7 shows the use of secondary carbon bio-carriers in the HIsarna/BOF route.
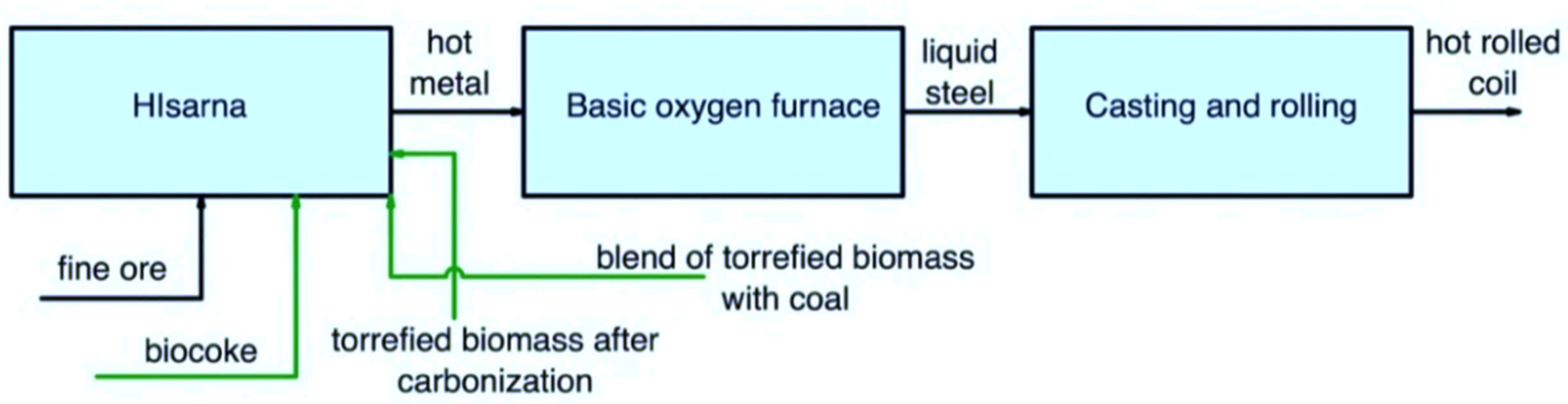
Figure 7. Main pathways for using secondary carbon bio-carriers in the HIsarna/BOF route.
Based on the analysis of the results, it is challenging to use torrefied biomass even at the highest possible temperature due to limitations in its properties. However, the first option of using torrefied biomass after carbonization can be considered for improving the properties. Secondly, a blend of coal with torrefied biomass (up to the highest possible torrefaction temperature) can be used. For further research, it could be of interest to replace coal with up to 50 wt.%. Additionally, the third option of using biocoke obtained using torrefied biomass is viable. At the same time, the amount of torrefied biomass in biocoke can reach up to 50 wt.% because the VM and FC will be at the required level even with such an amount of a substitute.
A similar recommendation for using biomass to replace pulverized coal with a particle size of <3 mm can be applied to the Hismelt process, which uses a two-stage rotary kiln process to preheat and pre-reduce iron-bearing raw materials (particle size < 6 mm) at 750 °C.
9. Ferroalloy Industry
9.1. Submerged Arc Furnace
The ferroalloy industry refers to iron alloys with a high proportion of additional elements, such as chromium, manganese, silicon, aluminum, and other elements. They are generally produced in a SAF at a temperature of >1500 °C. A three-phase electrode (AC power supply) is inserted into a mixture of ferroalloys and carbon-bearing reductants [92]. Carbon-bearing materials (coke or anthracite) are used to produce ferroalloys through a carbothermic reduction, which acts as a reducing agent. Biobased reductants can potentially replace fossil-based ones, mitigating anthropogenic CO2 and GHG emissions and increasing the efficiency of the smelting process. This leads to a search for new ways to produce and use alternative bioreductants to produce ferroalloys. The most important properties required for carbonaceous reducing agents are high reactivity, electrical resistance, high conversion rates, low sulfur content, high bulk density, and specific energy. Table 9 gives some of the important properties of conventional carbonaceous materials for use in ferroalloy production.
| Parameters | Conventional Coke |
|---|---|
| Fixed carbon, wt.% | 86–88 |
| Volatile matter, wt.% | ≤1 |
| Ash, wt.% | 10–12 |
| Reactivity with CO2 at 1060 °C, %C/s | (0.2–0.5)10−2 |
| Thermal cohesion strength, % | 93–97 |
| Thermal abrasion strength, % | 82–89 |
| Electrical resistance for carbon material with size, mm: | 10–20 |
| Electrical resistance at 1000 °C, U∙m | 0.003–0.008 |
| Electrical resistance at 1400 °C, U∙m | 0.003–0.009 |
Surup et al. [95] investigated the pyrolysis treatment of various types of biomass at high temperatures to obtain biochars, which can be subsequently used to produce ferroalloys. It was concluded that with a heat treatment > 2400 °C, it is possible to obtain biochar from renewable sources with a reactivity close to conventional coke. In addition, the co-pyrolysis of biomass with bio-oil was studied, and the prospect of obtaining biochars with a reactivity comparable to metallurgical coke was shown.
Electrical resistance is also an important indicator for carbon-bearing materials. According to another paper by Surup et al. [93], conventional coke used in a SAF is expected to have an electrical resistivity of 7–10 mΩm at room temperature and 1–10 mΩm at 1400 °C for a particle size of 5–20 mm. Coke substitutes, i.e., charcoal, have an electrical resistance of more than 106 mΩ at room temperature, which decreases with increasing temperature. The electrical resistance of charcoal obtained after carbonization at 950 °C was similar to that of coal char, varying from 1.7 mΩm to 3.4 mΩm [93].
Bazaluk et al. [7] investigated the properties of biocoke obtained at different carbonization temperatures and with varying amounts of biomass pellets for use in BFs and non-BFs. It was found that the electrical resistance of carbon-bearing materials decreases with an increase in the carbonization temperature, which is associated with the formation and/or increase of carbon crystallites. The electrical resistance was in the range of 12.0–15.9 mΩm for 950 °C and 10.3–13.8 mΩm for 1100 °C.
The selected obtained results [96][97] are the basis for the approach to use biocoke as a reductant to produce ferroalloys, and from the point of view of reducing the dependence on fossil fuel and mitigating emissions associated with the metallurgical industry, in particular the production of ferroalloys. Biocoke is also suitable for use in terms of such indicators as the amount of FC of more than 85% and the VM content.
According to Surup et al. [98], charcoal can replace conventional carbon-bearing reductants by more than 40% in the SAF, while complete replacement requires an additional heat treatment of charcoal.
9.2. Recommendations for the Use of Secondary Carbon Bio-Carriers
In ferroalloy production, the application of torrefied biomass is challenging because it does not achieve the required properties due to the low torrefaction temperature. However, biocoke has good potential for use in a SAF. Figure 8 is a layout of the main pathway for the use of secondary carbon bio-carriers in ferroalloy production.
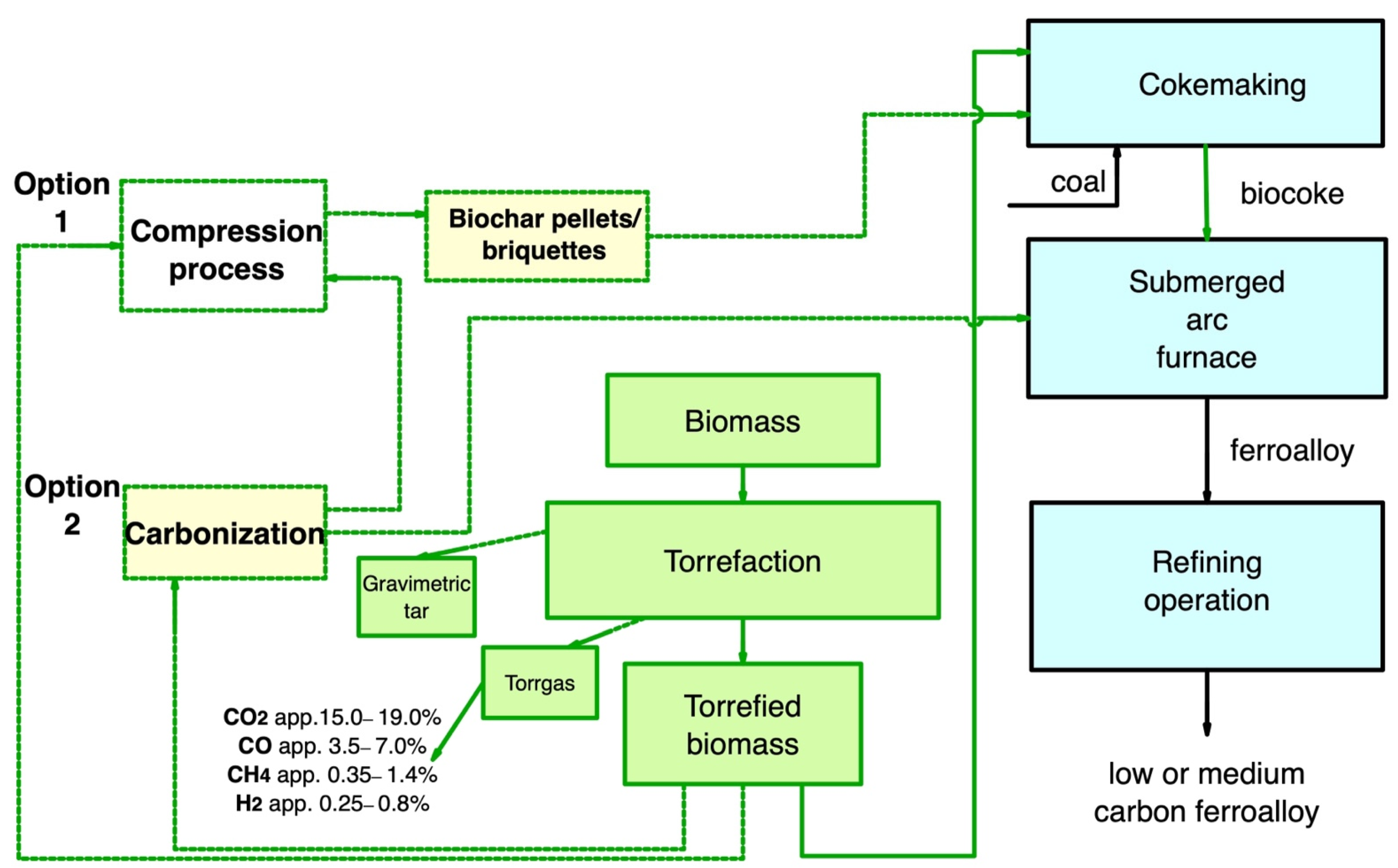
Figure 8. Main pathway for using secondary carbon bio-carriers in ferroalloy production.
There are several ways to use secondary carbon bio-carriers:
- -
-
Torrefied biomass to produce biocoke and further use of biocoke to produce ferroalloys. At the same time, it is of interest to replace up to 50% of coal within the coal blend;
- -
-
Subject torrefied biomass to carbonization to increase FC, reduce VM yield, and achieve the required electrical resistance values close to that of conventional fuel. Furthermore, after carbonization, secondary carbon bio-carriers can be used in the SAF;
- -
-
Torrefied biomass, also after carbonization, can be subjected to subsequent compaction, and then the resulting briquettes can be used to produce biocoke.
References
- European Steel Technology Platform (ESTEP): Clean Steel Partnership Roadmap. Available online: https://www.estep.eu/assets/Uploads/CSP-SRIA-Oct2021-clean.pdf (accessed on 22 November 2022).
- Kim, G.-M.; Lisandy, K.Y.; Isworo, Y.Y.; Kim, J.-H.; Jeon, C.-H. Investigation into the Effects of Ash-Free Coal Binder and Torrefied Biomass Addition on Coke Strength and Reactivity. Fuel 2018, 212, 487–497.
- Sun, W.; Wang, Q.; Zheng, Z.; Cai, J. Material–Energy–Emission Nexus in the Integrated Iron and Steel Industry. Energy Convers. Manag. 2020, 213, 112828.
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of Biomass in Integrated Steelmaking—Status Quo, Future Needs and Comparison to Other Low-CO2 Steel Production Technologies. Appl. Energy 2018, 213, 384–407.
- Ghosh, B.; Sahoo, B.K.; Chakraborty, B.; Manjhi, K.K.; Das, S.K.; Sahu, J.N.; Varma, A.K. Influence of Coke Structure on Coke Quality Using Image Analysis Method. Int. J. Coal Sci. Technol. 2018, 5, 473–485.
- Janhsen, U.; Sante, L.; Steiler, J.M.; Sert, D.; Negro, P.; Wilmers, R.R.; Bennington, C.R.; Hitchenson, C. Reduction of Iron Ores. In Coke Quality for Blast Furnace Operation with High Levels of Coal/Oil Injection; European Commission, Directorate-General for Research and Innovation: Luxembourg, 2002; p. 205.
- Bazaluk, O.; Kieush, L.; Koveria, A.; Schenk, J.; Pfeiffer, A.; Zheng, H.; Lozynskyi, V. Metallurgical Coke Production with Biomass Additives: Study of Biocoke Properties for Blast Furnace and Submerged Arc Furnace Purposes. Materials 2022, 15, 1147.
- Mansor, A.M.; Theo, W.L.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Potential Commercialisation of Biocoke Production in Malaysia—A Best Evidence Review. Renew. Sustain. Energy Rev. 2018, 90, 636–649.
- Castro-Díaz, M.; Vega, M.F.; Díaz-Faes, E.; Barriocanal, C.; Musa, U.; Snape, C. Evaluation of Demineralized Lignin and Lignin-Phenolic Resin Blends to Produce Biocoke Suitable for Blast Furnace Operation. Fuel 2019, 258, 116125.
- Montiano, M.G.; Díaz-Faes, E.; Barriocanal, C.; Alvarez, R. Influence of Biomass on Metallurgical Coke Quality. Fuel 2014, 116, 175–182.
- Tsalidis, G.A.; Di Marcello, M.; Spinelli, G.; de Jong, W.; Kiel, J.H.A. The Effect of Torrefaction on the Process Performance of Oxygen-Steam Blown CFB Gasification of Hardwood and Softwood. Biomass Bioenergy 2017, 106, 155–165.
- Kieush, L.; Schenk, J.; Pfeiffer, A.; Koveria, A.; Rantitsch, G.; Hopfinger, H. Investigation on the Influence of Wood Pellets on the Reactivity of Coke with CO2 and Its Microstructure Properties. Fuel 2022, 309, 122151.
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887.
- MacPhee, J.A.; Gransden, J.F.; Giroux, L.; Price, J.T. Possible CO2 Mitigation via Addition of Charcoal to Coking Coal Blends. Fuel Process. Technol. 2009, 90, 16–20.
- Babich, A.; Senk, D. Coke in the Iron and Steel Industry. In New Trends in Coal Conversion; Woodhead Publishing: Sawston, UK, 2019; pp. 367–404. ISBN 978-0-08-102201-6.
- MacPhee, J.A.; Grandsen, J.F.; Giroux, L.; Price, J.T. CO2 Mitigation via Addition of Charcoal to Coking Coal Blends. In Proceedings of the International Conference on Coal Science and Technology, Nottingham, UK, 28–31August 2007.
- Guerrero, A.; Diez, M.A.; Borrego, A.G. Influence of Charcoal Fines on the Thermoplastic Properties of Coking Coals and the Optical Properties of the Semicoke. Int. J. Coal Geol. 2015, 147–148, 105–114.
- Kudo, S.; Mori, A.; Soejima, R.; Murayama, F.; Karnowo; Nomura, S.; Dohi, Y.; Norinaga, K.; Hayashi, J. Preparation of Coke from Hydrothermally Treated Biomass in Sequence of Hot Briquetting and Carbonization. ISIJ Int. 2014, 54, 2461–2469.
- Castro-Díaz, M.; Uguna, C.N.; Florentino, L.; Díaz-Faes, E.; Stevens, L.A.; Barriocanal, C.; Snape, C.E. Evaluation of Hydrochars from Lignin Hydrous Pyrolysis to Produce Biocokes after Carbonization. J. Anal. Appl. Pyrolysis 2017, 124, 742–751.
- Fick, G.; Mirgaux, O.; Neau, P.; Patisson, F. Using Biomass for Pig Iron Production: A Technical, Environmental and Economical Assessment. Waste Biomass Valor 2014, 5, 43–55.
- Lu, L.; Ishiyama, O. Iron Ore Sintering. In Iron Ore; Elsevier: Amsterdam, The Netherlands, 2015; pp. 395–433. ISBN 978-1-78242-156-6.
- Luengen, H.B.; Endemann, G.; Schmoele, P. Measures to Reduce CO2 and Other Emissions in the Steel Industry in Germany and Europe. World Iron Steel 2013, 1, 8–15.
- Forsmo, S.P.E.; Forsmo, S.-E.; Samskog, P.-O.; Björkman, B.M.T. Mechanisms in Oxidation and Sintering of Magnetite Iron Ore Green Pellets. Powder Technol. 2008, 183, 247–259.
- Ooi, T.C.; Aries, E.; Ewan, B.C.R.; Thompson, D.; Anderson, D.R.; Fisher, R.; Fray, T.; Tognarelli, D. The Study of Sunflower Seed Husks as a Fuel in the Iron Ore Sintering Process. Miner. Eng. 2008, 21, 167–177.
- Koveria, A.; Kieush, L.; Svietkina, O.; Perkov, Y. Metallurgical Coke Production with Biomass Additives. Part 1. A Review of Existing Practices. Can. Metall. Q. 2020, 59, 417–429.
- Xiong, L.; Peng, Z.; Gu, F.; Ye, L.; Wang, L.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Combustion Behavior of Granulated Coke Breeze in Iron Ore Sintering. Powder Technol. 2018, 340, 131–138.
- Khanna, R.; Li, K.; Wang, Z.; Sun, M.; Zhang, J.; Mukherjee, P.S. Biochars in Iron and Steel Industries. In Char and Carbon Materials Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2019; pp. 429–446. ISBN 978-0-12-814893-8.
- Okimori, Y.; Ogawa, M.; Takahashi, F. Potential of Co 2 Emission Reductions by Carbonizing Biomass Waste from Industrial Tree Plantation in South Sumatra, Indonesia. Mitig. Adapt. Strateg. Glob. Chang. 2003, 8, 261–280.
- Wei, R.; Zhang, L.; Cang, D.; Li, J.; Li, X.; Xu, C.C. Current Status and Potential of Biomass Utilization in Ferrous Metallurgical Industry. Renew. Sustain. Energy Rev. 2017, 68, 511–524.
- Zandi, M.; Martinez-Pacheco, M.; Fray, T.A.T. Biomass for Iron Ore Sintering. Miner. Eng. 2010, 23, 1139–1145.
- Ooi, T.C.; Thompson, D.; Anderson, D.R.; Fisher, R.; Fray, T.; Zandi, M. The Effect of Charcoal Combustion on Iron-Ore Sintering Performance and Emission of Persistent Organic Pollutants. Combust. Flame 2011, 158, 979–987.
- Jha, G.; Soren, S.; Mehta, K.D. Life Cycle Assessment of Sintering Process for Carbon Footprint and Cost Reduction: A Comparative Study for Coke and Biomass-Derived Sintering Process. J. Clean. Prod. 2020, 259, 120889.
- Lovel, R.; Vining, K.; Dell’Amico, M. Iron Ore Sintering with Charcoal. Miner. Process. Extr. Metall. 2007, 116, 85–92.
- Abreu, G.C.; de Carvalho, J.A.; da Silva, B.E.C.; Pedrini, R.H. Operational and Environmental Assessment on the Use of Charcoal in Iron Ore Sinter Production. J. Clean. Prod. 2015, 101, 387–394.
- Ji, Z.; Fan, X.; Gan, M.; Chen, X.; Li, Q.; Jiang, T. Assessment on the Application of Commercial Medium-Grade Charcoal as a Substitute for Coke Breeze in Iron Ore Sintering. Energy Fuels 2016, 30, 10448–10457.
- Gan, M.; Li, Q.; Ji, Z.; Fan, X.; Lv, W.; Chen, X.; Tian, Y.; Jiang, T. Influence of Surface Modification on Combustion Characteristics of Charcoal and Its Performance on Emissions Reduction in Iron Ore Sintering. ISIJ Int. 2017, 57, 420–428.
- Cheng, Z.; Yang, J.; Zhou, L.; Liu, Y.; Wang, Q. Characteristics of Charcoal Combustion and Its Effects on Iron-Ore Sintering Performance. Appl. Energy 2016, 161, 364–374.
- Niesler, M.; Stecko, J.; Stelmach, S.; Kwiecińska-Mydlak, A. Biochars in Iron Ores Sintering Process: Effect on Sinter Quality and Emission. Energies 2021, 14, 3749.
- Wiinikka, H.; Sepman, A.; Ögren, Y.; Lindblom, B.; Nordin, L.-O. Combustion Evaluation of Renewable Fuels for Iron-Ore Pellet Induration. Energy Fuels 2019, 33, 7819–7829.
- Mousa, A.; Ahmed, H.; Viswanathan, N.; Larsson, M. Recent Trends in Ironmaking Blast Furnace Technology to Mitigate CO2 Emissions: Tuyeres Injection. In Ironmaking and Steelmaking Processes: Greenhouse Emissions, Control and Reduction; Springer International Publishing: Cham, Switzerland, 2016; pp. 173–197.
- Ahmed, H.M.; Viswanathan, N.; Bjorkman, B. Composite Pellets—A Potential Raw Material for Iron-Making. Steel Res. Int. 2014, 85, 293–306.
- Ueki, Y.; Yoshiie, R.; Naruse, I.; Ohno, K.; Maeda, T.; Nishioka, K.; Shimizu, M. Reaction Behavior during Heating Biomass Materials and Iron Oxide Composites. Fuel 2013, 104, 58–61.
- Hu, Q.; Yao, D.; Xie, Y.; Zhu, Y.; Yang, H.; Chen, Y.; Chen, H. Study on Intrinsic Reaction Behavior and Kinetics during Reduction of Iron Ore Pellets by Utilization of Biochar. Energy Convers. Manag. 2018, 158, 1–8.
- Praes, G.E.; de Arruda, J.D.; Lemos, L.R.; Tavares, R.P. Assessment of Iron Ore Pellets Production Using Two Charcoals with Different Content of Materials Volatile Replacing Partially Anthracite Fines. J. Mater. Res. Technol. 2019, 8, 1150–1160.
- Abd Rashid, R.Z.; Salleh, H.M.; Ani, M.H.; Yunus, N.A.; Akiyama, T.; Purwanto, H. Reduction of Low Grade Iron Ore Pellet Using Palm Kernel Shell. Renew. Energy 2014, 63, 617–623.
- Srivastava, U.; Kawatra, S.K.; Eisele, T.C. Production of Pig Iron by Utilizing Biomass as a Reducing Agent. Int. J. Miner. Process. 2013, 119, 51–57.
- Han, H.; Duan, D.; Yuan, P.; Li, D. Biomass Reducing Agent Utilisation in Rotary Hearth Furnace Process for DRI Production. Ironmak. Steelmak. 2015, 42, 579–584.
- Toloue Farrokh, N.; Suopajärvi, H.; Mattila, O.; Sulasalmi, P.; Fabritius, T. Characteristics of Wood-Based Biochars for Pulverized Coal Injection. Fuel 2020, 265, 117017.
- Knepper, M.; Babich, A.; Senk, D.; Buergler, T.; Feilmayr, C.; Kieberger, N. Waste Plastics Injection: Reaction Kinetics and Effect on the Blast Furnace Process. In Proceedings of the 6th International Congress on the Science and Technology of Ironmaking-ICSTI, Rio de Janeiro, RJ, Brazil, 14–18 October 2012; pp. 798–810.
- Babich, A.; Senk, D.; Fernandez, M. Charcoal Behaviour by Its Injection into the Modern Blast Furnace. ISIJ Int. 2010, 50, 81–88.
- de Castro, J.A.; de Mattos Araújo, G.; da Mota ID, O.; Sasaki, Y.; Yagi, J.I. Analysis of the Combined Injection of Pulverized Coal and Charcoal into Large Blast Furnaces. J. Mater. Res. Technol. 2013, 2, 308–314.
- Wang, C.; Mellin, P.; Lövgren, J.; Nilsson, L.; Yang, W.; Salman, H.; Hultgren, A.; Larsson, M. Biomass as Blast Furnace Injectant—Considering Availability, Pretreatment and Deployment in the Swedish Steel Industry. Energy Convers. Manag. 2015, 102, 217–226.
- Suopajärvi, H.; Fabritius, T. Towards More Sustainable Ironmaking—An Analysis of Energy Wood Availability in Finland and the Economics of Charcoal Production. Sustainability 2013, 5, 1188–1207.
- Norgate, T.; Haque, N.; Somerville, M.; Jahanshahi, S. Biomass as a Source of Renewable Carbon for Iron and Steelmaking. ISIJ Int. 2012, 52, 1472–1481.
- Proskurina, S.; Heinimö, J.; Schipfer, F.; Vakkilainen, E. Biomass for Industrial Applications: The Role of Torrefaction. Renew. Energy 2017, 111, 265–274.
- Mandova, H.; Leduc, S.; Wang, C.; Wetterlund, E.; Patrizio, P.; Gale, W.; Kraxner, F. Possibilities for CO2 Emission Reduction Using Biomass in European Integrated Steel Plants. Biomass Bioenergy 2018, 115, 231–243.
- Mattila, O.; Vuorenmaa, V.; Paananen, T. Grinding and Transport Properties of Wood Char Together with Blast Furnace PCI. In Proceedings of the 5th International Conference on Process Development in Iron and Steelmaking (SCANMET V), Luleå, Sweden, 12–15 June 2016.
- Du, S.-W.; Chen, W.-H.; Lucas, J.A. Pretreatment of Biomass by Torrefaction and Carbonization for Coal Blend Used in Pulverized Coal Injection. Bioresour. Technol. 2014, 161, 333–339.
- Phanphanich, M.; Mani, S. Impact of Torrefaction on the Grindability and Fuel Characteristics of Forest Biomass. Bioresour. Technol. 2011, 102, 1246–1253.
- Chen, W.-H.; Cheng, W.-Y.; Lu, K.-M.; Huang, Y.-P. An Evaluation on Improvement of Pulverized Biomass Property for Solid Fuel through Torrefaction. Appl. Energy 2011, 88, 3636–3644.
- Chen, W.-H.; Du, S.-W.; Tsai, C.-H.; Wang, Z.-Y. Torrefied Biomasses in a Drop Tube Furnace to Evaluate Their Utility in Blast Furnaces. Bioresour. Technol. 2012, 111, 433–438.
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The Potential of Using Biomass-Based Reducing Agents in the Blast Furnace: A Review of Thermochemical Conversion Technologies and Assessments Related to Sustainability. Renew. Sustain. Energy Rev. 2013, 25, 511–528.
- Großpietsch, K.H.; Lüngen, H.B.; Dauwels, G.; Ferstl, A.; Karjalahti, T.; Negro, P.; van der Velden, B.; Willmers, R. Coke Quality Requirements by European Blast Furnace Operators on the Turn of the Millennium. In Proceedings of the 4th European Coke and Ironmaking Congress, Paris, France, 19–21 June 2000; Volume 1, pp. 2–11.
- Álvarez, R.; Díez, M.A.; Barriocanal, C.; Díaz-Faes, E.; Cimadevilla, J.L.G. An Approach to Blast Furnace Coke Quality Prediction. Fuel 2007, 86, 2159–2166.
- Babich, A.; Senk, D.; Gudenau, H.W. Ironmaking; Mainz GmbH Aachen: Aachen, Germany, 2008.
- Scarpinella, C.A.; Cyro, T.; Tagusagawa, S.Y.; Mourao, M.B.; Lenz e Silva, F.B. Charcoal ironmaking: A contribution for CO2 mitigation. In Proceedings of the Fray International Syymposium, Cancun, Mexico, 27 November–1 December 2011; Metals and Materials Processing in a Clean Environment. pp. 109–121.
- Adilson de Castro, J.; de Medeiros, G.A.; de Oliveira, E.M.; de Campos, M.F.; Nogami, H. The Mini Blast Furnace Process: An Efficient Reactor for Green Pig Iron Production Using Charcoal and Hydrogen-Rich Gas: A Study of Cases. Metals 2020, 10, 1501.
- Hanrot, F.; Sert, D.; Delinchant, J.; Pietruck, R.; Bürgler, T.; Babich, A.; Fernández, M.; Alvarez, R.; Diez, M.A. CO2 Mitigation for Steelmaking Using Charcoal and Plastics Wastes as Reducing Agents and Secondary Raw Materials. In Proceedings of the 1st Spanish National Conference on Advances in Materials Recycling and Eco—Energy, Madrid, Spain, 12–13 November 2009; pp. 181–184.
- Steel Statistical Yearbook 2019. Available online: https://worldsteel.org/wp-content/uploads/Steel-Statistical-Yearbook-2019-concise-version.pdf (accessed on 22 November 2022).
- Rehfeldt, M.; Worrell, E.; Eichhammer, W.; Fleiter, T. A Review of the Emission Reduction Potential of Fuel Switch towards Biomass and Electricity in European Basic Materials Industry until 2030. Renew. Sustain. Energy Rev. 2020, 120, 109672.
- Yunos, N.F.M.; Zaharia, M.; Idris, M.A.; Nath, D.; Khanna, R.; Sahajwalla, V. Recycling Agricultural Waste from Palm Shells during Electric Arc Furnace Steelmaking. Energy Fuels 2012, 26, 278–286.
- Huang, X.-A.; Ng, K.W.; Giroux, L.; Duchesne, M. Carbonaceous Material Properties and Their Interactions with Slag During Electric Arc Furnace Steelmaking. Met. Mater. Trans. B 2019, 50, 1387–1398.
- Bianco, L.; Baracchini, G.; Cirilli, F.; Sante, L.D.; Moriconi, A.; Moriconi, E.; Agorio, M.M.; Pfeifer, H.; Echterhof, T.; Demus, T.; et al. Sustainable Electric Arc Furnace Steel Production: GREENEAF. BHM Berg-Und Hüttenmännische Mon. 2013, 158, 17–23.
- Fidalgo, B.; Berrueco, C.; Millan, M. Chars from Agricultural Wastes as Greener Fuels for Electric Arc Furnaces. J. Anal. Appl. Pyrolysis 2015, 113, 274–280.
- Meier, T.; Hay, T.; Echterhof, T.; Pfeifer, H.; Rekersdrees, T.; Schlinge, L.; Elsabagh, S.; Schliephake, H. Process Modeling and Simulation of Biochar Usage in an Electric Arc Furnace as a Substitute for Fossil Coal. Steel Res. Int. 2017, 88, 1600458.
- Nwachukwu, C.M.; Wang, C.; Wetterlund, E. Exploring the Role of Forest Biomass in Abating Fossil CO2 Emissions in the Iron and Steel Industry—The Case of Sweden. Appl. Energy 2021, 288, 116558.
- Echterhof, T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222.
- Wieder, K.; Böhm, C.; Wurm, J.; Vuletic, B. Confronting the Coke Shortage with the COREX and FINEX Technology. BHM Berg- Und Hüttenmännische Mon. 2004, 149, 379–384.
- Rammer, B.; Millner, R.; Boehm, C. Comparing the CO2 Emissions of Different Steelmaking Routes. BHM Berg- Und Hüttenmännische Mon. 2017, 162, 7–13.
- Hammerschmid, M.; Müller, S.; Fuchs, J.; Hofbauer, H. Evaluation of Biomass-Based Production of below Zero Emission Reducing Gas for the Iron and Steel Industry. Biomass Conv. Bioref. 2021, 11, 169–187.
- Mahi, F.T.; Nassaralla, C.L. Iron Production. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; p. B9780128035818035000. ISBN 978-0-12-803581-8.
- Prachethan Kumar, P.; Raju, B.S.L.; Ranjan, M. Characteristics of Coal Required for Superior Performance of Corex Ironmaking. Ironmak. Steelmak. 2011, 38, 412–416.
- Hasanbeigi, A.; Arens, M.; Price, L. Alternative Emerging Ironmaking Technologies for Energy-Efficiency and Carbon Dioxide Emissions Reduction: A Technical Review. Renew. Sustain. Energy Rev. 2014, 33, 645–658.
- Adeleke, A.A.; Odusote, J.K.; Lasode, O.A.; Ikubanni, P.P.; Malathi, M.; Paswan, D. Densification of Coal Fines and Mildly Torrefied Biomass into Composite Fuel Using Different Organic Binders. Heliyon 2019, 5, e02160.
- Yang, F.; Meerman, H.; Faaij, A. Harmonized Comparison of Virgin Steel Production Using Biomass with Carbon Capture and Storage for Negative Emissions. Int. J. Greenh. Gas Control 2021, 112, 103519.
- THE FINEX® Process Economical and Environmentally Safe Ironmaking. Available online: https://www.primetals.com/fileadmin/user_upload/content/01_portfolio/1_ironmaking/finex/THE_FINEX_R__PROCESS.pdf (accessed on 17 November 2022).
- Khasraw, D.; Spooner, S.; Hage, H.; Meijer, K.; Li, Z. Devolatilisation Characteristics of Coal and Biomass with Respect to Temperature and Heating Rate for HIsarna Alternative Ironmaking Process. Fuel 2021, 284, 119101.
- Abdul Quader, M.; Ahmed, S.; Dawal, S.Z.; Nukman, Y. Present Needs, Recent Progress and Future Trends of Energy-Efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) Program. Renew. Sustain. Energy Rev. 2016, 55, 537–549.
- Zhang, X.; Jiao, K.; Zhang, J.; Guo, Z. A Review on Low Carbon Emissions Projects of Steel Industry in the World. J. Clean. Prod. 2021, 306, 127259.
- Khasraw, D.; Theint Htet, T.; Yang, X.; Degirmenci, V.; Hage, H.; Meijer, K.; Li, Z. Gasification and Structural Behaviour of Different Carbon Sources and Resultant Chars from Rapid Devolatilization for HIsarna Alternative Ironmaking Process. Fuel 2022, 309, 122210.
- Htet, T.T.; Yan, Z.; Spooner, S.; Degirmenci, V.; Meijer, K.; Li, Z. Gasification and Physical-Chemical Characteristics of Carbonaceous Materials in Relation to HIsarna Ironmaking Process. Fuel 2021, 289, 119890.
- Surup, G.; Vehus, T.; Eidem, P.-A.; Trubetskaya, A.; Nielsen, H.K. Characterization of Renewable Reductants and Charcoal-Based Pellets for the Use in Ferroalloy Industries. Energy 2019, 167, 337–345.
- Surup, G.R.; Trubetskaya, A.; Tangstad, M. Charcoal as an Alternative Reductant in Ferroalloy Production: A Review. Processes 2020, 8, 1432.
- Monsen, B.; Tangstad, M.; Solheim, I.; Syvertsen, M.; Ishak, R.; Midtgaard, M. Charcoal for Manganese Alloy Production. In Proceedings of the 11th International Ferroalloys Congress, New Delhi, India, 18–21 February 2007; pp. 297–310.
- Surup, G.R.; Foppe, M.; Schubert, D.; Deike, R.; Heidelmann, M.; Timko, M.T.; Trubetskaya, A. The Effect of Feedstock Origin and Temperature on the Structure and Reactivity of Char from Pyrolysis at 1300–2800 °C. Fuel 2019, 235, 306–316.
- Solar, J.; Hernandez, A.; Lopez-Urionabarrenechea, A.; de Marco, I.; Adrados, A.; Caballero, B.M.; Gastelu, N. From Woody Biomass Waste to Biocoke: Influence of the Proportion of Different Tree Components. Eur. J. Wood Prod. 2017, 75, 485–497.
- Sommerfeld, M.; Friedrich, B. Replacing Fossil Carbon in the Production of Ferroalloys with a Focus on Bio-Based Carbon: A Review. Minerals 2021, 11, 1286.
- Surup, G.; Kaffash, H.; Ma, Y.; Trubetskaya, A.; Pettersen, J.; Tangstad, M. Life Cycle Based Climate Emissions of Charcoal Conditioning Routes for the Use in the Ferro-Alloy Production. Energies 2022, 15, 3933.
More
Information
Subjects:
Metallurgy & Metallurgical Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
02 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




