
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ahmed S BaHammam | -- | 3972 | 2022-12-01 07:24:27 | | | |
| 2 | Rita Xu | -60 word(s) | 3912 | 2022-12-01 08:01:20 | | |
Video Upload Options
Narcolepsy is a known auto-immune disease that presents mainly in the teenage years with irresistible sleep attacks. Patients with narcolepsy, especially NT1, have been found to have a high prevalence of obesity and other metabolic derangements. Compared to controls, patients with narcolepsy are more likely to be obese and have higher BMIs and waist circumferences. According to recent research, weight gain in narcolepsy patients may be higher during the disease's outset. Furthermore, the available data did not show any appreciable alterations in the levels of CSF melanin-concentrating hormone, plasma and CSF leptin, or serum growth hormone in relation to weight gain. Other mechanisms have been proposed, including a reduction in sympathetic tone, hormonal changes, changes in eating behavior and physical activity, and genetic predisposition. The association between increased body mass index and narcolepsy is well-recognized; the relationship between narcolepsy and other metabolic measures, such as body fat/muscle distribution and metabolic rate independent of BMI, is not well documented, and the available evidence is inconsistent. Future longitudinal studies with larger sample sizes are needed to assess BMR in patients with narcolepsy under a standard protocol at the outset of narcolepsy, with regular follow-up.
1. Introduction
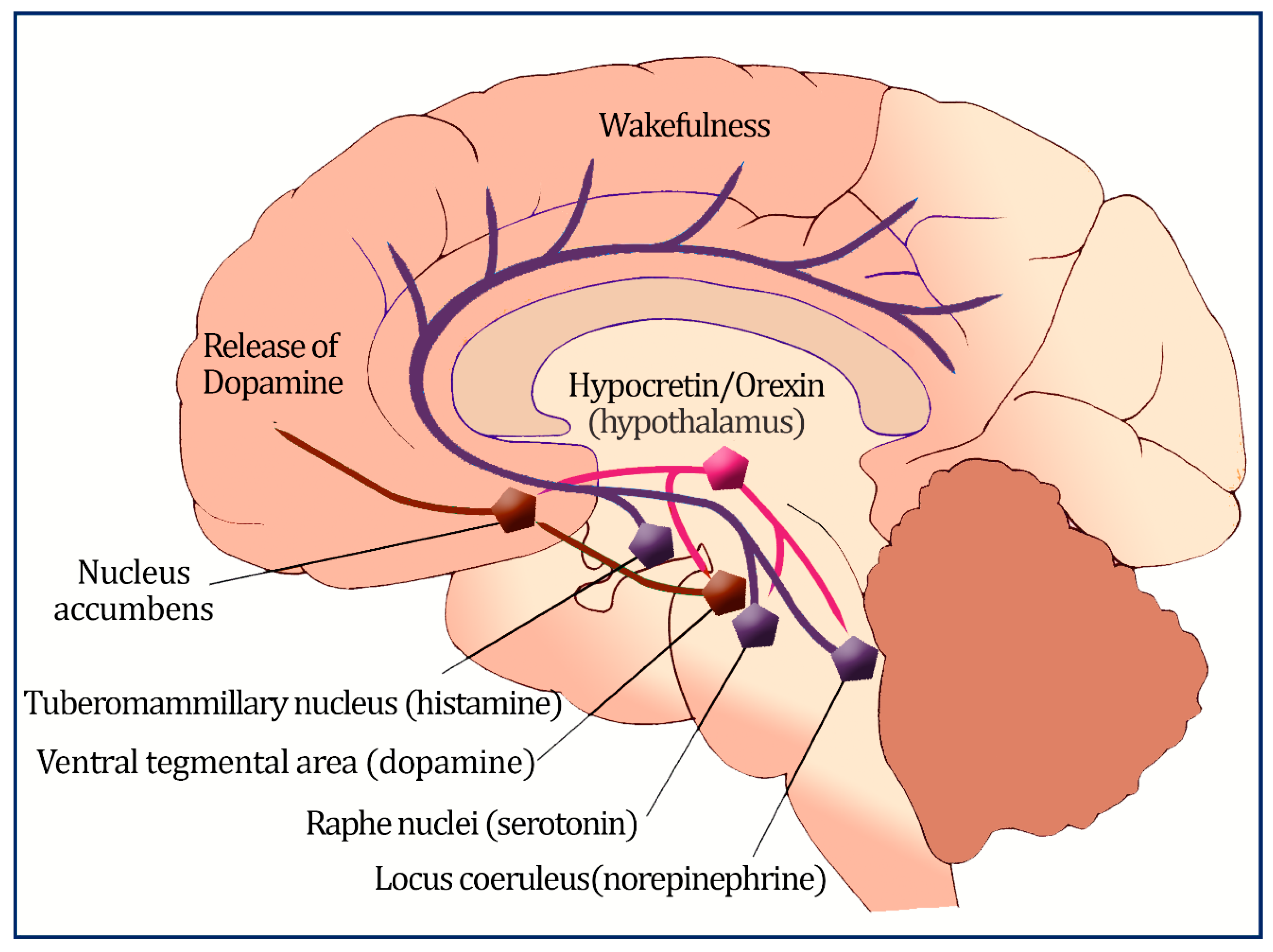
2. Prevalence of Obesity in Patients with Narcolepsy
| Study/Year Country | Study Design | No of Cases | Prevalence of Overweight (OW), Obesity (OB)%, or Mean BMI ± SD in Cases | No of Controls | Prevalence of Overweight (OW), Obesity (OB)%, or Mean BMI ± SD in Controls | p-Value |
|---|---|---|---|---|---|---|
| Filardi [17], 2020, Italy | Case-control | 38 | OW 28.95 OB 39.47 |
21 | OW 4.76 OB 28.57 |
<0.05 |
| Barateau [18], 2019, France | Case-control | 92 | OW 38.04 OB 23.91 |
109 | OW 27.52 OB 4.59 |
<0.000 |
| Vandi [19], 2019, Italy | Case-control | 27 | OW 62.96 OB 18.5 |
19 | OW 21.1 OB 21.1 |
0.010 |
| Drissi [20], 2018, Sweden | Case-control | 19 | 24.72 ± 6.37 | 17 | 21.22 ± 2.42 | 0.039 |
| Wang [21], 2016, China | Case-control | 65 | OW 26.15 OB 38.46 |
79 | OW 5.06 OB 3.80 |
0.002 <0.001 |
| Kovalska [22], 2016, Czech Republic |
Case-control | 42 | 31.47 ± 5.41 | 46 | 27.53 ± 6.11 | 0.0021 |
| Donadio [23], 2014, Italy |
Case-control | 19 | 25 ± 4 | 19 | 23 ± 3 | <0.05 |
| Dauvillers [24], 2012, France | Case-control | 50 | BMI 25.11 [17.01–38.30] | 42 | BMI 21.40 [18.30–29.10] | 0.0001 |
| Poli [25],2009, Italy | Case-control | 14 | 28 ± 4.4 | 14 | 24.2 ± 2.8 | 0.012 |
| Arnulf [26], 2006, France | Case-control | 93 | 27.6 ± 0.6 | 111 | 25.0 ± 0.4 | <0.05 |
| Dahmen [27], 2001, Germany | Case-control | 132 | 28.2 ± 5.5 | 104 | 24.5 ± 4.7 | <0.0001 |
4. Proposed Theories of Weight Gain in Narcolepsy
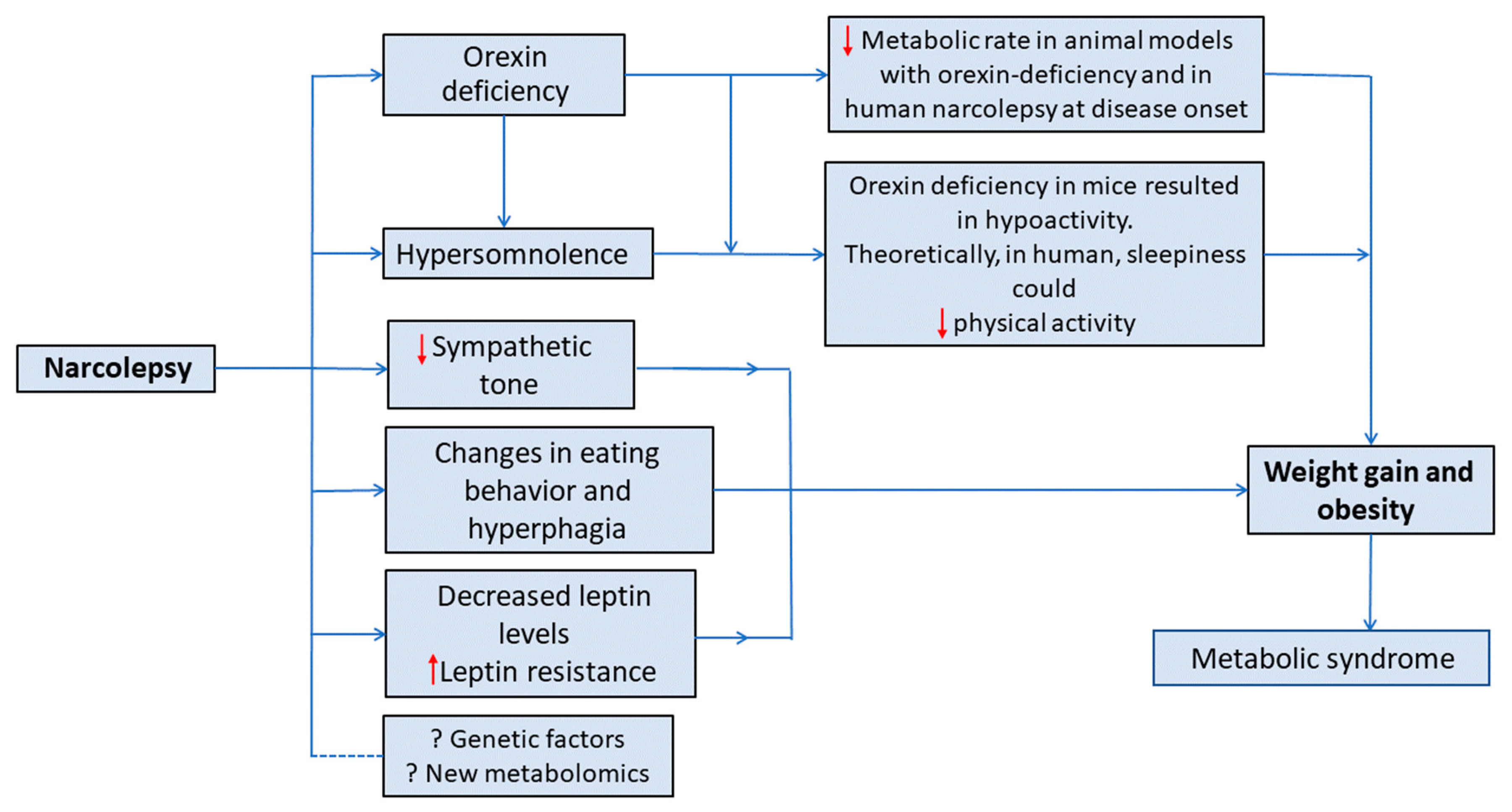
4.1. Orexin’s Role in Metabolism
4.2. Orexin and Eating Behavior
4.3. Leptin, Ghrelin, and Other Hormonal Changes
5. Changes in Metabolic Rate (Energy Expenditure) in Patients with Narcolepsy
It has been reported that preadipocytes in the brown adipose tissue of animal models with orexin deficiency may become incapable of differentiating, which in turn reduces thermogenesis and energy expenditure [87]. Orexin has also been demonstrated to control the metabolism of muscle glucose via the activation of muscle sympathetic neurons and beta(2)-adrenergic transmission [88]. Moreover, orexin receptor-2-deficient mice revealed lower energy expenditure when fed a high-fat diet [89]. Therefore, it has been proposed that orexin may cause a lower metabolic rate in some patients with narcolepsy resulting in obesity despite eating fewer calories.
A recent systematic review investigated metabolic profiles in narcolepsy; four studies that measured BMR-RMR were assessed and found no statistically significant difference in the BMR-RMR between patients with narcolepsy (n = 53) and controls (n = 75) [90]. Nonetheless, a meta-analysis was not performed because of the small number of studies.
In summary, measuring metabolic rate may lead to substantial insights into the pathophysiology of obesity in patients with narcolepsy. However, the limited number of studies evaluating metabolic rate in the literature and the disagreement about its reduction in patients with confirmed low orexin levels makes an association between the development of obesity and metabolic rate reduction in narcolepsy less likely. To confirm this, it would be helpful to establish a standard protocol for measuring BMR from the onset of narcolepsy, with regular follow-up of BMR and BMI in a large number of patients compared with controls matched for BMI, age, and sex, and in this regard, further research may be of additional value.6. Conclusions and Future Directions
Obesity and increased BMI and waist circumference are more prevalent among patients with narcolepsy than controls. Current evidence suggests that weight gain in narcolepsy may be higher early during the disease onset. However, the exact mechanisms of this weight gain are not known. Current evidence, though limited, does not support changes in BMR and RMR in patients with narcolepsy compared with controls except at disease onset. Moreover, current evidence did not document significant changes in different hormonal profiles related to weight gains, such as serum GH, plasma and CSF leptin, and CSF melanin-concentrating hormone levels.
Nevertheless, more work is needed to characterize the metabolic effects of orexin, including regulation of food intake with its relation to ghrelin and leptin hormones, fat and glucose metabolism, autonomic control, and energy homeostasis. Additionally, future longitudinal studies with larger sample sizes are needed to assess BMR in patients with narcolepsy under a standard protocol at the outset of narcolepsy, with regular follow-up of BMR and BMI compared to controls matched for BMI, age, and sex. Furthermore, clustering patients into different phenotypes may significantly impact the understanding of narcolepsy-related obesity.
Meanwhile, early screening for overweight and obesity in patients with narcolepsy is crucially valuable, as early non-pharmacological and pharmacological interventions could overcome the weight gain accompanying narcolepsy and reduce obesity-related complications. Patients with narcolepsy should receive proper health education about obesity and its effects. In addition, advice for a healthy lifestyle, including a healthy diet and physical exercises, could be implemented early in the management plan. Furthermore, following patients with narcolepsy in the clinic may include a periodic assessment of increased BMI and an investigation of metabolic derangements.References
- Aserinsky, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273–274.
- Longstreth, W.T., Jr.; Koepsell, T.D.; Ton, T.G.; Hendrickson, A.F.; van Belle, G. The epidemiology of narcolepsy. Sleep 2007, 30, 13–26.
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders:(ICSD-3); American Academy of Sleep Medicine: Darien, IL, USA, 2014.
- Kornum, B.R.; Knudsen, S.; Ollila, H.M.; Pizza, F.; Jennum, P.J.; Dauvilliers, Y.; Overeem, S. Narcolepsy. Nat. Rev. Dis. Primers 2017, 3, 16100.
- Quaedackers, L.; Pillen, S.; Overeem, S. Recognizing the Symptom Spectrum of Narcolepsy to Improve Timely Diagnosis: A Narrative Review. Nat. Sci. Sleep 2021, 13, 1083–1096.
- Mahoney, C.E.; Cogswell, A.; Koralnik, I.J.; Scammell, T.E. The neurobiological basis of narcolepsy. Nat. Rev. Neurosci. 2019, 20, 83–93.
- Siegel, J.M. Narcolepsy: A key role for hypocretins (orexins). Cell 1999, 98, 409–412.
- Li, J.; Hu, Z.; de Lecea, L. The hypocretins/orexins: Integrators of multiple physiological functions. Br. J. Pharmacol. 2014, 171, 332–350.
- Muroya, S.; Funahashi, H.; Yamanaka, A.; Kohno, D.; Uramura, K.; Nambu, T.; Shibahara, M.; Kuramochi, M.; Takigawa, M.; Yanagisawa, M.; et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca2+ signaling in a reciprocal manner to leptin: Orexigenic neuronal pathways in the mediobasal hypothalamus. Eur. J. Neurosci. 2004, 19, 1524–1534.
- Peyron, C.; Tighe, D.K.; van den Pol, A.N.; de Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998, 18, 9996–10015.
- Heinonen, M.V.; Purhonen, A.K.; Makela, K.A.; Herzig, K.H. Functions of orexins in peripheral tissues. Acta Physiol. 2008, 192, 471–485.
- Jennum, P.; Thorstensen, E.W.; Pickering, L.; Ibsen, R.; Kjellberg, J. Morbidity and mortality of middle-aged and elderly narcoleptics. Sleep Med. 2017, 36, 23–28.
- Jennum, P.J.; Plazzi, G.; Silvani, A.; Surkin, L.A.; Dauvilliers, Y. Cardiovascular disorders in narcolepsy: Review of associations and determinants. Sleep Med. Rev. 2021, 58, 101440.
- Futenma, K.; Takaesu, Y.; Nakamura, M.; Hayashida, K.; Takeuchi, N.; Inoue, Y. Metabolic-Syndrome-Related Comorbidities in Narcolepsy Spectrum Disorders: A Preliminary Cross-Sectional Study in Japan. Int. J. Environ. Res. Public Health 2022, 19, 6285.
- Poli, F.; Pizza, F.; Mignot, E.; Ferri, R.; Pagotto, U.; Taheri, S.; Finotti, E.; Bernardi, F.; Pirazzoli, P.; Cicognani, A.; et al. High prevalence of precocious puberty and obesity in childhood narcolepsy with cataplexy. Sleep 2013, 36, 175–181.
- Mohammadi, S.; Moosaie, F.; Saghazadeh, A.; Mahmoudi, M.; Rezaei, N. Metabolic profile in patients with narcolepsy: A systematic review and meta-analysis. Sleep Med. 2021, 81, 268–284.
- Filardi, M.; Demir, N.; Pizza, F.; Vandi, S.; Antelmi, E.; Noce, S.; Bruni, O.; Plazzi, G. Prevalence and neurophysiological correlates of sleep disordered breathing in pediatric type 1 narcolepsy. Sleep Med. 2020, 65, 8–12.
- Barateau, L.; Chenini, S.; Evangelista, E.; Jaussent, I.; Lopez, R.; Dauvilliers, Y. Clinical autonomic dysfunction in narcolepsy type 1. Sleep 2019, 42, zsz187.
- Vandi, S.; Rodolfi, S.; Pizza, F.; Moresco, M.; Antelmi, E.; Ferri, R.; Mignot, E.; Plazzi, G.; Silvani, A. Cardiovascular autonomic dysfunction, altered sleep architecture, and muscle overactivity during nocturnal sleep in pediatric patients with narcolepsy type 1. Sleep 2019, 42, zsz169.
- Morales Drissi, N.; Romu, T.; Landtblom, A.M.; Szakacs, A.; Hallbook, T.; Darin, N.; Borga, M.; Leinhard, O.D.; Engstrom, M. Unexpected Fat Distribution in Adolescents With Narcolepsy. Front. Endocrinol. 2018, 9, 728.
- Wang, Z.; Wu, H.; Stone, W.S.; Zhuang, J.; Qiu, L.; Xu, X.; Wang, Y.; Zhao, Z.; Han, F. Body weight and basal metabolic rate in childhood narcolepsy: A longitudinal study. Sleep Med. 2016, 25, 139–144.
- Kovalska, P.; Kemlink, D.; Nevsimalova, S.; Maurovich Horvat, E.; Jarolimova, E.; Topinkova, E.; Sonka, K. Narcolepsy with cataplexy in patients aged over 60 years: A case-control study. Sleep Med. 2016, 26, 79–84.
- Donadio, V.; Liguori, R.; Vandi, S.; Pizza, F.; Dauvilliers, Y.; Leta, V.; Giannoccaro, M.P.; Baruzzi, A.; Plazzi, G. Lower wake resting sympathetic and cardiovascular activities in narcolepsy with cataplexy. Neurology 2014, 83, 1080–1086.
- Dauvilliers, Y.; Jaussent, I.; Krams, B.; Scholz, S.; Lado, S.; Levy, P.; Pepin, J.L. Non-dipping blood pressure profile in narcolepsy with cataplexy. PLoS ONE 2012, 7, e38977.
- Poli, F.; Plazzi, G.; Di Dalmazi, G.; Ribichini, D.; Vicennati, V.; Pizza, F.; Mignot, E.; Montagna, P.; Pasquali, R.; Pagotto, U. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep 2009, 32, 1491–1497.
- Arnulf, I.; Lin, L.; Zhang, J.; Russell, I.J.; Ripley, B.; Einen, M.; Nevsimalova, S.; Bassetti, C.; Bourgin, P.; Nishino, S.; et al. CSF versus serum leptin in narcolepsy: Is there an effect of hypocretin deficiency? Sleep 2006, 29, 1017–1024.
- Dahmen, N.; Bierbrauer, J.; Kasten, M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 85–89.
- Ponziani, V.; Gennari, M.; Pizza, F.; Balsamo, A.; Bernardi, F.; Plazzi, G. Growing Up with Type 1 Narcolepsy: Its Anthropometric and Endocrine Features. J. Clin. Sleep Med. 2016, 12, 1649–1657.
- Zhang, M.; Thieux, M.; Inocente, C.O.; Vieux, N.; Arvis, L.; Villanueva, C.; Lin, J.S.; Plancoulaine, S.; Guyon, A.; Franco, P. Characterization of rapid weight gain phenotype in children with narcolepsy. CNS Neurosci. Ther. 2022, 28, 829–841.
- Franco, P.; Dauvilliers, Y.; Inocente, C.O.; Guyon, A.; Villanueva, C.; Raverot, V.; Plancoulaine, S.; Lin, J.S. Impaired histaminergic neurotransmission in children with narcolepsy type 1. CNS Neurosci. Ther. 2019, 25, 386–395.
- Dauvilliers, Y.; Delallee, N.; Jaussent, I.; Scholz, S.; Bayard, S.; Croyal, M.; Schwartz, J.C.; Robert, P. Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions. Sleep 2012, 35, 1359–1366.
- Anaclet, C.; Parmentier, R.; Ouk, K.; Guidon, G.; Buda, C.; Sastre, J.P.; Akaoka, H.; Sergeeva, O.A.; Yanagisawa, M.; Ohtsu, H.; et al. Orexin/hypocretin and histamine: Distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J. Neurosci. 2009, 29, 14423–14438.
- Parmentier, R.; Ohtsu, H.; Djebbara-Hannas, Z.; Valatx, J.L.; Watanabe, T.; Lin, J.S. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: Evidence for the role of brain histamine in behavioral and sleep-wake control. J. Neurosci. 2002, 22, 7695–7711.
- Schuld, A.; Beitinger, P.A.; Dalal, M.; Geller, F.; Wetter, T.C.; Albert, E.D.; Hebebrand, J.; Pollmacher, T. Increased body mass index (BMI) in male narcoleptic patients, but not in HLA-DR2-positive healthy male volunteers. Sleep Med. 2002, 3, 335–339.
- Yamamoto, Y.; Ueta, Y.; Date, Y.; Nakazato, M.; Hara, Y.; Serino, R.; Nomura, M.; Shibuya, I.; Matsukura, S.; Yamashita, H. Down regulation of the prepro-orexin gene expression in genetically obese mice. Brain Res. Mol. Brain Res. 1999, 65, 14–22.
- Almeneessier, A.S.; Alballa, N.S.; Alsalman, B.H.; Aleissi, S.; Olaish, A.H.; BaHammam, A.S. A 10-Year Longitudinal Observational Study Of Cataplexy In A Cohort Of Narcolepsy Type 1 Patients. Nat. Sci. Sleep 2019, 11, 231–239.
- Cremaschi, R.C.; Hirotsu, C.; Tufik, S.; Coelho, F.M. Narcolepsy type 1 and type 2—A 10-year follow-up: Body mass index and comorbidities. Sleep Med. 2017, 32, 285–286.
- Abulmeaty, M.M.A.; BaHammam, A.S.; Aljuraiban, G.S.; Almajwal, A.M.; Aldosari, M.S. Measured resting metabolic rate, respiratory quotient, and body composition in patients with narcolepsy: A preliminary report of a case-control study. Sci. Rep. 2020, 10, 11024.
- Parmar, A.; Yeh, E.A.; Korczak, D.J.; Weiss, S.K.; Lu, Z.; Zweerink, A.; Toulany, A.; Murray, B.J.; Narang, I. Depressive symptoms, sleep patterns, and physical activity in adolescents with narcolepsy. Sleep 2019, 42, zsz111.
- Chabas, D.; Foulon, C.; Gonzalez, J.; Nasr, M.; Lyon-Caen, O.; Willer, J.C.; Derenne, J.P.; Arnulf, I. Eating disorder and metabolism in narcoleptic patients. Sleep 2007, 30, 1267–1273.
- Fronczek, R.; Overeem, S.; Reijntjes, R.; Lammers, G.J.; van Dijk, J.G.; Pijl, H. Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin-deficient human narcolepsy. J. Clin. Sleep Med. 2008, 4, 248–254.
- Donjacour, C.E.; Aziz, N.A.; Roelfsema, F.; Frolich, M.; Overeem, S.; Lammers, G.J.; Pijl, H. Effect of sodium oxybate on growth hormone secretion in narcolepsy patients and healthy controls. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E1069–E1075.
- Schuld, A.; Hebebrand, J.; Geller, F.; Pollmacher, T. Increased body-mass index in patients with narcolepsy. Lancet 2000, 355, 1274–1275.
- Chang, X.; Suo, L.; Xu, N.; Zhao, Y. Orexin-A Stimulates Insulin Secretion Through the Activation of the OX1 Receptor and Mammalian Target of Rapamycin in Rat Insulinoma Cells. Pancreas 2019, 48, 568–573.
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585.
- Yamanaka, A.; Sakurai, T.; Katsumoto, T.; Yanagisawa, M.; Goto, K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res. 1999, 849, 248–252.
- Haynes, A.C.; Jackson, B.; Chapman, H.; Tadayyon, M.; Johns, A.; Porter, R.A.; Arch, J.R. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul. Pept. 2000, 96, 45–51.
- Yamanaka, A.; Beuckmann, C.T.; Willie, J.T.; Hara, J.; Tsujino, N.; Mieda, M.; Tominaga, M.; Yagami, K.; Sugiyama, F.; Goto, K.; et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 2003, 38, 701–713.
- Sakurai, T. Orexins and orexin receptors: Implication in feeding behavior. Regul. Pept. 1999, 85, 25–30.
- Almeneessier, A.S.; Alzoghaibi, M.; BaHammam, A.A.; Ibrahim, M.G.; Olaish, A.H.; Nashwan, S.Z.; BaHammam, A.S. The effects of diurnal intermittent fasting on the wake-promoting neurotransmitter orexin-A. Ann. Thorac. Med. 2018, 13, 48–54.
- Lubkin, M.; Stricker-Krongrad, A. Independent feeding and metabolic actions of orexins in mice. Biochem. Biophys. Res. Commun. 1998, 253, 241–245.
- Liu, L.; Wang, Q.; Liu, A.; Lan, X.; Huang, Y.; Zhao, Z.; Jie, H.; Chen, J.; Zhao, Y. Physiological Implications of Orexins/Hypocretins on Energy Metabolism and Adipose Tissue Development. ACS Omega 2020, 5, 547–555.
- Coborn, J.E.; DePorter, D.P.; Mavanji, V.; Sinton, C.M.; Kotz, C.M.; Billington, C.J.; Teske, J.A. Role of orexin-A in the ventrolateral preoptic area on components of total energy expenditure. Int. J. Obes. 2017, 41, 1256–1262.
- Nakamura, M.; Nagamine, T. Neuroendocrine, Autonomic, and Metabolic Responses to an Orexin Antagonist, Suvorexant, in Psychiatric Patients with Insomnia. Innov. Clin. Neurosci. 2017, 14, 30–37.
- Yoshikawa, F.; Shigiyama, F.; Ando, Y.; Miyagi, M.; Uchino, H.; Hirose, T.; Kumashiro, N. Chronotherapeutic efficacy of suvorexant on sleep quality and metabolic parameters in patients with type 2 diabetes and insomnia. Diabetes Res. Clin. Pract. 2020, 169, 108412.
- Toi, N.; Inaba, M.; Kurajoh, M.; Morioka, T.; Hayashi, N.; Hirota, T.; Miyaoka, D.; Emoto, M.; Yamada, S. Improvement of glycemic control by treatment for insomnia with suvorexant in type 2 diabetes mellitus. J. Clin. Transl. Endocrinol. 2019, 15, 37–44.
- Morrison, S.F.; Madden, C.J.; Tupone, D. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. Adipocyte 2012, 1, 116–120.
- Zink, A.N.; Bunney, P.E.; Holm, A.A.; Billington, C.J.; Kotz, C.M. Neuromodulation of orexin neurons reduces diet-induced adiposity. Int. J. Obes. 2018, 42, 737–745.
- Kok, S.W.; Overeem, S.; Visscher, T.L.; Lammers, G.J.; Seidell, J.C.; Pijl, H.; Meinders, A.E. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes. Res. 2003, 11, 1147–1154.
- Pollak, C.P.; Green, J. Eating and its relationships with subjective alertness and sleep in narcoleptic subjects living without temporal cues. Sleep 1990, 13, 467–478.
- Joly-Amado, A.; Cansell, C.; Denis, R.G.; Delbes, A.S.; Castel, J.; Martinez, S.; Luquet, S. The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 725–737.
- Micioni Di Bonaventura, E.; Botticelli, L.; Tomassoni, D.; Tayebati, S.K.; Micioni Di Bonaventura, M.V.; Cifani, C. The Melanocortin System behind the Dysfunctional Eating Behaviors. Nutrients 2020, 12, 3502.
- Fortuyn, H.A.; Swinkels, S.; Buitelaar, J.; Renier, W.O.; Furer, J.W.; Rijnders, C.A.; Hodiamont, P.P.; Overeem, S. High prevalence of eating disorders in narcolepsy with cataplexy: A case-control study. Sleep 2008, 31, 335–341.
- Dimitrova, A.; Fronczek, R.; Van der Ploeg, J.; Scammell, T.; Gautam, S.; Pascual-Leone, A.; Lammers, G.J. Reward-seeking behavior in human narcolepsy. J. Clin. Sleep. Med. 2011, 7, 293–300.
- van Holst, R.J.; van der Cruijsen, L.; van Mierlo, P.; Lammers, G.J.; Cools, R.; Overeem, S.; Aarts, E. Aberrant Food Choices after Satiation in Human Orexin-Deficient Narcolepsy Type 1. Sleep 2016, 39, 1951–1959.
- Kelly, N.R.; Shomaker, L.B.; Radin, R.M.; Thompson, K.A.; Cassidy, O.L.; Brady, S.; Mehari, R.; Courville, A.B.; Chen, K.Y.; Galescu, O.A.; et al. Associations of sleep duration and quality with disinhibited eating behaviors in adolescent girls at-risk for type 2 diabetes. Eat. Behav. 2016, 22, 149–155.
- van Holst, R.J.; Janssen, L.K.; van Mierlo, P.; Lammers, G.J.; Cools, R.; Overeem, S.; Aarts, E. Enhanced food-related responses in the ventral medial prefrontal cortex in narcolepsy type 1. Sci. Rep. 2018, 8, 16391.
- Mehr, J.B.; Mitchison, D.; Bowrey, H.E.; James, M.H. Sleep dysregulation in binge eating disorder and "food addiction": The orexin (hypocretin) system as a potential neurobiological link. Neuropsychopharmacology 2021, 46, 2051–2061.
- Barson, J.R. Orexin/hypocretin and dysregulated eating: Promotion of foraging behavior. Brain Res. 2020, 1731, 145915.
- Gonzalez, J.A.; Jensen, L.T.; Iordanidou, P.; Strom, M.; Fugger, L.; Burdakov, D. Inhibitory Interplay between Orexin Neurons and Eating. Curr. Biol. 2016, 26, 2486–2491.
- Hara, J.; Beuckmann, C.T.; Nambu, T.; Willie, J.T.; Chemelli, R.M.; Sinton, C.M.; Sugiyama, F.; Yagami, K.; Goto, K.; Yanagisawa, M.; et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 2001, 30, 345–354.
- Dauvilliers, Y.; Montplaisir, J.; Molinari, N.; Carlander, B.; Ondze, B.; Besset, A.; Billiard, M. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001, 57, 2029–2033.
- Dahmen, N.; Becht, J.; Engel, A.; Thommes, M.; Tonn, P. Prevalence of eating disorders and eating attacks in narcolepsy. Neuropsychiatr. Dis. Treat. 2008, 4, 257–261.
- Del Bianco, C.; Ulivi, M.; Liguori, C.; Pisani, A.; Mercuri, N.B.; Placidi, F.; Izzi, F. Alexithymia, impulsiveness, emotion, and eating dyscontrol: Similarities and differences between narcolepsy type 1 and type 2. Sleep Biol. Rhythms. 2022.
- Alzoghaibi, M.A.; Pandi-Perumal, S.R.; Sharif, M.M.; BaHammam, A.S. Diurnal intermittent fasting during Ramadan: The effects on leptin and ghrelin levels. PLoS ONE 2014, 9, e92214.
- Villano, I.; La Marra, M.; Di Maio, G.; Monda, V.; Chieffi, S.; Guatteo, E.; Messina, G.; Moscatelli, F.; Monda, M.; Messina, A. Physiological Role of Orexinergic System for Health. Int. J. Environ. Res. Public Health 2022, 19, 8353.
- Dahmen, N.; Engel, A.; Helfrich, J.; Manderscheid, N.; Lobig, M.; Forst, T.; Pfutzner, A.; Tonn, P. Peripheral leptin levels in narcoleptic patients. Diabetes Technol. Ther. 2007, 9, 348–353.
- Donjacour, C.E.; Pardi, D.; Aziz, N.A.; Frolich, M.; Roelfsema, F.; Overeem, S.; Pijl, H.; Lammers, G.J. Plasma total ghrelin and leptin levels in human narcolepsy and matched healthy controls: Basal concentrations and response to sodium oxybate. J. Clin. Sleep Med. 2013, 9, 797–803.
- Heier, M.S.; Jansson, T.S.; Gautvik, K.M. Cerebrospinal fluid hypocretin 1 deficiency, overweight, and metabolic dysregulation in patients with narcolepsy. J. Clin. Sleep Med. 2011, 7, 653–658.
- Huda, M.S.; Mani, H.; Durham, B.H.; Dovey, T.M.; Halford, J.C.; Aditya, B.S.; Pinkney, J.H.; Wilding, J.P.; Hart, I.K. Plasma obestatin and autonomic function are altered in orexin-deficient narcolepsy, but ghrelin is unchanged. Endocrine 2013, 43, 696–704.
- Kok, S.W.; Meinders, A.E.; Overeem, S.; Lammers, G.J.; Roelfsema, F.; Frolich, M.; Pijl, H. Reduction of plasma leptin levels and loss of its circadian rhythmicity in hypocretin (orexin)-deficient narcoleptic humans. J. Clin. Endocrinol. Metab. 2002, 87, 805–809.
- Nishino, S.; Ripley, B.; Overeem, S.; Nevsimalova, S.; Lammers, G.J.; Vankova, J.; Okun, M.; Rogers, W.; Brooks, S.; Mignot, E. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann. Neurol. 2001, 50, 381–388.
- Tsuneki, H.; Tokai, E.; Nakamura, Y.; Takahashi, K.; Fujita, M.; Asaoka, T.; Kon, K.; Anzawa, Y.; Wada, T.; Takasaki, I.; et al. Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes 2015, 64, 459–470.
- Tsuneki, H.; Murata, S.; Anzawa, Y.; Soeda, Y.; Tokai, E.; Wada, T.; Kimura, I.; Yanagisawa, M.; Sakurai, T.; Sasaoka, T. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia 2008, 51, 657–667.
- Shiuchi, T.; Haque, M.S.; Okamoto, S.; Inoue, T.; Kageyama, H.; Lee, S.; Toda, C.; Suzuki, A.; Bachman, E.S.; Kim, Y.B.; et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009, 10, 466–480.
- Engel, A.; Helfrich, J.; Manderscheid, N.; Musholt, P.B.; Forst, T.; Pfutzner, A.; Dahmen, N. Investigation of insulin resistance in narcoleptic patients: Dependent or independent of body mass index? Neuropsychiatr. Dis. Treat. 2011, 7, 351–356.
- Sellayah, D.; Bharaj, P.; Sikder, D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011, 14, 478–490.
- Shiuchi, T.; Haque, M.S.; Okamoto, S.; Inoue, T.; Kageyama, H.; Lee, S.; Toda, C.; Suzuki, A.; Bachman, E.S.; Kim, Y.B.; et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009, 10, 466–480.
- Kakizaki, M.; Tsuneoka, Y.; Takase, K.; Kim, S.J.; Choi, J.; Ikkyu, A.; Abe, M.; Sakimura, K.; Yanagisawa, M.; Funato, H. Differential Roles of Each Orexin Receptor Signaling in Obesity. iScience 2019, 20, 1–13.
- Mohammadi, S.; Moosaie, F.; Saghazadeh, A.; Mahmoudi, M.; Rezaei, N. Metabolic profile in patients with narcolepsy: A systematic review and meta-analysis. Sleep Med. 2021, 81, 268–284.




