
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adel Badria | -- | 1630 | 2022-11-30 19:29:17 | | | |
| 2 | Lindsay Dong | -18 word(s) | 1612 | 2022-12-01 10:31:35 | | |
Video Upload Options
Hierarchical structures are an essential part of numerous types of architecture in nature. They are defined as the presence of different structural elements with different length scales in a single body. This different length scale gives each hierarchical structure its “order, n” and characteristic properties. The higher the (n) the more sophisticated hierarchical structures; where n = 0 refers to continuum materials with only a single length scale. Noteworthy, several composites are considered low-ordered hierarchical structures. The idea of building blocks for hierarchical structures intersects perfectly with the modularity concept in click chemistry. Click chemistry is a powerful tool for constructing nano, micro and macro structures through two different approaches: (A) the first approach: through direct crosslinking of (pico-building blocks) monomers give a final micro/macro structure such as hydrogels; (B) the second approach: through nano-building blocks formation using click chemistry (e.g., dendrimers and dendrons) followed by connecting and crosslinking those formed nano-building blocks again using click chemistry to form bigger structures
1. Click Chemistry
| Advantage | Explanation |
|---|---|
| High Selectivity | - |
| High yield | - |
| Mild conditions | Usage of mild solvents and reactants |
| Fast reaction | The exothermicity of both thermodynamic and kinetics of the reaction allowed fast and stable final products |
| Minimum byproducts | - |
| Easy purification | No need for complicated chromatography or other purification techniques |
| Modularity | The ability of forming diverse library of chemical structures from simple chemical modules |
| Regiospecific | Minimum stereoisomers are being formed |
Historically, the first reaction to being identified as click chemistry is the copper-catalyzed azide-alkyne reaction to form a 5-membered ring. Nowadays, the click chemistry approach is being handled as a toolbox that different groups worldwide are adding to and modifying with different new reactions. So far, four categories have been identified to represent the different click chemistry reactions.
2. Hierarchical Structures and Click Chemistry
For any hierarchical structures in nature there have been repeated smaller building blocks which have been arranged in a certain manner to provide the required functionality for this hierarchical construction.
2.1. The First Approach
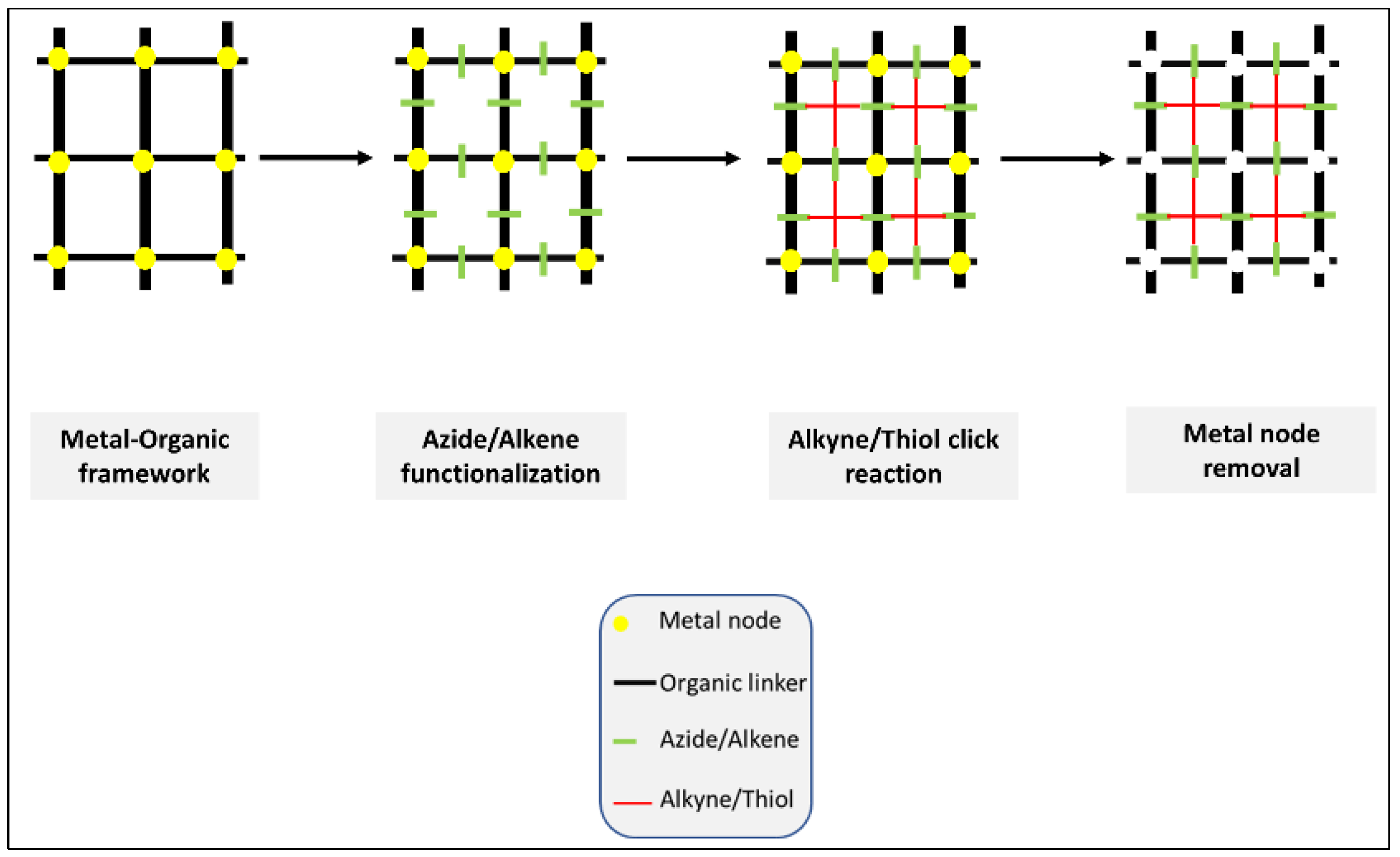
2.2. The Second Approach
2.2.1. Picometer ➔ 0D-Nanometer
2.2.2. 0D/1D-Nanometer ➔ 2D-Nanometer
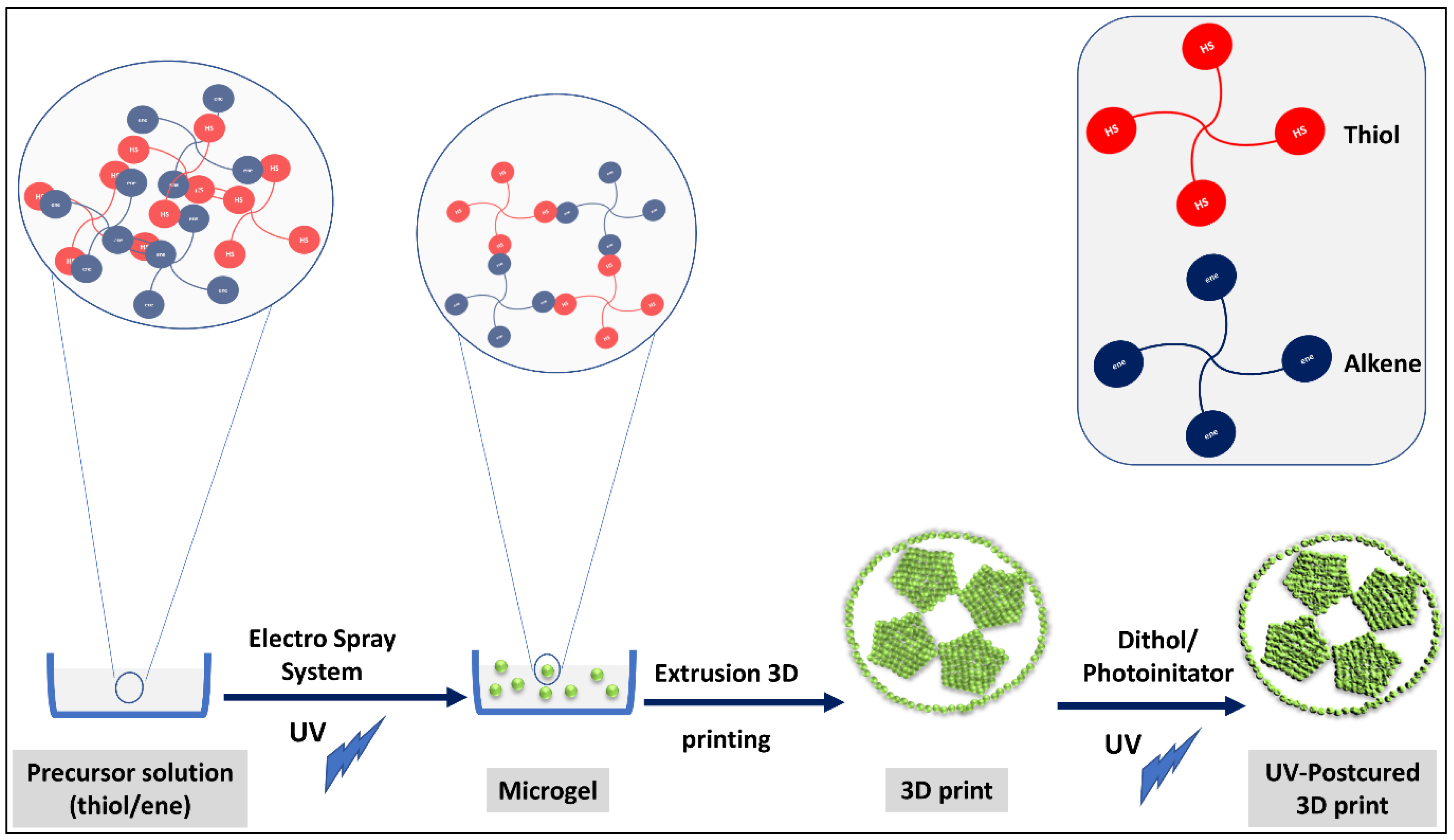
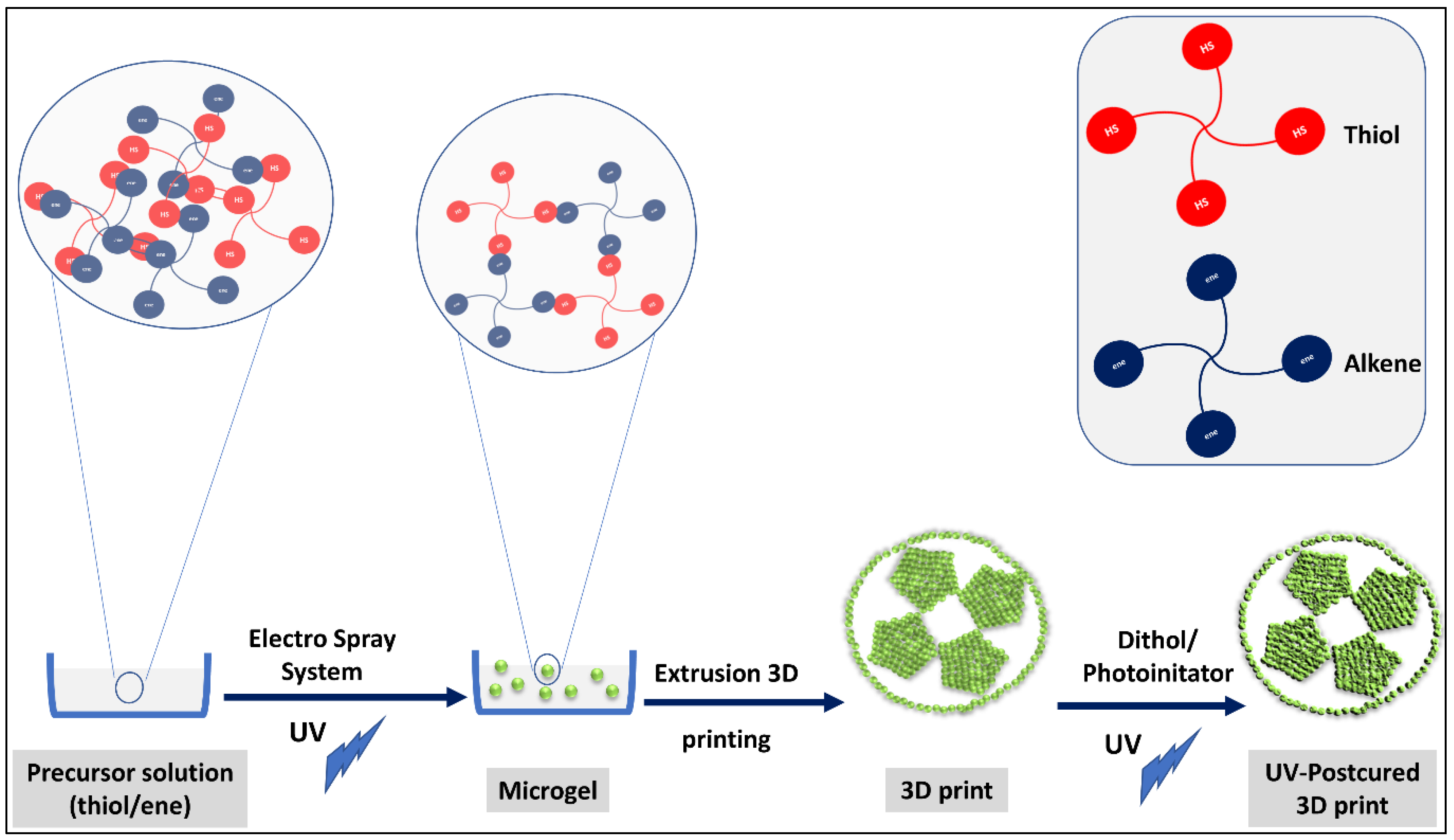
Soft/Soft Nano Building Blocks
Hard/Hard Nano Building Blocks
Soft/Hard Nano Building Blocks
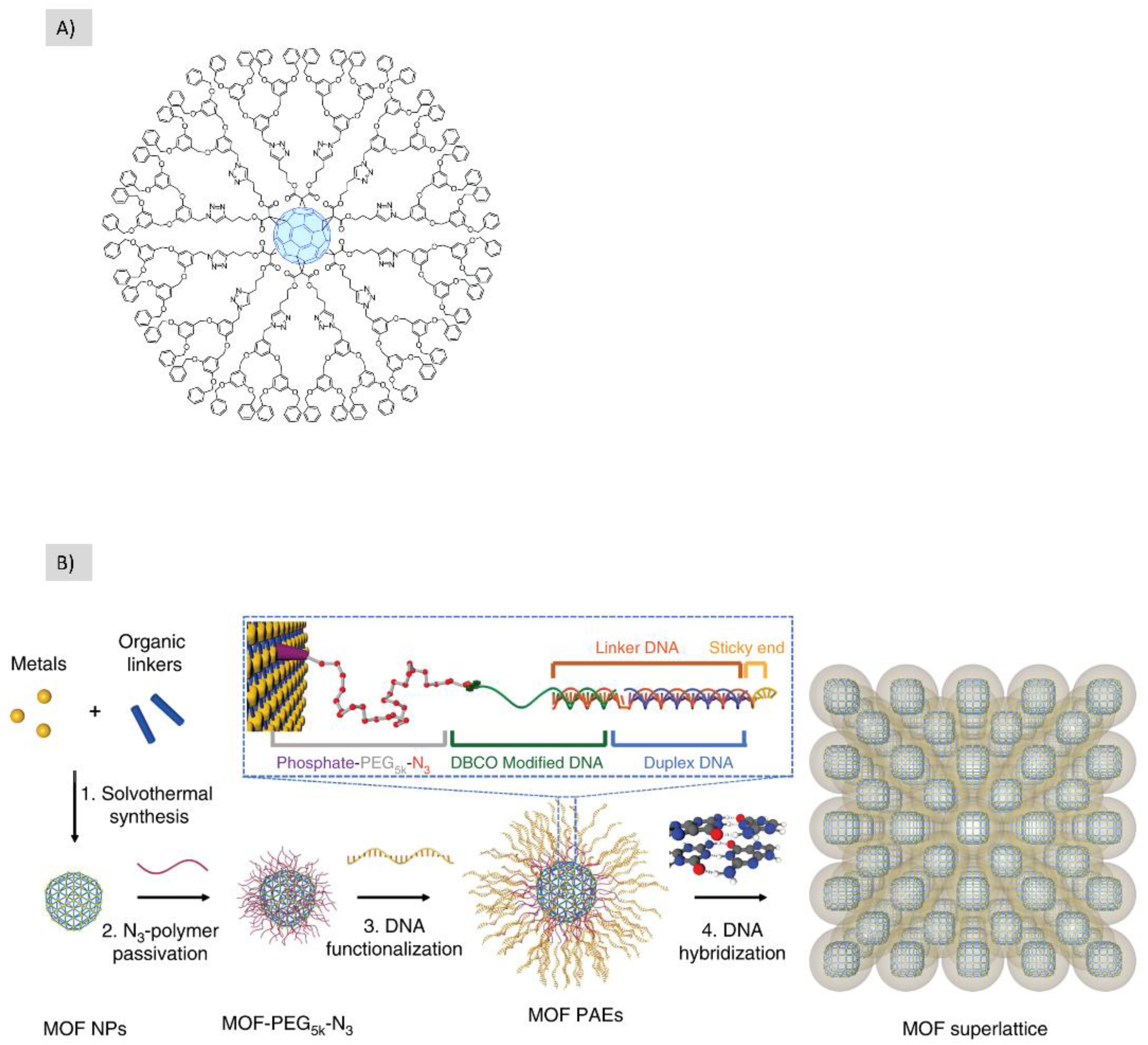
2.2.3. 2D-Nanometer ➔ 3D
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021.
- Luisa García-Betancourt, M.; Ramírez Jiménez, S.I.; González-Hodges, A.; Nuñez Salazar, Z.E.; Leilani Escalante-García, I.; Ramírez Aparicio, J. Low Dimensional Nanostructures: Measurement and Remediation Technologies Applied to Trace Heavy Metals in Water. In Trace Metals in the Environment-New Approaches and Recent Advances; IntechOpen: Piscataway, NJ, USA, 2021.
- Hassan, Z.; Matt, Y.; Begum, S.; Tsotsalas, M.; Bräse, S. Assembly of Molecular Building Blocks into Integrated Complex Functional Molecular Systems: Structuring Matter Made to Order. Adv. Funct. Mater. 2020, 30, 1907625.
- Xin, S.; Chimene, D.; Garza, J.E.; Gaharwar, A.K.; Alge, D.L. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater. Sci. 2019, 7, 1179–1187.
- Binder, W.H.; Sachsenhofer, R. ‘Click’ Chemistry in Polymer and Material Science: An Update. Macromol. Rapid Commun. 2008, 29, 952–981.
- Sanchez-Sanchez, A.; Pérez-Baena, I.; Pomposo, J. Advances in Click Chemistry for Single-Chain Nanoparticle Construction. Molecules 2013, 18, 3339–3355.
- Martens, S.; Holloway, J.O.; Du Prez, F.E. Click and Click-Inspired Chemistry for the Design of Sequence-Controlled Polymers. Macromol. Rapid Commun. 2017, 38, 1700469.
- Yi, G.; Son, J.; Yoo, J.; Park, C.; Koo, H. Application of click chemistry in nanoparticle modification and its targeted delivery. Biomater. Res. 2018, 22, 13.
- Yan, W.; Seifermann, S.M.; Pierrat, P.; Bräse, S. Synthesis of highly functionalized C 60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25–54.
- Contal, E.; Klymchenko, A.S.; Mély, Y.; Meunier, S.; Wagner, A. Core functionalization of polydiacetylene micelles by a “click” reaction. Soft Matter 2011, 7, 1648–1650.
- Walden, G.; Liao, X.; Riley, G.; Donell, S.; Raxworthy, M.J.; Saeed, A. Synthesis and Fabrication of Surface-Active Microparticles Using a Membrane Emulsion Technique and Conjugation of Model Protein via Strain-Promoted Azide–Alkyne Click Chemistry in Physiological Conditions. Bioconjug. Chem. 2019, 30, 531–535.
- Tomalia, D.A. In quest of a systematic framework for unifying and defining nanoscience. J. Nanopart. Res. 2009, 11, 1251–1310.
- Tomalia, D.A. Dendrons/dendrimers: Quantized, nano-element like building blocks for soft-soft and soft-hard nano-compound synthesis. Soft Matter 2010, 6, 456–474.
- Vestberg, R.; Malkoch, M.; Kade, M.; Wu, P.; Fokin, V.V.; Barry Sharpless, K.; Drockenmuller, E.; Hawker, C.J. Role of architecture and molecular weight in the formation of tailor-made ultrathin multilayers using dendritic macromolecules and click chemistry. J. Polym. Sci. Part Polym. Chem. 2007, 45, 2835–2846.
- Upadhyay, A.P.; Behara, D.K.; Sharma, G.P.; Bajpai, A.; Sharac, N.; Ragan, R.; Pala, R.G.S.; Sivakumar, S. Generic Process for Highly Stable Metallic Nanoparticle-Semiconductor Heterostructures via Click Chemistry for Electro/Photocatalytic Applications. ACS Appl. Mater. Interfaces 2013, 5, 9554–9562.
- Williams, M.G.; Teplyakov, A.V. Building high-coverage monolayers of covalently bound magnetic nanoparticles. Appl. Surf. Sci. 2016, 388, 461–467.
- Le Neindre, M.; Nicolaÿ, R. Polythiol copolymers with precise architectures: A platform for functional materials. Polym. Chem. 2014, 5, 4601.
- Hahn, U.; Vögtle, F.; Nierengarten, J.-F. Synthetic Strategies towards Fullerene-Rich Dendrimer Assemblies. Polymers 2012, 4, 501–538.
- Nierengarten, J.-F. Fullerene hexa-adduct scaffolding for the construction of giant molecules. Chem. Commun. 2017, 53, 11855–11868.
- Muñoz, A. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nat. Chem. 2016, 8, 50–57.
- Wang, S.; Park, S.S.; Buru, C.T.; Lin, H.; Chen, P.-C.; Roth, E.W.; Farha, O.K.; Mirkin, C.A. Colloidal crystal engineering with metal–organic framework nanoparticles and DNA. Nat. Commun. 2020, 11, 2495.
- Shehzad, K.; Xu, Y.; Gao, C.; Duan, X. Three-dimensional macro-structures of two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 5541–5588.
- Wang, K.; Ma, Q.; Qu, C.-X.; Zhou, H.-T.; Cao, M.; Wang, S.-D. Review on 3D Fabrication. Nanoscale Autex Res. J. 2022.
- Gaihre, B.; Potes, M.A.; Serdiuk, V.; Tilton, M.; Liu, X.; Lu, L. Two-dimensional nanomaterials-added dynamism in 3D printing and bioprinting of biomedical platforms: Unique opportunities and challenges. Biomaterials 2022, 284, 121507.
- Das, M.; Ambekar, R.S.; Panda, S.K.; Chakraborty, S.; Tiwary, C.S. 2D nanomaterials in 3D/4D-printed biomedical devices. J. Mater. Res. 2021, 36, 4024–4050.
- Lee, K.M.; Oh, Y.; Yoon, H.; Chang, M.; Kim, H. Multifunctional Role of MoS 2 in Preparation of Composite Hydrogels: Radical Initiation and Cross-Linking. ACS Appl. Mater. Interfaces 2020, 12, 8642–8649.
- Zhou, K.; Tang, G.; Gao, R.; Guo, H. Constructing hierarchical core-shell structures for regulating thermal and fire safety properties of polystyrene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2018, 107, 144–154.




